Abstract
Hypocrellin A (HA), well known as one of the best natural pigments and bioactive agent to treat skin diseases, is further anticipated to play a vital role in photodynamic therapy (PDT) in anticancer and antiviral treatments. In this study, an HA-producing strain ZZZ816 (Shiraia sp.) was isolated from the moso bamboo (Phyllostachys edulis) seeds, and gamma irradiation was used to mutagenize spores of the original strain. After treatment with cobalt-60 gamma (60Coγ) with different doses (20, 50, 80, 100, 150, 180, 300, and 500 Gy), the 100 Gy was selected as the optimal condition, which led to 77.2 % lethality of spores and 35 % positive mutant frequency. The extracted compound of the most excellent HA-producing strain (H-4-2) was precisely analyzed by a combination of seven detection methods, and the maximum HA content was shown to reach 2018.3 mg/L. HA production in H-4-2 increased by 414.9 % compared to that of original strain ZZZ816 (392 mg/L) and was significantly higher than all the other industrial HA-producing strains in published reports.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hypocrellins, which are generally regarded as important photosensitizers, belong to the class of perylenequinonoid compounds. They were initially extracted from parasitic fungi Shiraia bambusicola P. Henn. and Hypocrella bambusae (Berk. & Broome) Sacc., which are important sources of traditional Chinese medicines (Fang et al. 2006; Zhang et al. 1989). Until now, four types of hypocrellin (A, B, C, and D) with similar structures and properties have been isolated (Pan et al. 2012; Wu et al. 1989) and hypocrellin A (HA) exhibits greater potency compared to the other hypocrellins (Zhang and Ma, 2003). In China, hypocrellins have been used medicinally to treat skin diseases in China for many years, and this medicine was named “Zhuhongjunsu ruangao” (Kocisova et al. 1999). Hypocrellins were also regarded as the new generation of photodynamic therapy (PDT) drugs, being especially suitable for the treatment of superficial diseases (Deng et al. 2013; Wang et al. 1999; Xie et al. 2002; Zhou et al. 2014). Besides, many studies showed that hypocrellins possess favorable light-induced anticancer (Deng et al. 2013; Deininger et al. 2002) and antiviral activities (Yang et al. 2001; Ali et al. 2001; Xu et al. 2001), especially against the human immunodeficiency virus (HIV) (Hudson et al. 1994). In addition to benefiting the pharmaceutical industry, hypocrellins also have extensive potential applications in the agricultural, cosmetic, food, and feed industries (Qiu et al. 2011; Shen et al. 2012; Su et al. 2010; Su et al. 2011).
To protect dwindling natural resources and improve the production of HA of Shiraia sp., strain improvement has been achieved in recent years by UV mutagenesis (Pan et al. 2012), liquid cultural condition optimization (Xiang, 2010; Yang et al. 2009), chemical synthesis, or the induction of microbial elicitors (Du et al. 2015; Du et al. 2012; Li et al. 2000; Wu et al. 1995). Gamma irradiation is a convenient mutation method that has been broadly applied for several decades in microbial breeding fields to produce diverse bioactive metabolites, such as enzymes, organic acids, vitamins, and amino acids (Jiang et al. 2010; Najafi et al. 2011; Qi et al. 2007; Weng et al. 2012; Xie et al. 2010). However, in filamentous fungi, there were relatively few reports of gamma irradiation being applied to increase the yield of secondary metabolites (Najafi et al. 2011; Yun et al. 2008). In this study, cobalt-60 gamma (60Coγ) irradiation would be utilized to mutagenize spores of Shiraia sp.
Thin-layer chromatography (TLC), liquid chromatography-mass spectrometry (LC-MS), and nuclear magnetic resonance (NMR) are generally applied in qualitative analysis of bioactive agents and appeared in this study for the strains with the highest positive mutant rate. High-performance liquid chromatography (HPLC) was prepared for quantitative analysis of hyporellin A. Ultraviolet-visible (UV-vis) absorption spectrum and photoluminance (PL) spectrum could swiftly display the optical performance of HA. By these ways, HA-producing strains were screened for high-yield and stable hereditary. The highly accurate assays for identifying this red pigment have never appeared in the previous studies.
Materials and methods
Chemicals and reagents
Standard HA was purchased from Sichuan Weiqi Biotechnology Co. Limited (Chengdu, China). HPLC-grade methanol, formic acid, and acetonitrile used for HPLC and deuterated chloroform used for NMR were purchased from Fisher Scientific Co. (Fair Lawn, New Jersey, USA). All of the other chemicals, including acetone and dichloromethane, used in the study were of analytical grade.
Strains, media, and culture conditions
The original strain Shiraia sp. ZZZ816 (ACCC38984) was isolated from the moso bamboo seed in previous work (Shen et al. 2014) and was seeded onto 2 % potato dextrose agar medium (PDA, containing 200 g/L potato, 20 g/L dextrose, and 20 g/L agar; pH 6.0) at 25 °C without light. The fungal strain was stocked in the Agricultural Culture Collection of China (ACCC).
The fresh mycelia of the fungal strain ZZZ816 were cultured on plates at 25 °C for more than 7 days. Six plugs of the growing culture plus the adhering mycelia that were 6 mm in diameter were subsequently added to 250-mL Erlenmeyer flasks containing 100 mL potato dextrose broth medium (PDB, containing 200 g/L potato and 20 g/L dextrose; pH 6.0). All liquid cultures were kept at 25 °C for 60 h with shaking (150 rpm). Inoculum (0.1 mL) was smooth-spread on PDA plates and incubated at 25 °C for 7 days (Shen et al. 2014). All experiments were performed in triplicate.
Preparation of spore suspensions and 60Coγ irradiation procedures
The PDA plates were separately submerged in 10 mL of sterile ultra-pure water (contained 0.02 % Tween-80), and the spores were suspended with the spore content adjusted to 106 spores/mL (Brandsberg and French 1972). The spore suspensions were prepared as described and exposed to different doses of gamma rays produced by 60Coγ (20, 50, 80, 100, 150, 180, 300, and 500 Gy) and emitted by the Beijing Radiation Center (Beijing, China). The dose rate was 24.7 Gy/min at the time of the experiment at room temperature. After treatment with different doses of 60Coγ, spores subjected to different test doses were eluted, and spore suspensions were translated to PDA plates and incubated at 25 °C for 7 days.
Lethality assay and determination of mutant frequency
The lethality rate was determined by calculating the number of lethal colonies divided by the number of total viable colonies. The lethality data were fitted to the equation: L = (D − D 0) / D ∗ 100 %, where L, D, and D 0 are, respectively, the lethality fraction, total number of viable colonies, and number of viable colonies after treatment with 60Coγ. The positive mutant frequency was determined by calculating the amount of enhanced HA from the mutant colonies divided by the total number of viable colonies. The corresponding negative mutant frequency was determined based on the colonies that exhibited reduced HA production or disappeared.
Extraction of intracellular hypocrellins from the original and mutant strains
The plates with high lethality rates obtained from 60Coγ treatment were selected, and after a 60-h incubation on PDA plates, the mutant colonies were further screened for HA production. Mycelia on PDA plates were transferred to PDB medium as described above. After cultivation for 7 days, the fermented mycelia were harvested by centrifugation at 12,000 rpm for 10 min at 4 °C after being rinsed three times with distilled water. The mycelia were vacuum freeze-dried and then ground into powder using liquid nitrogen. The mycelium pellets (1 g) were accurately weighed and chemically extracted with 50 mL acetone via Soxhlet extraction for 12 h at 75 °C. The acetone was dried under vacuum conditions on a rotavapor at 42 °C. Finally, the residues were re-dissolved in 10 mL methanol, and the resulting hypocrellin content was determined.
HPLC analysis
An Agilent 1200 HPLC system was used to analyze the hypocrellin content in extracts and was equipped with a Zorbax Extend-C18 column (5 μm × 4.6 mm × 250 mm). The operating condition was a flow rate of 1.0 mL/min, and the extracts were eluted with a gradient strategy of 50 % A (acetonitrile) and 50 % (v/v) B (water + 0.1 % formic acid) to 65 % A and 35 % (v/v) B in 15 min and then immediately changed to 50 % A and 50 % (v/v) B for 5 min. For each sample, the injection volume was 20 μL, and the external standard method (Kong et al. 2012) was applied for the quantitative analysis with authentic HA as the standard, using the detection wavelength of 265 nm.
TLC, LC-MS, and NMR analysis
To further confirm the structure of the compound, the extracts were purified by TLC (dichloromethane/methanol = 70:1 (v/v)) on silica gel. After dichloromethane extraction, the purified sample was freeze-dried and dissolved in methanol for LC-MS (AB SCIEX QTRAP® 6500) analysis. Additionally, the dried samples were dissolved in CDCl3 (Cai et al. 2011) for further NMR (BRUKER-500) analysis (including 1H NMR and 13C NMR).
UV-vis absorption spectrum and PL emission spectrum analysis for exploration of optical performance
To further identify and explore the optical performance of the extracted pigment, we determined the UV-vis absorption spectrum using a Hitachi U-3900H and the PL spectrum using a Hitachi F-4600. According to Beer-Lambert law (A = εbc), with A assigned a suitable value of 0.434, the sample should be dissolved in methanol with a molarity of 1.14 × 10−4 mol/L.
Hereditary stability of mutant strains with increased hypocrellin production
The selected mutant strain with increased hypocrellin production was examined based on the stability of hypocrellin production for nine generations by successive inoculation on PDB medium, and HPLC was used to quantify the HA content.
Results
Strain selection and sensitivity of Shiraia sp. to 60Coγ irradiation
After exposure to different dosage of 60Coγ irradiation (20, 50, 80, 100, 150, 180, 300, and 500 Gy), eight different strain groups were obtained from the original strain ZZZ816. The sensitivity of irradiated and non-irradiated strains to gamma irradiation was detected by calculating the number of spores after incubating at 25 °C for 60 h on PDA medium (Fig. 1). The results revealed that the increasing 60Coγ irradiation doses could reduce the number of spores. When the irradiation dose reached 50 Gy, the lethality rate of spores was higher than 50 %. When reaching 180 Gy, the mortality rate increased to 85.6 %. The lethality rate of spores reached 100 % when exposed to 500 Gy (Fig. 2).
Spores in PDA plates of both irradiated and non-irradiated strains of Shiraia sp. with different 60Coγ irradiation doses. a Wild-type strain ZZZ816 as control. b Mutant strain following 20-Gy gamma irradiation. c Mutant strain following 50-Gy gamma irradiation. d Mutant strain following 80-Gy gamma irradiation. e Mutant strain following 100-Gy gamma irradiation. f Mutant strain following 150-Gy gamma irradiation. g Mutant strain following 180-Gy gamma irradiation. h Mutant strain following 300-Gy gamma irradiation. i Mutant strain following 500-Gy gamma irradiation
Lethality rate, survival rate, and positive mutant frequency in different 60Coγ irradiation doses. The green pillar represents the survival rate, the pink pillar represents the lethality rate, and the blue pillar represents the positive mutant frequency from 50- to 180-Gy 60Coγ irradiation doses. The lethality rate of spores was 77.2 %, and the positive mutant frequency was 35 % following 100 Gy of 60Coγ irradiation
Preliminary screening of mutant strains with increased hypocrellin production
Mutant strains (treatment by 20–180-Gy dose 60Coγ irradiation) with 50–90 % lethality rate (Fig. 2) were screened preliminarily by the number of the survived spores, formation of pigments, biomass of fermented mycelia, and dry weight of crude extract (Table 1). According to the above indices, 100 Gy was selected as the optimal 60Coγ irradiation dose, leading to 77.2 % lethality of spores and a 35 % positive mutant frequency (Fig. 2), and under this dosage, six mutant strains which showed high capacity of producing hypocrellins were selected for the first round (Fig. 3a).
Final screening of mutant strains and the identification of hypocrellins
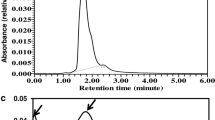
Three of the six selected mutants (named H-4-1, H-4-2, H-4-3) that produced abundant red pigment (Fig. 3) were further tested. To confirm whether hypocrellins were synthesized from our mutant strains, rapid identification of perylenequinonoid derivatives was performed using a chemical color response test (Hu and Shen, 1992), and Fig. 4a shows that the pigments from these three mutants were dark purple when FeCl3 was added, turned red under acidic conditions, and turned green under alkaline conditions. HPLC was used for the preliminary identification of the pigments, and the results are displayed in Fig. 4b, indicating that red pigment was extracted from mutant strain H-4-2, original strain ZZZ816, and HA standard. Our samples produced a red pigment that emerged as a sharp peak on the HPLC chromatogram at 16.304 min, which had the same retention time as an authentic sample of HA under these elution conditions. To further confirm the structure of the compound, LC-MS and NMR analyses were performed after TLC purification. The LC-MS analysis was performed on an AB SCIEX QTRAP® 6500 mass spectrometer, establishing the molecular formula as C30H26O10 (with the [M + H] at m/z = 547.2 and [M + Na] at m/z = 569.2), which is the same formula as that of standard HA (Fig. 5a). The freeze-dried samples were dissolved in CDCl3 for 1H NMR and 13C NMR analysis. The results indicated that 1H NMR (600 MHz, δ) of the main peak was 1.71 (s, 3H), 1.89 (s, 3H), 2.64 (d, 1H), 3.45 (d, 1H), 3.52 (s, 3H), 4.07 (s, 3H), 4.11 (s, 3H), 4.27 (s, 3H) 6.55 (s, 1H), 6.58 (s, 1H), 15.91 (s, 1H), and 15.96 (s, 1H); 13C NMR (150 MHz, δ) of the main peak was as follows: 26.9, 30.8, 41.8, 56.5, 56.6, 60.7, 61.9, 62.1, 78.6, 101.9, 102.1, 106.7, 107.0, 117.6, 118.1, 125.0, 125.3, 127.6, 128.5, 133.2, 134.1, 150.6, 150.9, 167.5, 167.6, 170.8, 171.9, 179.8, 180.2, and 207.4. They were conformed with 1H NMR (Fig. 5b) and 13C HMR (Fig. 5d) spectrum of standard HA separately, and the chemical structures were generated by ChemDraw® (Fig. 5e) (Kishi et al. 1991). To verify the optical performance and prepare to further study the PDT of HA, we examined the normalized UV-vis absorption spectra and its corresponding PL spectra at an excitation wavelength of 520 nm (Fig. 5c). The normalized UV-visible absorption spectrum of HA displays maxima at 478 and 582 nm, which broaden the absorption spectrum and hone in on the phototherapy window (600–900 nm) (Bonnet, 2014; Zhou et al. 2009). The normalized PL spectrum presents a peak at 602 nm and a shoulder peak at 641 nm, which is consistent with the spectrum of standard HA (Zhou et al. 2014). In addition, the broad range of the spectrum fits the order of PDT for superficial diseases (480–600 nm) (Deng et al. 2013; Wang et al. 1999; Xie et al. 2002; Zhou et al. 2014).
Chemical color response test and HPLC of crude extracts. a Color reaction of the pigments produced by strains of Shiraia sp.: 1 pigment acetone extract with FeCl3 solution (1 mol/L, 0.1 mL); 2 pigment acetone extract with hydrochloric acid solution (1 mol/L, 0.1 mL); 3 pigment acetone extract with sodium hydroxide solution (1 mol/L, 0.1 mL). b HPLC of extractions: 1 standard hypocrellin A; 2 cell acetone extracts of Shiraia sp. ZZZ816; 3 cell acetone extracts of mutant strain H-4-2
Qualitative analysis of HA. a LC-MS analysis and positive ion spectra with one adduct proton, m/z [M + H] 547.4, molecular formula, C30H26O10. b 1H NMR analysis. c Normalized UV-vis absorption (black line) and corresponding normalized PL emission (red line) of HA in methanol. The normalized UV-vis absorption showed maxima at 478 and 582 nm, and the normalized PL spectrum presented a peak at 602 nm and a shoulder peak at 641 nm. d 13C NMR analysis. e Chemical structures generated by ChemDraw®
Standard HA was used to create a standard curve using the same HPLC procedure, and the extracted HA samples from fermentation broth were properly diluted by HPLC analysis to quantify HA production. Table 2 shows that HA production in H-4-2 (2018.3 mg/L) increased by 414.9 % compared to that of original strain ZZZ816 (392.0 mg/L).
Genetic stability of the HA-producing strain H-4-2
In the present study, H-4-2 was selected as the optimal mutant producing increased HA. The stability of this mutant to produce HA was examined by successive subculture for nine generations. After each subculture, the strain was tested for its ability to produce HA by HPLC, and the mutant maintained a steady production yield after nine generations (Table 3).
Comparison of H-4-2 and ZZZ816
To confirm the HA production of the optimal mutant, the growing rate, the color of colonies, and the biomass of strain H-4-2 were compared with those of the original strain ZZZ816 (Fig. 6). Figure 6a shows that colonies of H-4-2 were larger than those of ZZZ816, and the color was much deeper than that of ZZZ816. Figure 6b shows that both the biomass and the colonial growth rate of H-4-2 were increased compared to those of ZZZ816, which was consistent with the change in HA production.
Discussion
Shiraia sp. ZZZ816 was isolated from the moso bamboo seeds as an endophytic fungus in our lab. Compared to the corresponding strains from fruit bodies or other host, ZZZ816 was able to produce high-yield hypocrellins (Shen et al. 2014), and its production status could remain stable. Therefore, strain ZZZ816 was selected as the original strain in this study.
For the industrial strains, 60Coγ irradiation had been reported to enhance the production of aflatoxins B1 and B2 in Aspergillus flavus twofold (Applegate and Chipley, 1974). The mutants of Phaffia rhodozyma indicated the increased production of astaxanthin by submerged fermentation with an optimal 3.5-kGy dosage, and the final yield was found to be 101.8 % higher than that of the original strain (Yun et al. 2008). In this article, the efficient biosynthesis of an HA-producing strain was also explored using 60Coγ irradiation for the first time, and a reliable mutant strain H-4-2 with the highest yield of HA was obtained. This result demonstrated that 60Coγ irradiation is a simple, rapid, and effective tool in mutation breeding of hypocrellin-producing strain and would have great potential application in other strains with the similar property.
Although there were seven detection methods (TLC, LC-MS, 1H NMR, 13C NMR, HPLC, UV-vis, and PL) employed in this study, the small peak besides the HA peak (Fig. 4b) could not be distinguished from the extracts. The molecular formula of this element was identified as C30H26O10 (data not shown in this paper) preliminarily by LC-MS and might be attributed into hypocrellin C (HC). However, the tiny output blocked the way of continued research in fermentation industry.
It is fascinating that the production of HA in H-4-2 increased more than fivefold just by 60Coγ irradiation, and we have not discovered any other effective methods to sincerely improve HA production from the original high-yield strains. Next, we would apply a combination of two or more mutagenesis methods to further increase the final yield, which has been significantly higher than all the other strains in published studies. Besides, the optimization of media constituents and submerged fermentation conditions still remains to be unexplored at the present time.
References
Ali SM, Chee SK, Yuen GY, Olivo M (2001) Hypericin and hypocrellin induced apoptosis in human mucosal carcinoma cells. J Photoch Photobio B 65(1):59–73. doi:10.1016/S1011-1344(01)00252-4
Applegate KL, Chipley JR (1974) Effects of 60CO gamma irradiation on aflatoxin B1 and B2 production by Aspergillus flavus. Mycologia 66(3):436–445
Bonnet S (2014) Shifting the light activation of metallodrugs to the red and near-infrared region in anticancer phototherapy. Comments Inorg Chem 35(4):179–213. doi:10.1080/02603594.2014.979286
Brandsberg JW, French ME (1972) In vitro susceptibility of isolates of Aspergillus fumigatus and Sporothrix schenckii to amphotericin B. Antimicrob Agents Chemother 2(5):402–404. doi:10.1128/AAC.2.5.402
Cai YJ, Liao XR, Liang XH, Ding YR, Sun J, Zhang DB (2011) Induction of hypocrellin production by triton X-100 under submerged fermentation with Shiraia sp. SUPER-H168. New Biotechnol 28(6):588–592. doi:10.1016/j.nbt.2011.02.001
Deininger MH, Weinschenk T, Morgalla MH, Meyermann R, Schluesener HJ (2002) Release of regulators of angiogenesis following hypocrellin-A and -B photodynamic therapy of human brain tumor cells. Biochem Biophys Res Commun 298(4):520–530. doi:10.1016/S0006-291X(02)02512-3
Deng H, Li T, Xie J, Huang N, Gu Y, Zhao J (2013) Synthesis and bio-evaluation of novel hypocrellin derivatives: potential photosensitizers for photodynamic therapy of age-related macular degeneration. Dyes Pigments 99(3):930–939. doi:10.1016/j.dyepig.2013.06.037
Du W, Liang J, Han Y, Yu J, Liang Z (2015) Nitric oxide mediates hypocrellin accumulation induced by fungal elicitor in submerged cultures of Shiraia bambusicola. Biotechnol Lett 37(1):153–159. doi:10.1007/s10529-014-1665-4
Du W, Liang Z, Zou X, Han Y, Liang J, Yu J, Chen W, Wang Y, Sun C (2012) Effects of microbial elicitor on production of hypocrellin by Shiraia bambusicola. Folia Microbiol 58(4):1–7. doi:10.1007/s12223-012-0203-9
Fang LZ, Qing C, Shao HJ, Yang YD, Dong ZJ, Wang F, Zhao W, Yang WQ, Liu JK (2006) Hypocrellin D, a cytotoxic fungal pigment from fruiting bodies of the ascomycete Shiraia bambusicola. J Antibiot 59(6):351–354. doi:10.1038/ja.2006.49
Hu X, Shen LD (1992) The isolation and structure identification of chemical constituents of Sharaia bambusicola. West China J Pharm Sci 7(1):1–4
Hudson JB, Zhou J, Chen J, Harris L, Yip L, Towers GHN (1994) Hypocrellin, from Hypocrella bambuase, is phototoxic to human immunodeficiency virus. Photochem Photobiol 60(3):253–255. doi:10.1111/j.1751-1097.1994.tb05100.x
Jiang YX, Zhu MF, Sun LH, Wang Y (2010) Study on the effect of 60Coγ ray irradiation on the stability of vitamin. Chin J Health Lab Technol 20(11):2796-2798
Kishi T, Tahara S, Taniguchi N, Tsuda M, Tanaka C, Takahashi S (1991) New perylenequinones from Shiraia bambusicola. Planta Med 57(57):376–379. doi:10.1055/s-2006-960121
Kocisova E, Jancura D, Sanchez-Cortes S, Miskovsky P, Chinsky L, Garcia-Ramos JV (1999) Interaction of antiviral and antitumor photoactive drug hypocrellin A with human serum albumin. J Biomol Struct Dyn 17(1):111–120. doi:10.1080/07391102.1999.10508345
Kong M, Chen ZL, Yin ZQ, Jian Z (2012) Simultaneous determination of hypocrellin A, hypocrellin B, and hypocrellin C by HPLC. China J Chin Mater Med 37(1):75–78. doi:10.4268/cjcmm20120116
Li L, Chen Y, Shen J (2000) New long-wavelength perylenequinones: synthesis and phototoxicity of hypocrellin B derivatives. BBA-Gen Subjects 1523(1):6–12. doi:10.1016/S0304-4165(00)00081-7
Najafi N, Ahmadi AR, Hosseini R, Golkhoo S (2011) Gamma irradiation as a useful tool for the isolation of astaxanthin-overproducing mutant strains of Phaffia rhodozyma. Can J Microbiol 57(9):730–734. doi:10.1139/w11-060
Pan WS, Ji YY, Yang ZY, Wang JW (2012) Screening of high-yield hypocrellin A producing mutants from Shiraia sp. S8 by protoplast mutagenesis and ultraviolet irradiation. Chin J Bioprocess Eng 10(6):18–23 10.3696.j
Qi WN, Lu JY, Zhao J, Gao JF (2007) Effects of 60Coγ ray irradiation on protective enzymes isozymes expression in winter wheat. J Northwest A and F Univ 7(35):123–126
Qiu Y, Lu Z, Sun P (2011) Preparation of hypocrellin liposome and determination of its extent of encapsulation. China Surfact Det Cosmet 41(6):422–425
Shen XY, Zheng DQ, Gao J, Hou CL (2012) Isolation and evaluation of endophytic fungi with antimicrobial ability from Phyllostachys edulis. Bangl J of Pharmacol 7(4):249–257. doi:10.3329/bjp.v7i4.12068
Shen XY, Cheng YL, Cai CJ, Fan L, Gao J, Hou CL (2014) Diversity and antimicrobial activity of culturable endophytic fungi isolated from moso bamboo seeds. PLoS One 9(4):e95838. doi:10.1371/journal.pone.0095838
Su YJ, Rao SQ, Cai YJ, Yang YJ (2010) Preparation and characterization of the inclusion complex of hypocrellin A with hydroxypropyl-beta-cyclodextrin. Eur Food Res Technol 231(5):781–788. doi:10.1007/s00217-010-1322-7
Su YJ, Si SH, Qiao LW, Cai YJ, Xu ZM, Yang YJ (2011) The effect of a hypocrellin A enriched diet on egg yolk quality and hypocrellin A distributions in the meat of laying hens. Eur Food Res Technol 232(6):935–940. doi:10.1007/s00217-011-1461-5
Wang ZJ, He YY, Huang CG, Huang JS, Huang YC, An JY, Gu Y, Jiang LJ (1999) Pharmacokinetics, tissue distribution and photodynamic therapy efficacy of liposomal-delivered hypocrellin A, a potential photosensitizer for tumor therapy. Photochem Photobiol B 70(5):773–780. doi:10.1111/j.1751-1097.1999.tb08282.x
Weng B, Jiang Z, Lu CX, Lei J, Xiao S, Huang Z (2012) Factors related to the amino acids quantitative traits of a white new Agaricus blazei strain selected with 60Coγ ray radiation and ultraviolet rays. Chin J Trop Crop 33(7):1170–1173
Wu D, Zhen J (1995) Novel therapeutic and diagnostic applications of hypocrellins and hypericins. J Photochem Photobiol B 61(6):529–539. doi:10.1111/j.1751-1097.1995.tb09903.x
Wu H, Lao XF, Wang QW, Lu RR, Shen C, Zhang F, Liu M, Jia L (1989) The shiraiachromes: novel fungal perylenequinone pigments from Shiraia bambusicola. J Nat Prod 52(5):948–951. doi:10.1021/np50065a006
Xiang XY (2010) Optimization of Shiraia bambusicola P. Henn. Under liquid fermentatio. J Biotechnol 20(4):73–75. doi:10.3969/j.issn.1004-311x.2010.04.135
Xie J, Ma JH, Zhao JQ (2002) Prediction on amphiphilicity of hypocrellin derivatives. Sci China Ser B 45(3):251–256. doi:10.1360/02yb9033
Xie XY, Zeng BQ, Zhou XQ, Zhi XU, Song R (2010) Study on 60Coγ irradiation mutation breeding of kojic acid production strain and its characterization. Sci Technol Food Ind 12:213–217 10.13386/j.issn1002-0306.2010.12.080
Xu SJ, Chen S, Zhang MH, Shen T, Zhao YP, Liu ZW (2001) Butylamino-demethoxy-hypocrellins and photodynamic therapy decreases human cancer in vitro and in vivo. BBA-Gen Subjects 1537(3):222–232. doi:10.1016/S0925-4439(01)00074-6
Yang HL, Xiao CX, Ma WX, He GQ (2009) The production of hypocrellin colorants by submerged cultivation of the medicinal fungus Shiraia bambusicola. Dyes Pigments 82(2):142–146. doi:10.1016/j.dyepig.2008.12.012
Yang HY, Zhang WG, Ma LP, Wang SW, Zhang ZY (2001) An approach to enhancing the phototoxicity of a novel hypocrellin congener to MGC803 cells. Dyes Pigments 51(2-3):103–110. doi:10.1016/S0143-7208(01)00059-6
Yun H, Lim S, Yang SH, Lee WY, Kwon J, Lim BL, Kim D (2008) Effect of gamma irradiation on the growth and patulin production of Penicillium griseofulvum in an apple model system. Food Sci Biotechnol 17(4):723–727
Zhang J, Ma L (2003) Recent advances of hypocrellin research. J Yunnan Univ 25(S1):184–188
Zhang MH, Chen S, An JY, Jiang LJ (1989) Separation and identification of Hypocrellin and fatty acids as ingredients in Hypocrella bambusae (B. et Br.) sacc. Chinese Sci Bull 34(12):1008–1014
Zhou L, Zhou JH, Dong C, Ma F, Wei SH, Shen J (2009) Water-soluble hypocrellin A nanoparticles as a photodynamic therapy delivery system. Dyes Pigments 82(1):90–94. doi:10.1016/j.dyepig.2008.11.009
Zhou L, Zhou L, Ge X, Zhou J, Wei S, Shen J (2014) Multicolor imaging and the anticancer effect of a bifunctional silica nanosystem based on the complex of graphene quantum dots and hypocrellin A. Chem Commun 51(2):421–424. doi:10.1039/C4CC06968D
Acknowledgments
Authors thank the reviewers for their helpful comments and suggestions, and this study was supported by the National Natural Science Foundation of China (No. 31470145) and Beijing Natural Science Foundation (No. 5132009).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Human and animal rights and informed consent
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Xin-Yao Liu and Xiao-Ye Shen contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, XY., Shen, XY., Fan, L. et al. High-efficiency biosynthesis of hypocrellin A in Shiraia sp. using gamma-ray mutagenesis. Appl Microbiol Biotechnol 100, 4875–4883 (2016). https://doi.org/10.1007/s00253-015-7222-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7222-9