Abstract
α-Amylase from Bacillus licheniformis ATCC 9945a (BliAmy) was proven to be very efficient in hydrolysis of granular starch below the temperature of gelatinization. By applying two-stage feeding strategy to achieve high-cell-density cultivation of Escherichia coli and extracellular production of BliAmy, total of 250.5 U/mL (i.e. 0.7 g/L) of enzyme was obtained. Thermostability of amylase was exploited to simplify purification. The hydrolysis of concentrated raw starch was optimized using response surface methodology. Regardless of raw starch concentration tested (20, 25, 30 %), BliAmy was very effective, achieving the final hydrolysis degree of 91 % for the hydrolysis of 30 % starch suspension after 24 h. The major A-type crystalline structure and amorphous domains of the starch granule were degraded at the same rates, while amylose-lipid complexes were not degraded. BliAmy presents interesting performances on highly concentrated solid starch and could be of value for starch-consuming industries while response surface methodology (RSM) could be efficiently applied for the optimization of the hydrolysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Amylases (EC 3.2.1.1: 1,4-α-D-glucan-glucanohydrolase) are the most widely used thermostable enzymes in the starch industry (Leveque et al. 2000). Due to their enhanced stability, they are amongst the most important industrial enzymes with potential application in starch saccharification, food, paper, pharmaceuticals, detergent and textile industries (Gupta et al. 2003; van der Maarel et al. 2002). Their main substrate, starch, represents an inexpensive source for the production of glucose, fructose and maltose syrups (Roy and Gupta 2004) and for obtaining the products of their fermentation, including biofuels. The importance of the enzymatic liquefaction of granular starch below the temperature of gelatinization has been well recognized, mainly due to the energy savings and the effective utilization of biomass, which reduces the overall cost of starch processing (Robertson et al. 2006; Uthumporn et al. 2010).
The hydrolysis of granular starch strongly depends on the starch structure and the amylase source (Oates 1997; Tawil et al. 2011). Several raw starch-degrading amylolytic enzymes have been described (Robertson et al. 2006; Vikso-Nielsen et al. 2006). Amongst these enzymes, α-amylases (E.C.3.2.1.1) are the main enzymes involved in the hydrolysis of α(1,4) bonds (Oates 1997). They are amongst the most important raw starch-degrading enzymes, although only a limited number of α-amylases seem to be able to degrade raw starch (Robertson et al. 2006; Sun. et al. 2010).
The application of raw starch-degrading α-amylases (RSDA) has been hampered by poor temperature stability (Vikso-Nielsen et al. 2006). Although thermostable enzymes have found a number of commercial applications due to their overall inherent stability (Haki and Rakshit 2003), screening strains capable of producing commercially acceptable yields of amylases remains a challenging task (Pandey et al. 2000). Characterization of amylases from new strains therefore continues to be of importance. Consequently, several novel thermostable bacterial α-amylases able to bind and degrade raw starch have been sequenced and biochemically characterized recently (Mok et al. 2013; Puspasari et al. 2013; Sharma and Satyanarayana 2012; Sun et al. 2010).
RSDA from Bacillus sp. usually need prolonged time of incubation for efficient raw starch hydrolysis, and often, better results were obtained at temperatures between 60 and 70 °C (Goyal et al. 2005). Furthermore, the starch-processing industry usually employs the mashes containing around 25–33 % starch. There is, thus, an urgent need to explore the amylases for efficient hydrolysis of raw starches under high concentration. To date, however, there were few amylases reported to have the ability to hydrolyse high-concentration raw starch suspensions effectively because of the inhibitory effect of the substrates or products on the enzyme activity (Liu and Xu 2008).
Escherichia coli expression system for extracellular production of thermostable, highly efficient RSDA from Bacillus licheniformis ATCC 9945a was developed previously (Božić et al. 2013). In this work, a two-stage feeding strategy was developed to reach the overproduction of α-amylase in a 2-L fermenter, resulting in a significant enhancement of the total α-amylase yield. Furthermore, response surface methodology (RSM) was used to optimize BliAmy doses, starch concentration and incubation times. The goal was to achieve the maximal efficiency of the process by reaching a high hydrolysis yield at sub-gelatinization temperature and optimum pH value, in the shortest possible time and to use the minimum amount of the enzyme. This methodology has the advantage of being less expensive and time-consuming than the classical methods, and it has already been successfully applied in optimization of enzymatic hydrolysis of several substrates including gelatinized starch (Kunamneni and Singh 2005). However, this is the first study in which optimizations were made for hydrolysis of highly concentrated raw corn starch with α-amylase. Results obtained suggest that BliAmy present interesting performances on solid starch and could be of value for starch-consuming industries.
Materials and methods
Bacterial strains, plasmids and media
The E. coli BL21 (DE3) strain harbouring pDA-amy plasmid (Božić et al. 2013) was used in this work. The gene encoding BliAmy (GenBank accession number JN042159.1.) was amplified by polymerase chain reaction from genomic DNA of B. licheniformis (ATCC® 9945a™). LB medium (10 g/L tryptone, 5 g/L yeast extract and 10 g/L NaCl) containing 100 μg/mL ampicillin was used for the preinoculum preparation. A composition of defined mineral medium utilizing glucose as the sole carbon source, which was used for inocula, and for bioreactor experiments and the composition of feed medium for high-cell-density fermentations and trace elements solution can be found elsewhere (Ruiz et al. 2009).
Cultivation conditions and analytical procedures
Preinoculum cultures were grown from glycerol stocks in a 100-mL shake flask containing 15 mL LB media and incubated overnight at 37 °C in a rotary shaker at 250 rpm. For inoculum, 5 mL of preinoculum cultures was transferred to a 0.5-L shake flask containing 100 mL of defined medium which was incubated at 37 °C for 5 h at 250 rpm. Inoculum (100 mL) was transferred to the bioreactor containing 900 mL of defined medium. All growths were carried out using a BIOSTAT B bioreactor (Sartorius) equipped with a 2-L fermentation vessel. The end of the batch phase was identified by a reduction in the oxygen consumption rate and an increase in pH.
The specific growth rate was kept at a constant value by an exponential feed medium addition which was calculated based on mass balances and substrate consumption kinetics (Pinsach et al. 2006). When the dry cell weight (DCW) reached a certain value (15 g/L), 0.2 mM IPTG was added as pulse, followed with constant glucose feeding rate of 15 mL/h. The pH was maintained at 7.00 ± 0.05 by adding 15 % NH3 solution to the reactor. The temperature was kept at 37 °C and lowered to 25 °C after the induction. The pO2 value was maintained at 50 % of air saturation by adapting the stirrer speed between 450 and 900 rpm and supplying air (enriched with pure oxygen when necessary) at a space velocity of 2 vvm. The fermentation broth was centrifuged at 10,000 rpm for 20 min at 4 °C using a SL 40R centrifuge (Thermo Scientific), and the cell-free supernatants were used as a crude enzyme preparation.
For the measurement of DCW, aliquots of the broth were withdrawn and centrifuged. The resulting pellets were washed twice with deionized water and dried at 110 °C until constant weight.
To quantify glucose and the recombinant amylase activity during cultures, broth samples were withdrawn and centrifuged and the supernatant was used. Glucose was analysed by dinitrosalicylic (DNS) acid method (Bernfeld 1955).
α-Amylase activity assay and determination of protein concentration
α-Amylase activity was determined as previously reported (Bozic et al. 2011) in 50 mM phosphate buffer pH 6.5 and 37 °C. The amount of liberated reducing sugar was determined by the DNS acid method (Bernfeld 1955). One unit of amylase activity was defined as the amount of enzyme that released 1 μmol of reducing end groups per minute at 37 °C. Maltose was used to construct a standard curve.
Protein concentration was determined by the Bradford method by using bovine serum albumin as the protein standard (Bradford 1976).
Enzyme purification
For partial purification of BliAmy, a total amount of 1600 mL fermentation broth (4.5 mg protein/mL, 250.5 U/mL) was concentrated 10 times by ultrafiltration using membrane with MWCO 10 kDa followed by heat treatment for 1 h at 60 °C and pH 9.0. Samples were centrifuged for 20 min at 10,000 rpm, 4 °C. For obtaining pure enzyme, 20 mL of partially purified concentrated fermentation broth (2000 U/mL, 1.2 mg/mL), adjusted to pH 7.5, was subjected to ion-exchange chromatography on Q Sepharose column on a fast protein liquid chromatography (FPLC) system (Pharmacia, Uppsala). The column was equilibrated in 10 mM Tris-HCl buffer pH 7.5. After applying the sample, the column was washed with starting buffer. Elution was performed using a gradient from 0 to 0.5 M NaCl, pH 7.5 in 10-column volumes. Fractions were assayed for purity by reducing SDS-PAGE (10 % gels). Percentage of BliAmy amongst the rest of extracellular proteins was estimated by ImageJ software (www.rsbweb.nih.gov/ij) (Bozic and Vujcic 2005).
Statistical optimization of hydrolysis process variables
A three-step design consisted of full factorial design (FFD), steepest ascent design (SAD) and central composite design (CCD).
For FFD, three independent variables, enzyme loading (X 1), solid starch content (X 2) and incubation time (X 3), were included in a two-level full factorial design. A total of 14 experiments were run where each variable was examined in two levels: −1 and +1 (Table 1). The corresponding first-order model equation for the full factorial experiment has the form:
where Y is the predicted response, β 0 is a constant coefficient, β i is the linear coefficient and X i is level of independent variables.
For SAD, the center point of the factorial design has been considered as the origin of the path. The steps along the path were proportional to the regression coefficients from the linear model given by Eq. (1), Table 2.
For CCD, the three independent variables were studied at five different levels (−α, −1, 0, 1, α) (Table 3). The factorial points were used to fit all linear and interaction terms. The axial points provided additional levels of the factor for purposes of estimation of the quadratic terms. Replicates of the test at the centre point were very important as they provided an independent estimate of the experimental error. A set of 19 experiments was performed. All variables were taken at a central coded value considered as zero. The minimum and maximum ranges of variables were used as explained in Table 3. Average yield of the hydrolysis of concentrated raw corn starch suspension was taken as a response (Y). The predicted optimum value was confirmed by the experiment using the selected optimum values of the variables. The quadratic model used for the analysis and for the predicting the optimum point is expressed below:
where Y is the predicted response, β 0 is a constant coefficient, β i is the linear coefficient, β ij is the interaction coefficient, β ii is the quadratic coefficient and X i is the level of independent variables.
The fit of the models and their significance were evaluated by variance analysis (ANOVA). Three-dimensional surface plots were drawn to show the effects of the independent variables on the hydrolysis, being described by a quadratic polynomial equation, fitted to the experimental data. The experimental results were analysed using Design-Expert® software version 9 (Stat-Ease Inc., Minneapolis, USA).
Hydrolysis of raw starch by BliAmy
Starch grains prepared as previously published (Bozic et al. 2011) and the enzyme were incubated in 50 mM phosphate buffer pH 6.5 at 60 °C for different incubation time. Temperature of 60 °C was chosen based on the literature data where gelatinization temperatures for corn starch range from 65.6–69 °C was reported (Sandhu and Singh 2007). Several starch concentrations (16.6–34 % dry basis) were used. The amount of BliAmy was optimized using 0.2–14.2 IU per milligram dry starch. Each reaction was centrifuged to separate starch residues from soluble sugars. Supernatants with soluble sugars were further treated with glucoamylase (Dextrozyme ® GA, Novozyme) at pH 5.0 at 50 °C to allow hydrolysis of all soluble sugars to glucose. Glucose was measured by the DNS acid method, and the extent of hydrolysis was expressed as the ratio of glucose from starch hydrolysis to the initial mass of starch (d.b.). All experiments were carried out in triplicate.
Using 30 % raw corn starch in 50 mM phosphate buffer pH 6.5 at 60 °C, the hydrolysis efficiency of BliAmy was compared with the commercial α-amylase Termamyl®120 L, Type L (Novozymes).
Analysis of the residual starch
X-ray diffraction analysis
X-ray powder diffraction (XRPD) was used for identification of crystalline phases of raw starch and residual starch withdrawn at different time intervals during hydrolysis. The samples (100 mg) were washed three times in deionized water by centrifugation for 2 min at 14,000 rpm to remove residual enzyme and dried at constant temperature (22 °C). The XRPD patterns were collected with Philips PW-1710 automated diffractometer (equipped with a diffracted beam curved graphite monochromator and a Xe-filled proportional counter) employing a Cu-tube (CuKα1,2 radiation) operated at 40 kV and 30 mA. Step scanning was performed with 2θ ranging from 3 to 30°, step size of 0.020° and the fixed counting time of 4 s per step. A fixed 2° divergence and 0.2 mm receiving slits and standard sample holders for reflection mode diffraction were used.
SEM characterization of hydrolysed granules
After 22 h of hydrolysis, the residues were washed three times in deionized water by centrifugation for 2 min at 14,000 rpm to remove residual enzyme and two times with anhydrous ethanol to dehydrate the solid residues then dried at room temperature for 2 days. Dried samples of hydrolysed and untreated starch were coated with gold using an ion sputtercoater and observed by the use of a scanning electron microscope JSM 6390LV with field emission gun operated at 3 kV. The granule size distribution as well as characterization of partially hydrolysed granules was determined by use of SMile View software according to a length scale provided by the user.
Results
Production of recombinant BliAmy in fed-batch cultures
Two-stage feeding strategy was applied to achieve high-cell-density cultivation of E. coli and production of extracellular recombinant α-amylase. During the pre-induction phase, the glucose feeding rate was increased exponentially, according to the exponential feeding method (Pinsach et al. 2006), and the cell growth was controlled at a specific growth rate of 0.16 h−1. When the DCW reached 15 g L−1 (intermediate cell density), the post-induction phase began and the glucose feeding rate was kept constant at 15 mL h−1. Cell growth continued after the IPTG was added and the DCW of E. coli cells reached 58 g at the end of the process (Fig. 1). Through this cultivation approach, the total amylase activity reached 250.5 U mL−1, which was 7-fold higher than that in batch culture. The final content of BliAmy was 0.7 g L−1.
Purification of recombinant BliAmy
To test whether there were differences in concentrated raw starch hydrolysis efficiency and adsorption, pure enzyme is needed. Due to the high thermal stability of BliAmy (Božić et al. 2013; Bozic et al. 2011), a very fast and simple purification was achieved by a heat treatment of the fermentation broth at 60 °C for 1 h, followed by centrifugation to remove precipitated host proteins (Fig. 2). Different pH values were tested on cell-free extracts, and results showed that a significant purification is achieved only at pH 9.0 compared to pH 5.0 and 7.0. Purity of enzyme preparation had no effect on adsorption to raw starch granules or efficacy. The hydrolysis yield of concentrated raw starch suspension between the pure and partially purified enzyme was the same (results not shown).
Purification of BliAmy. kDa: Molecular masses of standard proteins. Lane 1: Positions of standard proteins molecular masses. Lane 2: Extracellular proteins of fermentation broth. Lane 3: Proteins after heat treatment of the fermentation broth at 60 °C for 1 h. Lane 4: Purified BliAmy after ion-exchange chromatography on Q Sepharose
Statistical optimization of hydrolysis process variables
The screening design was performed by using RSM in order to determine the optimal values of process variables to reach the maximal yield and minimal costs. In other words, the aim was to optimize the process to obtain maximum efficiency of hydrolysis in the shortest possible time and to use the minimum amount of the enzyme.
In the first optimization step, FFD was used to analyse the effects of enzyme loading (X 1), starch content (X 2) and incubation time (X 3) on hydrolysis yield (Table 1). The corresponding first-order model equation fitted to the experimental data has the form:
Statistical testing of the model done by ANOVA showed that enzyme loading and incubation time have very significant effect (P < 0.001) on final hydrolysis yield, (Supplementary Table S1).
From the first-order model Eq. (3), it was predicted that increasing the enzyme loading and incubation time, while decreasing starch content, should enhance the hydrolysis yield. However, it is evident that the maximum of hydrolysis lies outside of the designed regions. Because of that, the steepest ascent design was employed to determine the direction of the experiment (Eq. (3)). Direction of steepest ascent was (Δ1, Δ3) = (0.92, 1). This experiment started at the center point of the current design and stretches beyond of the current design (Table 2). Regarding the results from the path of the steepest ascent, it can be clearly seen that the hydrolysis yield reaches maximum when using the enzyme loading of 6.95 IU/mg of starch after 43 h of incubation. Results obtained also implied that such long incubation time could be reduced by choosing an increased enzyme/substrate ratio. This approximation was used to create experimental scheme for further optimization of the hydrolysis process by CCD.
In CCD, factors were set to five levels: ±1 (factorial points), ±α (axial points) and the centre point (0). By applying the multiple regression analysis on experimental data, a second-order polynomial model was obtained (Eq 4):
The statistics evaluation of quadratic model by ANOVA showed the accuracy and general applicability of the polynomial model for description of the responses of the experiments (Supplementary Table S2).
3D response surface plot (Fig. 3) shows the changes of hydrolysis yield of 20, 25 and 30 % solid substrate with the enzyme loading and incubation time varying within the experimental ranges. The response surface shows that hydrolysis increase with time and enzyme loading and reaches a plateau surface (maximum), i.e. 100 % of hydrolysis. Figure 3 showed clearly that the optimum may be obtained with different combinations of enzyme loading and incubation time.
BliAmy is highly effective in the hydrolysis of highly concentrated raw corn starch suspension, being able to degrade 73, 63 and 56 % of a 20, 25 and 30 % starch suspension, respectively, within only 5 h at 60 °C, by using enzyme loading of 11.5 IU/mg. Moreover, the hydrolysis yield reached 99 % for 20, 95 % for 25 % and 91 % for 30 % solid substrate after 24 h at 60 °C.
Model validation and applicability
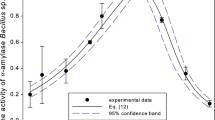
The optimal conditions for high raw corn starch hydrolysis were extracted by Design-Expert software through graphical model optimization (Fig. 3). To confirm the applicability of the model, confirmation runs under the optimal condition were conducted. Five confirmation runs in three replicates with different time incubation and with the same enzyme loading (11.5 IU/mg) on 30 % starch were carried out. The actual values obtained through experiments were compared with the values predicted by the multi-optimization model. After only 5 h of incubation, BliAmy (11.5 IU/mg) hydrolysed 55.7 % of a 30 % raw corn starch, while after 24 h of incubation, the hydrolysis degree was 91 %, as shown in Fig. 4. The corresponding predicted values were 57.1 and 90 % for period of 5 and 24 h, respectively (Fig. 4), which was comparable to the actual values obtained in the experiments.
In addition, we compared BliAmy and commercial α-amylase from B. licheniformis (Termamyl® 120 L) under optimal conditions determined by RSM for 30 % raw corn starch hydrolysis by adding 11.5 IU/mg of both enzyme preparations (Fig. 4). After 5 h of incubation, Termamyl® 120 L hydrolysed 57.7 % of a 30 % raw corn starch, while hydrolysis degree reached its maximal value of 76.9 % after 24 h hydrolysis. Minor further hydrolysis was observed after prolonged incubation time (31 h). These results demonstrate that BliAmy hydrolysis rate was 13 % higher under the optimal conditions.
Analysis of residual starch
After 91 % of hydrolysis, 10 % of starch residues remained calculated by weight of starch before and after the hydrolysis. Cystalline structure of starch residues was analysed by X-ray diffraction on the samples originating from 30 % starch suspensions after 5 and 24 h. As shown in Fig. 5a, 30 % raw corn starch has A-type crystallinity with characteristic peaks at Bragg angle (2θ) = 15, 17, 18 and 23° and a Vh-type, from which intensity of the peak is at 2θ around 20° and corresponds to amylose-lipid complexes formed between amylose and endogeneous fatty acids present in corn starch.
For both times tested, no significant increase in crystallinity level was observed, as indicated by constant diffracted intensities and scattering background (Fig. 5a) which indicated that BliAmy most likely degraded both amorphous and crystalline areas at the same rates. This is in agreement with a granule by granule mode of attack and can be concluded from the SEM micrographies (Fig. 5b). Vh-type structure is remarkably stable and more visible in the 24 h BliAmy residue obtained from 30 % starch suspensions (Fig. 5a) which suggests that the Vh-type structure is much more resistant to α-amylases than A-type.
Discussion
Fermentation controlled by constant supply of carbon source at exponential stage of growth while providing enough oxygenation enables reaching high cell densities. This approach offers a tool for increasing yield of recombinant enzyme production. Two-stage feeding strategy was applied to achieve high-cell-density cultivation of E. coli and production of extracellular recombinant α-amylase. In the first phase (pre-induction phase), the glucose feeding rate was increased exponentially, according to the exponential feeding method (Pinsach et al. 2006), keeping the constant specific cell growth rate of 0.16 h−1. When the DCW reached intermediate cell density, second stage of feeding (post-induction phase) began and the glucose feeding rate was kept constant. Lower constant feeding rate was applied during the post-induction phase in order to prevent the accumulation of nutrients in the medium as a consequence of changes in host cell physiology and metabolism after induction. The final high yield of BliAmy (0.7 g L−1) enabled optimization of hydrolysis process by RSM.
The application in the starch industry does not require high-purity amylases and generally makes use of crude or partially purified enzyme preparations. This was further confirmed to be the case with BliAmy where no differences in concentrated raw starch hydrolysis efficiency and adsorption between pure and partially purified enzyme was observed (data not shown). Therefore, the finding that the amylase can be easily isolated as enriched fraction by simply heating fermentation broth could facilitate large-scale production of this biocatalyst and consequently reduce the production costs to which chromatography columns and resins contributes significantly (Loncar and Fraaije 2014).
The optimal load of the enzyme (X 1), the solid starch content (X 2) and the incubation time (X 3) of the hydrolysis was optimized by RSM. FFD with two levels for all design variables are the most frequently used experimental design when the number of variables is less than five (Trichon et al. 2007). FFD method estimates the main effects of factors and their interactions simultaneously (Montgomery 2009). In this work, a FFD was used in the first phase to find the optimal region of hydrolysis process. The values of factors were determined by preliminary experiments (data not shown) from which it was demonstrated that 100 % of hydrolysis was obtained by using low concentration of starch (1 %). Recently, several novel thermostable bacterial α-amylases able to degrade raw starch have been biochemically characterized. One of them, raw-starch-digesting α-amylase from Geobacillus thermoleovorans (strain PizzoT) was able to hydrolyse 99 % of 1 % raw corn starch within 3 h (Finore et al. 2011), while the others did not reach 100 % hydrolysis rates in the reaction conditions tested (Liu and Xu 2008; Puspasari et al. 2013).
For higher concentration of starch (>20 %), it was uncertain that complete hydrolysis was even possible. For that reason, the value of enzyme doses and incubation times was assumed based on the preliminary experiments with 1 % starch. Statistical testing of the model (Eq. (3)) done by ANOVA showed that substrate content have less significant effect on hydrolysis yield in the ranges studied.
However, from the model (Eq. (3)), it was obvious that the maximum of hydrolysis lies outside of the designed regions. Instead of changing the values one by one in FFD to reach the possible maximum of hydrolysis, a directional search method was used in order to shorten the search for desired regions of values. Regarding the results from the path of steepest ascent, it can be clearly seen that the complete hydrolysis can be reached and that long incubation time could be reduced by choosing an increased enzyme/substrate ratio. Based on these results, experimental scheme for CCD for further optimization of the hydrolysis process was created.
A second-order response surface model should be reliable to provide a good prediction throughout the region of interest. A CCD is made rotatable by choice of α. The value of α depends on the number of experimental runs in the factorial portion of CCD and can be determined as α = [2k] 1/4. For rotatable CCD with three factorial points, α = 1.7 (Montgomery 2009). The main and interaction effects of these factors were subsequently evaluated based on fitting a quadratic response model in this region. Statistical evaluation by ANOVA indicated a high significance of the quadratic model.
Regardless of the raw starch concentration tested (20, 25, 30 %), BliAmy was very effective in achieving the final hydrolysis degree of 91 % for the hydrolysis of 30 % starch suspension after 24 h. This study has shown that the RSM could efficiently be applied for optimization of the hydrolysis of highly concentrated raw corn starch as an economical way of obtaining the maximum amount of information in a short period of time and with the fewest number of experiments.
BliAmy allows complete hydrolysis after prolonged incubation, unlike some other highly efficient bacterial RSDA, such as α-amylase from Anoxybacillus flavothermus (Tawil et al. 2012). There are a few papers in the literature concerning bacterial α-amylase capable for hydrolysis of raw starches under high concentration (30 %) (Mehta and Satyanarayana 2014; Tawil et al. 2012; Vikso-Nielsen et al. 2006), and not including B. licheniformis amylase to the best of our knowledge. Possibly, it is due to its well-known uses at high temperatures; however, its high stability is one of the major benefits for its application at sub-gelatinization temperatures for prolonged time. Quantitative comparison is sometimes difficult since enzyme activity units are determined differently across the literature, and there is still a lack of information about actual enzyme doses applied for the hydrolysis. Nevertheless, BliAmy is more efficient than α-amylase from Anoxybacillus flavothermus which lead to 77 % hydrolysis of a 31 % raw corn starch suspension after 96 h at 61 °C (Tawil et al. 2012) or α-amylase from Geobacillus thermoleovorans that hydrolysed 40 % of raw corn starch (30 % slurry) at 60 °C (Mehta and Satyanarayana 2014).
When compared with commercial α-amylase from B. licheniformis (Termamyl® 120 L) under the same reaction condition of 30 % raw corn starch hydrolysis, BliAmy showed significantly higher efficiency. Besides, BliAmy does not require Ca2+ or any other metal ion for its activity and stability, which represents another advantage for its application. The primary structure of the BliAmy (Božić et al. 2013) shares 96 % identity with the commercially used B. lichenisformis α-amylase (WO/2001/096537). In the literature, two critical positions for the thermostability of the highly thermostable α-amylase from B. licheniformis have been located at positions His133 and Ala209 (also present in Termamyl® 120 L) which was subsequently confirmed by mutations (Declerck et al. 1995). However, Gln133 and Thr209 have been found in BliAmy which might be an explanation of the basis of high thermostability of this enzyme and consequently to a higher efficiency for raw starch hydrolysis after prolonged time of incubation at 60 °C.
Hydrolysis of raw starch is a heterogeneous reaction, involving a reaction between an enzyme in solution and a solid substrate. Complex semicrystalline and hierarchical starch structure limits the diffusion of enzyme and restricts its accessibility to breakable linkages. The morphology and the surface of the substrate, the amylose content, the crystalline structure or the presence of the amylose-lipid complexes were shown to be limiting factors for the hydrolysis of the starch granule (Lei et al. 2012; Tawil et al. 2012). BliAmy was shown to be very efficient on concentrated raw starch suspensions by hydrolyzing amorphous and crystalline regions concomitantly, while the Vh-type structure was much more resistant to α-amylases than A-type, as already noticed (Gernat et al. 1993).
Within a starch preparation, the granules are not equally susceptible to enzymatic degradation, which is possibly a function of the manner in which amylases adsorb to a granule. Despite the specific mode of attack, starch hydrolysis occurs granule by granule, with an attacked granule being completely hydrolysed, as already observed for hydrolysis of wheat starch and other starches in solid state (Colonna et al. 1988; Oates 1997). The visualization of the degraded corn starch granules showed that the BliAmy molecules proceed from the surface toward the centre (centripetal hydrolysis). Then, the core is completely degraded from within by erosion of its periphery (centrifugal hydrolysis) in a manner that was already noticed for B. licheniformis α-amylase (Helbert et al. 1996).
The final degree of starch solubilization reached 91 % after 24 h in a 30 % starch suspension and can be modulated by changing enzyme doses vs. incubation time upon need. Although highly efficient BliAmy can alone efficiently hydrolyse almost completely 30 % raw corn starch, it may also be tried in synergism with some glucoamylase to bring a complete hydrolysis to glucose.
Strong correlation between the hydrolysis of raw starch and the adsorption to raw starch is closely associated with the presence of either a starch-binding domain (SBD) or surface-binding sites (SBSs) (Janecek et al. 2014; Mitsuiki et al. 2005). Since BliAmy lacks a separate SBD, whether raw starch digestion depend on the existence of an enzyme SBS(s) or is possible simply through the enzyme active site and/or its neighbourhood is yet to be elucidated.
References
Bernfeld P (1955) Amylases, α and β. In: De Murray P (ed) Methods in enzymology, vol I. Deutcher Academic Press INC, San Diego California, pp. 149–158
Božić N, Puertas J-M, Lončar N, Sans Duran C, López-Santín J, Vujčić Z (2013) The DsbA signal peptide-mediated secretion of a highly efficient raw-starch-digesting, recombinant α-amylase from Bacillus licheniformis ATCC 9945a. Process Biochem 48(3):438–442
Božić N, Ruiz J, Lopez-Santin J, Vujčić Z (2011) Production and properties of the highly efficient raw starch digesting alpha-amylase from a Bacillus licheniformis ATCC 9945a. Biochem Eng J 53(2):203–209. doi:10.1016/j.bej.2010.10.014
Božić N, Vujčić Z (2005) Detection and quantification of leucyl arninopeptidase after native electrophoresis using leucine-p-nitroanilide. Electrophoresis 26(12):2476–2480. doi:10.1002/elps.200500047
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Colonna P, Buleon A, Lemarie F (1988) Action of Bacillus subtilis alpha-amylase on native wheat starch. Biotechnol Bioeng 31(9):895–904. doi:10.1002/bit.260310902
Declerck N, Joyet P, Trosset JY, Garnier J, Gaillardin C (1995) Hyperthermostable mutants of Bacillus licheniformis alpha-amylase: multiple amino acid replacements and molecular modelling. Protein Eng 8(10):1029–1037
Finore I, Kasavi C, Poli A, Romano I, Oner ET, Kirdar B, Dipasquale L, Nicolaus B, Lama L (2011) Purification, biochemical characterization and gene sequencing of a thermostable raw starch digesting alpha-amylase from Geobacillus thermoleovorans subsp stromboliensis subsp nov. World J Microb Biot 27(10):2425–2433. doi:10.1007/s11274-011-0715-5
Gernat C, Radosta S, Anger H, Damaschun G (1993) Crystalline parts of three different conformations detected in native and enzymatically degraded starches. Starch - Stärke 45(9):309–314. doi:10.1002/star.19930450905
Goyal N, Gupta JK, Soni SK (2005) A novel raw starch digesting thermostable alpha-amylase from Bacillus sp I-3 and its use in the direct hydrolysis of raw potato starch. Enzyme Microb Tech 37(7):723–734. doi:10.1016/j.enzmictec.2005.04.017
Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial alpha-amylases: a biotechnological perspective. Process Biochem 38(11):1599–1616. doi:10.1016/S0032-9592(03)00053-0
Haki GD, Rakshit SK (2003) Developments in industrially important thermostable enzymes: a review. Bioresource Technol 89(1):17–34. doi:10.1016/S0960-8524(03)00033-6
Helbert W, Schulein M, Henrissat B (1996) Electron microscopic investigation of the diffusion of Bacillus licheniformis alpha-amylase into corn starch granules. Int J Biol Macromol 19(3):165–169
Janeček S, Svensson B, MacGregor EA (2014) Alpha-amylase: an enzyme specificity found in various families of glycoside hydrolases. Cell Mol Life Sci 71(7):1149–1170. doi:10.1007/s00018-013-1388-z
Kunamneni A, Singh S (2005) Response surface optimization of enzymatic hydrolysis of maize starch for higher glucose production. Biochem Eng J 27(2):179–190. doi:10.1016/j.bej.2005.08.027
Lei Y, Peng H, Wang Y, Liu YT, Han F, Xiao YZ, Gao Y (2012) Preferential and rapid degradation of raw rice starch by an alpha-amylase of glycoside hydrolase subfamily GH13_37. Appl Microbiol Biot 94(6):1577–1584. doi:10.1007/s00253-012-4114-0
Leveque E, Janecek S, Haye B, Belarbi A (2000) Thermophilic archaeal amylolytic enzymes. Enzyme Microb Tech 26(1):3–14. doi:10.1016/S0141-0229(99)00142-8
Liu XD, Xu Y (2008) A novel raw starch digesting alpha-amylase from a newly isolated Bacillus sp YX-1: purification and characterization. Bioresource Technol 99(10):4315–4320. doi:10.1016/j.biortech.2007.08.040
Lončar N, Fraaije MW (2014) Not so monofunctional-a case of thermostable Thermobifida fusca catalase with peroxidase activity. Appl Microbiol Biotechnol. doi:10.1007/s00253-014-6060-5
Mehta D, Satyanarayana T (2014) Domain C of thermostable alpha-amylase of Geobacillus thermoleovorans mediates raw starch adsorption. Appl Microbiol Biotechnol 98(10):4503–4519. doi:10.1007/s00253-013-5459-8
Mitsuiki S, Mukae K, Sakai M, Goto M, Hayashida S, Furukawa K (2005) Comparative characterization of raw starch hydrolyzing alpha-amylases from various Bacillus strains. Enzyme Microb Tech 37(4):410–416. doi:10.1016/j.enzmictec.2005.02.022
Mok SC, Teh AH, Saito JA, Najimudin N, Alam M (2013) Crystal structure of a compact alpha-amylase from Geobacillus thermoleovorans. Enzyme Microb Tech 53(1):46–54. doi:10.1016/j.enzmictec.2013.03.009
Montgomery DC (2009) Design and analysis of experiments, Eight edn. John Wiely and Sons, Inc., Singapore
Oates CG (1997) Towards an understanding of starch granule structure and hydrolysis. Trends Food Sci Tech 8(11):375–382. doi:10.1016/S0924-2244(97)01090-X
Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, Mohan R (2000) Advances in microbial amylases. Biotechnol Appl Biochem 31:135–152. doi:10.1042/Ba19990073
Pinsach J, de Mas C, Lopez-Santin J (2006) A simple feedback control of Escherichia coli growth for recombinant aldolase production in fed-batch mode. Biochem Eng J 29(3):235–242. doi:10.1016/j.bej.2006.01.001
Puspasari F, Radjasa OK, Noer AS, Nurachman Z, Syah YM, van der Maarel M, Dijkhuizen L, Janecek S, Natalia D (2013) Raw starch-degrading alpha-amylase from Bacillus aquimaris MKSC 6.2: isolation and expression of the gene, bioinformatics and biochemical characterization of the recombinant enzyme. J Appl Microbiol 114(1):108–120. doi:10.1111/jam.12025
Robertson GH, Wong DWS, Lee CC, Wagschal K, Smith MR, Orts WJ (2006) Native or raw starch digestion: a key step in energy efficient biorefining of grain. J Agric Food Chem 54(2):353–365. doi:10.1021/Jf051883m
Roy I, Gupta MN (2004) Hydrolysis of starch by a mixture of glucoamylase and pullulanase entrapped individually in calcium alginate beads. Enzyme Microb Tech 34(1):26–32. doi:10.1016/j.enzmictec.2003.07.001
Ruiz J, Pinsach J, Alvaro G, Gonzalez G, de Mas C, Resina D, Lopez-Santin J (2009) Alternative production process strategies in E. coli improving protein quality and downstream yields. Process Biochem 44(9):1039–1045. doi:10.1016/j.procbio.2009.05.007
Sandhu KS, Singh N (2007) Some properties of corn starches II: physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem 101(4):1499–1507. doi:10.1016/j.foodchem.2006.01.060
Sharma A, Satyanarayana T (2012) Cloning and expression of acidstable, high maltose-forming, Ca2 + -independent alpha-amylase from an acidophile Bacillus acidicola and its applicability in starch hydrolysis. Extremophiles 16(3):515–522. doi:10.1007/s00792-012-0451-2
Sun HY, Zhao PJ, Ge XY, Xia YJ, Hao ZK, Liu JW, Peng M (2010) Recent advances in microbial raw starch degrading enzymes. Appl Biochem Biotech 160(4):988–1003. doi:10.1007/s12010-009-8579-y
Tawil G, Vikso-Nielsen A, Rolland-Sabate A, Colonna P, Buleon A (2011) In depth study of a new highly efficient raw starch hydrolyzing alpha-amylase from Rhizomucor sp. Biomacromolecules 12(1):34–42. doi:10.1021/Bm100913z
Tawil G, Vikso-Nielsen A, Rolland-Sabate A, Colonna P, Buleon A (2012) Hydrolysis of concentrated raw starch: a new very efficient alpha-amylase from Anoxybacillus flavothermus. Carbohyd Polym 87(1):46–52. doi:10.1016/j.carbpol.2011.07.005
Trichon S, Bonte M, van den Boogaard A, Ponthot J-P A (2007) Study for efficiently solving optimisation problems with an increasing number of design variables. NUMIFORM, 481–486, http://purl.utwente.nl/publications/59674
Uthumporn U, Zaidul ISM, Karim AA (2010) Hydrolysis of granular starch at sub-gelatinization temperature using a mixture of amylolytic enzymes. Food Bioprod Process 88(C1):47–54. doi:10.1016/j.fbp.2009.10.001
van der Maarel MJ, van der Veen B, Uitdehaag JC, Leemhuis H, Dijkhuizen L (2002) Properties and applications of starch-converting enzymes of the alpha-amylase family. J Biotechnol 94(2):137–155
Vikso-Nielsen A, Andersen C, Hoff T, Pedersen S (2006) Development of new alpha-amylases for raw starch hydrolysis. Biocatal Biotransfor 24(1–2):121–127. doi:10.1080/10242420500519191
WO 2001 Pre-oxidezed alpha-amylase, 096537
Acknowledgments
This work was supported by the Serbian Ministry of Education, Science and Technological development, project grant number 172048 and International Centre for Genetic Engineering and Biotechnology (ICGEB) research project grant number CRP/YUG11-02.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 304 kb)
Rights and permissions
About this article
Cite this article
Šokarda Slavić, M., Pešić, M., Vujčić, Z. et al. Overcoming hydrolysis of raw corn starch under industrial conditions with Bacillus licheniformis ATCC 9945a α-amylase. Appl Microbiol Biotechnol 100, 2709–2719 (2016). https://doi.org/10.1007/s00253-015-7101-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7101-4