Abstract
In our previous study, we produced phenylpyruvic acid (PPA) in one step from l-phenylalanine by using an Escherichia coli whole-cell biocatalyst expressing an l-amino acid deaminase (l-AAD) from Proteus mirabilis KCTC2566. However, the PPA titer was low due to the degradation of PPA and low substrate specificity of l-AAD. In this study, metabolic engineering of the l-phenylalanine degradation pathway in E. coli and protein engineering of l-AAD from P. mirabilis were performed to improve the PPA titer. First, three aminotransferase genes were knocked out to block PPA degradation, which increased the PPA titer from 3.3 ± 0.2 to 3.9 ± 0.1 g/L and the substrate conversion ratio to 97.5 %. Next, l-AAD was engineered via error-prone polymerase chain reaction, followed by site-saturation mutation to improve its catalytic performance. The triple mutant D165K/F263M/L336M produced the highest PPA titer of 10.0 ± 0.4 g/L, with a substrate conversion ratio of 100 %, which was 3.0 times that of wild-type l-AAD. Comparative kinetics analysis showed that compared with wild-type l-AAD, the triple mutant had higher substrate-binding affinity and catalytic efficiency. Finally, an optimal fed-batch biotransformation process was developed to achieve a maximal PPA titer of 21 ± 1.8 g/L within 8 h. This study developed a robust whole-cell E. coli biocatalyst for PPA production by integrating metabolic and protein engineering, strategies that may be useful for the construction of other biotransformation biocatalysts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
α-Keto acids are vital intermediate compounds with a wide range of applications in the pharmaceutical, food, and chemical industries (Fuchs et al. 2009; Pailla et al. 2000). Among these compounds, phenylpyruvic acid (PPA) is particularly useful. In the pharmaceutical industry, PPA is used to produce d-phenylalanine, the raw material of antidiabetic, antineoplastic, anti-AIDS compounds, and peptide antibiotics (Pantaleone et al. 2001; Zhu et al. 2014). In addition, PPA is used to synthesize phenyllactic acid, an antimicrobial agent, food preservative, and flavor compound (Li et al. 2008; Zheng et al. 2011).
Currently, PPA is chemically synthesized with glycine, benzaldehyde, benzyl chloride, and carbonic oxide (des Abbayes and Salaun 2003; Villablanca and Cilento 1987). This chemical strategy is not only environmentally unfriendly but also produces various toxic by-products. PPA can also be produced via microbial fermentation with microorganisms such as Zygosaccharomyces rouxii, Proteus vulgaris, Morganella morganii, and Corynebacterium glutamicum (Coban et al. 2014). Although microbial fermentation is environmentally friendly, it produces relatively small amounts of PPA. Biotransformation is an alternative for PPA production. The production of PPA via the oxidation of d-phenylalanine by d-amino acid oxidase (EC 1.4.3.3) has been reported (Buto et al. 1994; Fernandez-Lafuente et al. 1998; Yoshimoto et al. 2014). This reaction produces toxic hydrogen peroxide; however, an additional catalase enzyme is necessary to minimize detrimental effects of that compound. On the contrary, l-amino acid deaminases (l-AADs) catalyze the stereospecific oxidative deamination of l-amino acids to generate their respective keto acids and ammonia without the formation of hydrogen peroxide (Geueke and Hummel 2002). Moreover, l-phenylalanine is cheaper than d-phenylalanine, which is an important consideration for industrial PPA production. Therefore, l-AADs are an ideal enzyme choice for PPA biosynthesis.
Proteus mirabilis contains two types of membrane-bound l-AADs. One type has broad substrate specificity and catalyzes the oxidation of aliphatic and aromatic l-amino acids to α-keto acids, typically l-phenylalanine. The other acts mainly on basic l-amino acids (Duerre and Chakrabarty 1975). In a previous study, we constructed an E. coli whole-cell catalyst expressing the first type of l-AAD from P. mirabilis KCTC2566 and produced PPA from l-phenylalanine with a maximal production and substrate conversion ratio of 3.3 ± 0.2 g/L and 82.5 ± 4 %, respectively (Hou et al. 2015). The relatively low PPA production of this system was attributed to low substrate specificity, significant substrate or product inhibition, and side reactions. In the present study, the metabolic pathway of PPA in E. coli was engineered to block PPA degradation and the l-AAD from P. mirabilis KCTC2566 was engineered for improved catalytic performance. Three aminotransferase genes, tyrB, aspC, and ilvE, were deleted to reduce PPA degradation, and l-AAD was then engineered through a combination of directed evolution and site-saturation mutagenesis to increase its catalytic performance. Finally, an optimal fed-batch biotransformation was developed to improve PPA production further.
Materials and methods
Plasmids, bacterial strains, and chemicals
The strains and plasmids used in this study are listed in Table 1. Enzymes (PrimeSTAR HS DNA polymerase, 2× Taq polymerase chain reaction [PCR] mix) and kits (Genomic Extraction Kit, DNA Purification Kit, and Competent Cell Preparation Kit) were supplied by TaKaRa (Dalian, China). With the exception of phenylpyruvic acid (Sigma-Aldrich, Shanghai, China), all chemical reagents were purchased from Shanghai Sangon Biological Engineering Technology and Services Co. Ltd. (Shanghai, China).
Deletion of three aminotransferase genes tyrB, aspC, and ilvE in E. coli
The gene knockout strains were constructed with a one-step disruption protocol (Datsenko and Wanner 2000). The upstream and downstream regions (approximately 1000 bp) flanking the target gene were designed and amplified from E. coli BL21 (DE3) genome DNA. The disruption cassettes were amplified from vector pKD13 and fused to the flanking regions via fusion PCR. The primers are shown in Table 2. The purified PCR products were transformed via electroporation into E. coli BL21 (DE3). Kanamycin-resistant (Kanr) transformants were selected in the presence of kanamycin (Shanghai Sangon Biological Engineering Technology and Services Co. Ltd). The resistance marker cassettes were removed from the chromosome with FLP recombinase by using the temperature-inducible plasmid pCP20. The single-deletion mutants were designated E. coli BL21 (DE3) (ΔtyrB), E. coli BL21 (DE3) (ΔaspC), and E. coli BL21 (DE3) (ΔilvE). The double- and triple-deletion strains were constructed in the same way and designated E. coli BL21 (DE3) (ΔtyrBΔaspC), E. coli BL21 (DE3) (ΔtyrBΔilvE), E. coli BL21 (DE3) (ΔaspCΔilvE), and E. coli BL21 (DE3) (ΔtyrBΔaspCΔilvE).
The amount of PPA degradation by the parental and engineered E. coli BL21 (DE3) mutants was determined under optimal biotransformation conditions (4 g/L PPA, 1.2 g/L cells (dry cell weight), pH 7.4, 40 °C, 6 h). The recombinant l-AAD was then transformed into the engineered strains, and whole-cell biotransformation was performed with various concentrations of l-phenylalanine (4, 12, and 20 g/L) to produce PPA.
Directed evolution of l-AAD via error-prone PCR
The l-AAD gene was amplified from the recombinant plasmid pET 20b (+)-AAD via error-prone PCR (ep-PCR) with a GeneMorph II Random Mutagenesis Kit (Agilent Technologies, Santa Clara, USA). The 50-μL reaction mixture for ep-PCR contained 5 μL 10× Mutazyme II reaction buffer, 1 μL 40 mM deoxyribonucleotide triphosphate mix (200 μM each, final), 0.5 μL primer mix (250 ng/μL each primer), 1 μL Mutazyme II DNA polymerase (2.5 U/μL), 1 μL template, and 41.5 μL water.
To produce high amplification yields after 30 cycles and a mutation frequency of 2–20 nucleotide changes per mutant, we used 0.1–100 ng target DNA as the template. The mutant library was transformed into E. coli BL21 (DE3) and cultured on Luria-Bertani (LB) solid medium containing 100 μg/mL ampicillin at 37 °C for 8 h. Variants from the mutant library were selected and cultured in 0.5-mL LB broth containing 100 μg/mL ampicillin and grown on 96-well plates at 37 °C overnight. Seed broth was inoculated into Terrific broth medium containing 0.2 % l-phenylalanine and 100 μg/mL ampicillin, and the cells were grown at 37 °C for 2 h, induced by the addition of isopropyl-β-d-thiogalactopyranoside (IPTG; 0.04 mM), and grown for an additional 5 h at 30 °C. Ferric chloride solution (1 %, w/v) was added to assess the presence of PPA (Massad et al. 1995). Positive mutants were selected and used for whole-cell transformation to check the PPA titer.
Site-directed mutagenesis of l-AAD
Site-directed mutagenesis was performed with a one-step PCR method by using a MutanBEST kit (TaKaRa). The reactions contained recombinant plasmid pET 20b (+)-AAD as the template and mutagenic oligonucleotides as primers (see Table 2). After purification, the PCR product was blunted with Blunting Kination Enzyme Mix and ligated with Ligation Solution I (TaKaRa). The mutant plasmids were transformed into E. coli BL21 (DE3) and confirmed through PCR and sequencing. The PPA titers of the successful transformants were measured under the optimal whole-cell biotransformation conditions (10 g/L l-phenylalanine, 4.2 g/L cells, pH 7.4, 40 °C, 6 h).
Preparation of the biocatalyst
The wild-type and mutants of l-AAD were transformed into the metabolically engineered E. coli BL21 (DE3). The recombinant E. coli BL21 (DE3) was inoculated into 20-mL LB medium containing ampicillin (100 μg/mL) and grown in a rotary shaker (200 rpm) at 37 °C overnight. A seed culture (1 %, v/v) was inoculated into 500-mL Terrific broth medium containing ampicillin (100 μg/mL) for expression. The overall optimal induction conditions were as follows: pH 8, 0.04 mM IPTG, optical density at 600 nm of 0.6, and induction at 20 °C for 12 h. The cells were obtained via centrifugation (Beckman Instruments, San Jose, CA) at 8000×g and 4 °C for 20 min, and washed twice with 20 mM phosphate buffer (pH 7.0). The cells were then resuspended in 20 mM phosphate buffer (pH 7.0). Biomass concentration was checked spectrophotometrically (UV-2450 PC; Shimadzu Co., Kyoto, Japan) at an optical density of 600.
Purification of l-AAD and its mutants
The solubilization and purification of the wild-type l-AAD and its mutants were performed according to a previously published method (Hou et al. 2015). Briefly, the induced cell pellets were suspended in purification buffers containing n-dodecyl-β-d-maltoside (0.01 %). After disruption with ultrasonication at 4 °C for 20 min, the detergent-solubilization solution was filtered through a 0.45-μM pore size membrane, and the filtrate was purified on a HisTrap™ FF 5 mL column with AKTA Explorer (GE Healthcare, Piscataway, NJ). Then, desalting was performed with an Ultra-4 Centrifugal Filter Device (Amicon, Shanghai, China). The concentration of protein was determined with a BCA protein assay kit (TianGen, Beijing, China).
Determination of kinetic parameters
The kinetic analysis of l-phenylalanine transformation to PPA with wild-type l-AAD and its mutants was performed according to a previously published method (Hou et al. 2015). The parameters were calculated with the Lineweaver-Burk plotting method shown in Eq. (1):
where V is the reaction rate (the amount of PPA produced by 1 mg recombinant l-AAD per min; μmol PPA min−1 mg−1 protein), V max is the maximum reaction rate (μmol PPA min−1 mg−1 protein), K m is the Michaelis constant (mM), and [S] is the l-phenylalanine concentration (mM).
Protein analysis
Protein was analyzed with sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The cells were prepared in denaturing buffer at 95 °C for 10 min, and 15 μL of the samples was loaded onto a gel composed of a 5 % (m/v) polyacrylamide stacking gel and an 8 % (m/v) polyacrylamide separating gel (Bio-Rad Laboratories, Hercules, CA).
Results
Metabolic engineering of the PPA degradation pathway in E. coli
Product degradation via side reactions often occurs in whole-cell biocatalyst systems (Fig. 1a), and substrates are inefficiently utilized to produce the target products (de Carvalho 2011). Measurements show that the rate of PPA degradation by E. coli BL21 (DE3) is approximately 110 ± 4 mg/L per gram cell (dry cell weight) (Fig. 1b). In E. coli, three aminotransferases—an aromatic amino acid aminotransferase encoded by tyrB, an aspartate aminotransferase encoded by aspC, and an isoleucine aminotransferase encoded by ilvE—participate in the degradation of PPA (see Fig. 1a) (Müller et al. 2006; Sun et al. 2011). The aromatic amino acid aminotransferase and aspartate aminotransferase are also involved in the synthesis of l-tyrosine (see Fig. 1a). Single-, double-, and triple-deletion mutants were constructed in E. coli BL21 (DE3) to determine the amount of PPA degradation that occurs in these strains (see Fig. 1b) and evaluate the effect of gene deletion on the growth of recombinant E. coli BL21 (DE3) (Fig. 1d).
Construction of deletion mutants of E. coli BL21 (DE3). a l-Phenylalanine pathway in E. coli BL21 (DE3). b The amount of PPA degradation by the wild type and its knockout mutants. c PPA production of whole-cell biotransformation by the wild-type and engineered E. coli BL21 (DE3) with different concentrations of l-phenylalanine. Square, wild type; circle, BL21 (DE3) (ΔtyrB); up triangle, BL21 (DE3) (ΔaspC); down triangle, BL21 (DE3) (ΔilvE); left triangle, BL21 (DE3) (ΔtyrBΔaspC); right triangle, BL21 (DE3) (ΔtyrBΔilvE); star, BL21 (DE3) (ΔaspCΔilvE); cross, BL21 (DE3) (ΔtyrBΔaspCΔilvE). d Cell growth profile of the recombinant E. coli BL21 (DE3). Square, wild type; circle, BL21 (DE3) (ΔtyrBΔaspCΔilvE)
The final cell concentration of the parental strain was 1.4 times than that of the recombinant E. coli BL21 (DE3) (ΔtyrBΔaspCΔilvE)—perhaps because the gene deletion mutants became auxotrophic for both l-phenylalanine and l-tyrosine. For the single-deletion mutants, the PPA degradation rate of E. coli BL21 (DE3) (ΔtyrB) was 64.5 % that of the parental strain, whereas deletion of aspC or ilvE had little effect on PPA degradation. As for the double-deletion mutants, the PPA degradation rate of E. coli BL21 (DE3) (ΔtyrBΔaspC), E. coli BL21 (DE3) (ΔtyrBΔilvE), and E. coli BL21 (DE3) (ΔaspCΔilvE) was 29.1, 43.6, and 73.6 % that of the parental strain. For the triple-deletion mutant E. coli BL21 (DE3) (ΔtyrBΔaspCΔilvE), PPA degradation rate was only 10 ± 0.3 mg/L per gram cell, which indicated that compared with other mutants, the triple-deletion mutant had less PPA degradation. Then, whole-cell biotransformation of l-phenylalanine with these mutants was performed (Fig. 1c). Higher PPA production in the mutant strains was consistent with the lower PPA degradation rate. Deletion of tyrB increased PPA production in the single-deletion mutant compared with the parental strain, whereas deletion of aspC and ilvE had little effect on PPA production. Among all the mutant strains, recombinant E. coli BL21 (DE3) (ΔtyrBΔaspCΔilvE) had the highest PPA production of 3.9 ± 0.1, 6.7 ± 0.7, and 6.8 ± 0.5 g/L with 4, 12, and 20 g/L of l-phenylalanine, which was 1.2, 1.2, and 1.3 times than that of the wild type, respectively. We also found that at the lowest tested concentration of l-phenylalanine (4 g/L), the substrate conversion ratio was 97.5 %. This ratio decreased to 55.8 and 34.0 % at higher concentration of l-phenylalanine (12 and 20 g/L) due to low substrate specificity, substrate inhibition, and product inhibition.
Directed evolution and site-directed mutagenesis of l-AAD
Due to the low similarity (19 %) of l-AAD to other l-amino amino oxidases, it is difficult to develop a homology model to improve the catalytic performance. Therefore, to improve the catalytic performance of l-AAD and the biotransformation efficiency of l-phenylalanine, we performed directed evolution of l-AAD with ep-PCR and constructed a mutant library. First, the ep-PCR conditions were optimized by varying the initial amount of template. Low, medium, or high mutation rates can be produced by varying the initial amount of target DNA or the number of amplification cycles with minimal mutational bias (Neylon 2004). Compared with low mutation rates, high mutation rates produce a greater number of unique sequences (Drummond et al. 2005).
The results showed that the PCR conditions resulted in an average of 1–6 amino acid substitutions (2–20 nucleotide changes) per mutant. The resulting library of approximately 10,000 colonies was screened to identify the mutants with higher biotransformation efficiencies. One mutant had PPA production (8.1 ± 0.25 g/L) that was 2.1 times that of the wild-type (3.9 ± 0.1 g/L; see Fig. 2a). The catalytic properties of wild-type l-AAD and this mutant were analyzed (Table 3) and compared with the wild type, the mutant exhibited a 30.0 % increase in K cat and an 8.0 % decrease in K m . The overall catalytic efficiency (as indicated by K cat/K m) of the mutant was 76 s−1 M−1, a 43.4 % improvement over the catalytic efficiency of the wild type. Sequence analysis showed that the mutant had four amino acid substitutions (D165G, S179L, F263V, and L336V). Two hydrophobic amino acids (phenylalanine and leucine) had changed to a different hydrophobic amino acid (valine), and one hydrophilic amino acid (aspartic acid) had changed to a different hydrophilic amino acid (glycine). However, one hydrophilic amino acid (serine) had changed to a hydrophobic amino acid (leucine). These results indicated that the amino acids D165, S179, F263, and L336 are critical to the catalytic efficiency of l-AAD. Hence, they were selected as targets for further evolution via site-saturation mutagenesis for the fine-tuning of the PPA production capacity of the mutants.
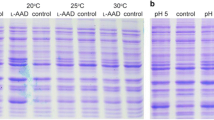
PPA production of whole-cell biotransformation by different mutants and SDS-PAGE analysis of the purification results. a PPA production of whole-cell biotransformation by different mutants under 10 g/L of l-phenylalanine. Results were obtained with a whole-cell E. coli biocatalyst containing different mutants of l-AAD. Square, wild-type; circle, D165G/S179L/F263V/L336V; up triangle, D165K; down triangle, F263M; diamond, L336M; left triangle, D165K/F263M; right triangle, D165K/L336M; hexagon, F263M/L336M; star, D165K/F263M/L336M; pentagon, wild type E. coli (D165K/F263M/L336M). b SDS-PAGE analysis of the purification results of different mutants
All l-AAD mutants were expressed in E. coli BL21 (DE3) (ΔtyrBΔaspCΔilvE), and similar expression levels were observed in sodium dodecyl sulfate gel analysis (data not shown).
Time courses for PPA production with the wild-type and mutants were compared (Fig. 2a). The D165K, F263M, and L336M mutants produced PPA titers of 7.7 ± 0.3, 8.5 ± 0.2, and 7.9 ± 0.3 g/L—2.0, 2.2, and 2.0 times that of the wild type, respectively. By contrast, any change to S179 resulted in PPA titers lower than that of the wild type, so S179 was not employed for further study. Based on these results, double and triple mutants were constructed, and their biotransformation capabilities were measured. The PPA titers of the double mutants D165K/F263M, D165K/L336M, and F263M/L336M were 2.4, 2.0, and 2.3 times that of the wild type, respectively. The triple mutant D165K/F263M/L336M produced the highest PPA titer of 10.0 ± 0.4 g/L, with a substrate conversion ratio of 100 %, which was 3.0 times that of the wild-type. Compared with the wild type, the triple mutant exhibited a 60.7 % increase in K cat and a 16.0 % decrease in K m . Furthermore, it displayed the highest catalytic efficiency of 102 s−1 M−1, which was 1.9-fold that of wild type. Furthermore, the best l-AAD mutant D165K/F263M/L336M was expressed in E. coli BL21 (DE3) and E. coli BL21 (DE3) (ΔtyrBΔaspCΔilvE), and similar expression levels were observed in sodium dodecyl sulfate gel analysis (data not shown). The engineered E. coli produced PPA titers of 1.25 times that of the wild type.
Fed-batch biotransformation process for PPA production
Although we deleted the PPA degradation pathway and improved the catalytic performance of l-AAD via molecular engineering, the PPA titer cannot increase further with more than 10 g/L l-phenylalanine due to substrate inhibition. To overcome this inhibition, we developed a fed-batch biotransformation strategy to increase the PPA titer by maintaining the l-phenylalanine concentration below 10 g/L. A specific amount of l-phenylalanine was added to the reaction mixture at various times. According to the time profiles of PPA synthesis and l-phenylalanine consumption in the batch biotransformation (Fig. 3a), almost all of the l-phenylalanine was used to produce PPA, and thus, substrate feeding can be performed by measuring PPA concentration. Beginning with 10 g/L of l-phenylalanine, the feeding interval was optimized. When feeding occurred every hour, the total l-phenylalanine added to the system was 31 ± 2.5 g/L and the maximal PPA production was 21 ± 1.8 g/L within 8 h. When feeding occurred every 2 h, the total l-phenylalanine added to the system was 24.3 ± 1.2 g/L and the maximal PPA production was 14.8 ± 1.6 g/L within 7 h. When feeding occurred every 4 h, the total l-phenylalanine added to the system was 18.8 ± 1.1 g/L and the maximal PPA production was 10.8 ± 1.0 g/L within 7 h. Therefore, feeding every hour was the optimal fed-batch method for PPA production (Fig. 3).
Discussion
Previously, we used both a purified enzyme and a whole-cell biocatalyst to produce PPA via one-step biotransformation from l-phenylalanine. Although the whole-cell biocatalyst provided substantial benefits such as simplified cell preparation, higher thermal stability, and the capacity to recycle, PPA production was not remarkably high owing to the degradation of PPA by side reactions. In this study, metabolic engineering of the PPA degradation pathway and protein engineering of l-AAD were performed to improve PPA production with an E. coli whole-cell biocatalyst.
Resting E. coli under nongrowing conditions suspended in buffer solution without carbon, nitrogen, or mineral sources is used as a biocatalyst to produce many compounds (Hara and Kino 2010; Narancic et al. 2013), but the enzymes in the cells still function under certain conditions, resulting in side reactions as well as degradation (Hossain et al. 2014b). In E. coli BL21 (DE3), three aminotransferases are involved in the degradation of PPA. To minimize PPA degradation by the whole-cell biocatalyst, we constructed single-, double-, and triple-gene deletion strains. Among all the strains, the triple-deletion mutant E. coli BL21 (DE3) (ΔtyrBΔaspCΔilvE) was the most effective in reducing PPA degradation, and therefore, PPA production improved. In general, the performance of the double-deletion strains was better than that of the single-deletion strains, which demonstrated the synergistic effect of these three genes on the degradation of PPA. We concluded that the aromatic amino acid aminotransferase is the primary enzyme for transforming PPA to l-phenylalanine.
The biotransformation efficiency of E. coli BL21 (DE3) (ΔaspC) was better than that of E. coli BL21 (DE3) (ΔilvE), which indicated that the aspartate aminotransferase was the second most significant enzyme in this reaction, whereas the isoleucine aminotransferase was the least vital. Although the deletion of ilvE had no obvious effect on PPA degradation, PPA degradation dramatically decreased when ilvE was deleted after the deletion of tyrB and aspC. The results also showed that the isoleucine aminotransferase was more effective without the aromatic amino acid and aspartate aminotransferases. A small amount of PPA degraded in the triple-deletion mutant, which may be attributed to unknown reactions in E. coli.
The results of directed evolution and site-directed mutagenesis of l-AAD showed that compared with the single mutants, the double mutants had higher substrate binding affinity and catalytic efficiency, and the triple mutant exhibited the highest catalytic efficiency overall. These outcomes indicated that the three mutations had a synergistic effect on enzyme activity toward l-phenylalanine. After fine-tuning the mutation sites, which were identified through random mutagenesis, we observed that the new mutants generated by site-saturation mutagenesis were more effective for biotransformation. Thus, a combination of directed evolution and site-directed mutation is an effective strategy for the quick and effective evolution of enzymes (Cherry et al. 1999; Hossain et al. 2014a).
For enzymatic transformation, the synthesis of PPA via the oxidation of d-phenylalanine (Brodelius et al. 1981) or d,l-phenylalanine (Szwajcer et al. 1982) by d-amino acid oxidases has been reported, but only 10–50 mM substrate could be catalyzed and the PPA production was low (Brodelius et al. 1981; Buto et al. 1994; Fernandez-Lafuente et al. 1998). Besides, these reactions produce hydrogen peroxide, which is toxic to d-amino acid oxidases. Many methods were used to eliminate hydrogen peroxide, like the addition of catalase (Fernandez-Lafuente et al. 1998), manganese oxide (Brodelius et al. 1981), and activated charcoal (Szwajcer et al. 1982), resulting in increased costs and complicated process. As for microbial fermentation, P. vulgaris was used to produce PPA and the titer reached 1.1 g L−1 under the optimal conditions (Coban et al. 2014). Compared with the PPA titers in the above studies, the production of PPA here was higher. Moreover, the reaction catalyzed by l-AAD from P. mirabilis does not produce hydrogen peroxide and l-phenylalanine is cheaper than d-phenylalanine.
In conclusion, we constructed a metabolically engineered E. coli whole-cell biocatalyst via the deletion of three aminotransferase genes to eliminate side reactions and increase PPA production. l-AAD was also engineered via ep-PCR and site-directed mutagenesis, and the final triple mutant showed increased substrate binding affinity and catalytic efficiency. Finally, fed-batch biotransformation was optimized to increase PPA production. Substrate and product inhibition are expected to be decreased further through structural engineering of l-AAD and kinetic modeling of the biotransformation system in future studies.
References
Brodelius P, Nilsson K, Mosbach K (1981) Production of α-keto acids Part I. Immobilized cells of Trigonopsis variabilis containing D-amino acid oxidase. Appl Biochem Biotech 6:293–307
Buto S, Pollegioni L, Dangiuro L, Pilone MS (1994) Evaluation of D-amino acid oxidase from Rhodotorula gracilis for the production of α-keto acids: a reactor system. Biotechnol Bioeng 44:1288–1294
Cherry JR, Lamsa MH, Schneider P, Vind J, Svendsen A, Jones A, Pedersen AH (1999) Directed evolution of a fungal peroxidase. Nat Biotechnol 17:379–384
Coban HB, Demirci A, Patterson PH, Elias RJ (2014) Screening of phenylpyruvic acid producers and optimization of culture conditions in bench scale bioreactors. Bioproc Biosyst Eng 37:2343–2352
Datsenko KA, Wanner BL (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. P Natl Acad Sci U S A 97:6640–6645
de Carvalho CCCR (2011) Enzymatic and whole cell catalysis: finding new strategies for old processes. Biotechnol Adv 29:75–83
des Abbayes H, Salaun JY (2003) Double carbonylation and beyond: systems at work and their organometallic models. Dalton Trans 6:1041–1052
Drummond DA, Iverson BL, Georgiou G, Arnold FH (2005) Why high-error-rate random mutagenesis libraries are enriched in functional and improved proteins. J Mol Biol 350:806–816
Duerre JA, Chakrabarty S (1975) L-Amino acid oxidases of Proteus rettgeri. J Bacteriol 121:656–663
Fernandez-Lafuente R, Rodriguez V, Guisan JM (1998) The coimmobilization of D-amino acid oxidase and catalase enables the quantitative transformation of D-amino acids (D-phenylalanine) into α-keto acids (phenylpyruvic acid). Enzyme Microb Tech 23:28–33
Fuchs M, Engel J, Campos M, Matejec R, Henrich M, Harbach H, Wolff M, Weismüller K, Menges T, Heidt MC, Welters ID, Kruell M, Hempelmann G, Mühling J (2009) Intracellular α-keto acid quantification by fluorescence-HPLC. Amino Acids 36:1–11
Geueke B, Hummel W (2002) A new bacterial L-amino acid oxidase with a broad substrate specificity: purification and characterization. Enzyme Microb Tech 31:77–87
Hara R, Kino K (2010) Industrial production of threo-3-hydroxy-L-aspartic acid using Escherichia coli resting cells. J Biotechnol 150:S373–S373
Hossain GS, Li J, Shin H-D, Du G, Wang M, Liu L, Chen J (2014a) One-step biosynthesis of α-keto-gamma-methylthiobutyric acid from L-methionine by an Escherichia coli whole-cell biocatalyst expressing an engineered L-amino acid deaminase from Proteus vulgaris. PLoS ONE 9, e114291
Hossain GS, Li J, Shin H-D, Liu L, Wang M, Du G, Chen J (2014b) Improved production of α-ketoglutaric acid (α-KG) by a Bacillus subtilis whole-cell biocatalyst via engineering of L-amino acid deaminase and deletion of the α-KG utilization pathway. J Biotechnol 187:71–77
Hou Y, Hossain GS, Li J, Shin H-D, Liu L, Du G (2015) Production of phenylpyruvic acid from L-phenylalanine using an L-amino acid deaminase from Proteus mirabilis: comparison of enzymatic and whole-cell biotransformation approaches. Appl Microbiol Biot 99:8391–8402
Li X, Jiang B, Pan B, Mu W, Zhang T (2008) Purification and partial characterization of Lactobacillus species SK007 lactate dehydrogenase (LDH) catalyzing phenylpyruvic acid (PPA) conversion into phenyllactic acid (PLA). J Agr Food Chem 56:2392–2399
Massad G, Zhao H, Mobley HL (1995) Proteus mirabilis amino acid deaminase: cloning, nucleotide sequence, and characterization of aad. J Bacteriol 177:5878–5883
Müller U, van Assema F, Gunsior M, Orf S, Kremer S, Schipper D, Wagemans A, Townsend CA, Sonke T, Bovenberg R, Wubbolts M (2006) Metabolic engineering of the E. coli L-phenylalanine pathway for the production of D-phenylglycine (D-Phg). Metab Eng 8:196–208
Narancic T, Kadivojevic J, Jovanovic P, Francuski D, Bigovic M, Maslak V, Savic V, Vasiljevic B, O'Connor KE, Nikodinovic-Runic J (2013) Highly efficient Michael-type addition of acetaldehyde to β-nitrostyrenes by whole resting cells of Escherichia coli expressing 4-oxalocrotonate tautomerase. Bioresource Technol 142:462–468
Neylon C (2004) Chemical and biochemical strategies for the randomization of protein encoding DNA sequences: library construction methods for directed evolution. Nucleic Acids Res 32:1448–1459
Pailla K, Blonde-Cynober F, Aussel C, De Bandt J-P, Cynober L (2000) Branched-chain keto-acids and pyruvate in blood: measurement by HPLC with fluorimetric detection and changes in older subjects. Clin Chem 46:848–853
Pantaleone DP, Geller AM, Taylor PP (2001) Purification and characterization of an L-amino acid deaminase used to prepare unnatural amino acids. J Mol Catal B-Enzym 11:795–803
Sun Z, Ning Y, Liu L, Liu Y, Sun B, Jiang W, Yang C, Yang S (2011) Metabolic engineering of the L-phenylalanine pathway in Escherichia coli for the production of S- or R-mandelic acid. Microbial cell factories 10:71
Szwajcer E, Brodelius P, Mosbach K (1982) Production of α-keto acids: 2. Immobilized whole cells of Providencia sp. PCM 1298 containing L-amino acid oxidase. Enzyme Microb Tech 4:409–413
Villablanca M, Cilento G (1987) Oxidation of phenylpyruvic acid. BBA-Gen Subjects 926:224–230
Yoshimoto M, Okamoto M, Ujihashi K, Oldta T (2014) Selective oxidation of D-amino acids catalyzed by oligolamellar liposomes intercalated with D-amino acid oxidase. Langmuir 30:6180–6186
Zheng Z, Ma C, Gao C, Li F, Qin J, Zhang H, Wang K, Xu P (2011) Efficient conversion of phenylpyruvic acid to phenyllactic acid by using whole cells of Bacillus coagulans SDM. PLoS ONE 6, e19030
Zhu LB, Zhou L, Huang N, Cui WJ, Liu ZM, Xiao K, Zhou ZM (2014) Efficient preparation of enantiopure D-phenylalanine through asymmetric resolution using immobilized phenylalanine ammonia-lyase from Rhodotorula glutinis JN-1 in a recirculating packed-bed reactor. PLoS ONE 9, e108586
Acknowledgments
This work was financially supported by the Enterprise-university-research prospective program Jiangsu Province (BY2013015-37) and 863 Program (2014AA021200, 2014AA021201).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Hou, Y., Hossain, G.S., Li, J. et al. Combination of phenylpyruvic acid (PPA) pathway engineering and molecular engineering of l-amino acid deaminase improves PPA production with an Escherichia coli whole-cell biocatalyst. Appl Microbiol Biotechnol 100, 2183–2191 (2016). https://doi.org/10.1007/s00253-015-7048-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-7048-5