Abstract
The filamentous fungus Beauveria bassiana is an economically important pathogen of numerous arthropod pests and is able to grow in submerged culture as filaments (mycelia) or as budding yeast-like blastospores. In this study, we evaluated the effect of dissolved oxygen and high glucose concentrations on blastospore production by submerged cultures of two isolates of B. bassiana, ESALQ1432 and GHA. Results showed that maintaining adequate dissolved oxygen levels coupled with high glucose concentrations enhanced blastospore yields by both isolates. High glucose concentrations increased the osmotic pressure of the media and coincided with higher dissolved oxygen levels and increased production of significantly smaller blastospores compared with blastospores produced in media with lower concentrations of glucose. The desiccation tolerance of blastospores dried to less than 2.6 % moisture was not affected by the glucose concentration of the medium but was isolate dependent. Blastospores of isolate ESALQ1432 produced in media containing 140 g glucose L−1 showed greater virulence toward whitefly nymphs (Bemisia tabaci) as compared with blastospores produced in media containing 40 g glucose L−1. These results suggest a synergistic effect between glucose concentration and oxygen availability on changing morphology and enhancing the yield and efficacy of blastospores of B. bassiana, thereby facilitating the development of a cost-effective production method for this blastospore-based bioinsecticide.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Unlike the per os infection mechanism of entomopathogenic bacteria and viruses needed to cause disease, the ascomycete fungus Beauveria bassiana (Bals.-Criv.) Vuill. sensu lato (Hypocreales: Cordycipitaceae) as well as other filamentous entomopathogenic fungi have uniquely evolved to infect their arthropod hosts by direct (per cutaneous) penetration of the cuticle followed by multiplication in the hemocoel (Feng et al. 1994; Humber 2008). Typically, these fungal pathogens grow hyphally as they penetrate the insect body and, upon reaching the hemocoel, they undergo a morphological transition differentiating into yeast-like hyphal bodies which are specialized to rapidly exploit the host nutrients and tissues by colonizing the host and evading its immune system (Wanchoo et al. 2009; Wang et al. 2013). When grown in liquid media, B. bassiana and related species form unicellular, budding yeast-like cells termed blastospores, which are similar to in vivo produced hyphal bodies (Bidochka et al. 1987; Jackson et al. 1997; Wanchoo et al. 2009). The rapid germination rate of liquid culture-produced blastospores (90 % in less than 10 h) compared with solid-substrate-produced conidia (16–24 h) suggests that the former would be more suitable for use as the primary infective structure in commercial bioinsecticides (Jackson et al. 1997, 2003; Mascarin et al. 2015). This dimorphic growth expressed as yeast-like and mycelial morphologies can be regulated by nutritional and physical conditions during growth in artificial liquid media (Jackson 1997; Jackson et al. 1997). For instance, the regulation of morphogenesis in the filamentous fungi Mucor rouxii (Calmette) Wehmer and Mucor bacilliformis Hesselt. (Mucorales: Mucoraceae) has been attributed to understanding the chemical and environmental cues that trigger yeast-mycelium transition (Ruiz-Herrera 1985).
B. bassiana is one of the most widely used mycopesticides for control of a broad spectrum of arthropod pests (Faria and Wraight 2007). The mass production of Beauveria species for use as a biorational pesticide relies primarily on solid-substrate fermentation to yield aerial conidia. However, solid-substrate fermentation is generally very labor intensive, has a high risk of contamination, and is difficult and costly to practice on a large scale (Jackson 1997; Jackson et al. 2010). Studies conducted with Isaria fumosorosea (Wise) Brown & Smith (formerly Paecilomyces fumosoroseus; Hypocreales: Cordycipitaceae) and B. bassiana have shown that nutrient-rich media supported the production of yeast-like blastospores (Bidochka et al. 1987; Jackson 2012; Jackson et al. 2003; Mascarin et al. 2015). Despite the large number of studies concerning the liquid culture growth of entomopathogenic fungi, cultures of B. bassiana have been reported to produce less than 1 × 109 blastospores mL−1 in >3 days fermentation with these blastospores generally not surviving drying and lacking storage stability (Chong-Rodriguez et al. 2011; Humphreys et al. 1989; Lane et al. 1991; Lohse et al. 2014; Pham et al. 2009; Samsinakova 1966; Vega et al. 2003). The commercial development of a production process for blastospores of B. bassiana requires a rapid, low-cost liquid culture production method that yields high concentrations of stable, effective blastospores.
Identifying nutritional and environmental factors that improve blastospore yields during liquid culture growth is a prerequisite for developing a cost-effective mass production processes. Nutrition, osmolarity, pH, inoculum size, and oxygen availability have been shown to be crucial parameters for the optimization of industrial fermentation processes using filamentous fungi by affecting their metabolism, dimorphic growth, and fitness (Duran et al. 2011; Li et al. 2011). Elevated levels of dissolved oxygen along with appropriate nutritional conditions during the growth of submerged cultures of I. fumosorosea and Metarhizium flavoviride Gams & Rozsypal sensu lato (Hypocreales: Clavicipitaceae) have proven to be critical for enhanced blastospore production (Issaly et al. 2005; Jackson 2012).
Previous studies with B. bassiana cultures showed that blastospore production was inversely correlated to the water activity of the liquid media with blastospore yields remaining very low and thus making the process not suitable for large-scale production (Humphreys et al. 1989; Inch and Trinci 1987; Ypsilos and Magan 2004). Recent studies have shown that high concentrations (1 × 109 blastospores mL−1) of stable, effective blastospores of B. bassiana can be rapidly produced in a short 2–3-day liquid culture fermentation (Mascarin et al. 2015). Within this context, the aim of this study was to improve the yield and fitness of blastospores of two isolates of B. bassiana, ESALQ 1432 and GHA, by evaluating the impact of dissolved oxygen coupled with high glucose concentrations on the growth rate, yield, stability, and insecticidal efficacy of blastospores produced using liquid culture fermentation.
Materials and methods
Ethics statement
The research did not involve manipulations of humans or endangered or protected species and were conducted under laboratory conditions. Therefore, no specific permissions were required for our work. The fungal isolates used for this study were obtained from culture collections located in Brazil and the USA. The insect colony was reared in a greenhouse under APHIS permit No. P526P-13-02631.
Culture maintenance and media development
A pure culture of B. bassiana strain ESALQ1432 was originally isolated from the citrus psyllid, Diaphorina citri Kuwayama (Hemiptera: Liviidae), in Piracicaba-SP, Brazil and was obtained from the microbial culture collection of the Laboratory of Invertebrate Pathology and Microbial Control, University of São Paulo. The B. bassiana isolate GHA (ARSEF6444) was originally isolated from Diabrotica undecimpunctata Barber (Coleoptera: Chrysomelidae) in Corvallis, Oregon, USA and was obtained from the ARSEF culture collection in Ithaca, NY. The GHA strain of B. bassiana is the active ingredient of the commercial product Mycotrol® (Laverlam International Cop., Butte, MT, USA). These fungal isolates were maintained as stock cultures containing spore suspensions in 10 % glycerol stored at −80 °C.
Frozen stock cultures of each isolate were used to inoculate potato dextrose agar (PDA; Difco®) in Petri dishes (Falcon®, 100 × 15 mm) that were incubated at 22 °C with 12:12 h (L/D) photoperiod for 2–3 weeks. Conidia were harvested from sporulated PDA plates by rinsing with 10 mL aqueous solution of sterile 0.04 % (v/v) Tween 80 (Polysorbate 80, Sigma® Chemical, St. Louis, MO, USA). The conidial suspension was used to inoculate a pre-culture medium to provide a final concentration of 1 × 106 conidia mL−1. Conidial and blastospore concentrations were measured microscopically at ×400 magnification using a Neubauer hemacytometer in a light microscope with Nomarski optics (BH2, Olympus America, Center Valley, PA, USA).
The pre-culture medium was used to produce blastospores for use as inoculum in the blastospore production experiments. The pre-culture medium contained basal salts, vitamins, and trace metals and were supplemented with glucose (Sigma®) 80 g L−1 and acid hydrolyzed casein (Hy-case™ MSF, Kerry Bioscience, New York, NY) at 25 g L−1 (Mascarin et al. 2015). The basal salts, vitamins, and trace metals added to each liter of double-deionized water (ddH2O) were: KH2PO4, 2.0 g; CaCl2·2H2O, 0.4 g; MgSO4·7H2O, 0.3 g; CoCl2·6H2O, 37 mg; FeSO4·7H2O, 50 mg; MnSO4·H2O, 16 mg; ZnSO4·7H2O, 14 mg; thiamin, riboflavin, pantothenate, niacin, pyridoxamine, thioctic acid, 500 mg each; folic acid, biotin, vitamin B12, 50 mg each (Jackson et al. 1997). Concentrated glucose solutions were autoclaved separately. The B. bassiana isolates were grown for 3 days in 100 mL of pre-culture medium using 250-mL baffled Erlenmeyer shake flasks (Bellco Glass, Vineland, NJ, USA) at 28 °C and 350 revolutions per minute (rpm) in a rotary shaker incubator (INNOVA 4000, New Brunswick Scientific, Edison, NJ, USA).
The blastospore production media consisted of the previously described basal salts medium amended with cottonseed flour at 25 g L−1 (Pharmamedia®, Traders Protein, Memphis, TN, USA) as the nitrogen source using various concentrations of glucose. The standard glucose concentration for the blastospore production media was 100 g L−1 glucose unless otherwise stated. All blastospore production media were inoculated with blastospores from 3-day-old pre-cultures to deliver a final blastospore concentration of 5 × 106 blastospores mL−1. The initial pH of the media was 5.5 and was uncontrolled during culture growth. Cultures were evaluated at 48 and 72 h for biomass accumulation and blastospore formation. Flasks were hand shaken, as needed, to reduce fungal growth on the flask walls. Two shake flasks (replicates) per experimental treatment were used for each experiment, and all experiments were independently repeated three times (n = 6).
Dissolved oxygen experiments
To evaluate the influence of dissolved oxygen concentration on culture growth and blastospore yields by B. bassiana isolates ESALQ1432 and GHA, cultures were grown in liquid media volumes of 50 or 100 mL and at agitation speeds of 175 or 350 rpm in modified 250-mL baffled Erlenmeyer flasks. The modified Erlenmeyer flasks were fitted with oxygen sensors (PSt3, PreSens, Regensburg, Germany) that provided continuous monitoring of dissolved oxygen (DO) tension in shake flask cultures by transferring DO data via Bluetooth at 15-min intervals to the SFR v2 system (PreSens), as previously described (Jackson 2012). The oxygen concentration \( {C}_{{\mathrm{O}}_2,\ \mathrm{L}} \) was measured as O2 molecules in the liquid phase, which was expressed as percent dissolved oxygen. The time of fermentation required to reach 50 % DO (DO50) was computed. All B. bassiana cultures were incubated at 28 °C in a rotary shaker incubator.
Glucose concentration experiments
Cultures of B. bassiana ESALQ1432 and GHA were grown in liquid media supplemented with glucose concentrations that varied from 20 to 220 g L−1 (intervals of 20 g L−1). Stock solutions containing 200–250 and 400–600 g glucose L−1 were autoclaved separately and added to the liquid media to achieve specified glucose concentrations. Fifty-milliliter culture volumes were grown in 250-mL baffled Erlenmeyer flasks in a rotary shaker incubator at 28 °C and 350 rpm to provide maximum DO.
The water activity (a w) of these media with differing glucose concentrations was measured using the AquaLab series 4TEV (Decagon Devices, Inc., Pullman, WA, USA) equilibrated at 25 °C. Water activity was used to calculate the expected initial osmotic pressure in the glucose-modified liquid media based on Norrish’s equation (Norrish 1966). Water activity and osmotic pressure are directly related by the following equation, П = −(R*T*ln(a w))/(V w ) where П is the osmotic pressure in megapascals (MPa), R is the perfect gas constant (8.3144720 mL MPa−1 mol−1 K−1), T is the temperature in Kelvin (25 °C = 298.15 K), and V w is the partial molar volume of water (18 mL mol−1).
To determine the effect of glucose concentration on cell size and morphology, we measured the length and width of 50 random blastospores from each treatment flask using an ocular micrometer (WHB10x-H/20, Olympus) at ×400 magnification. The width of both oblong and ovoid cells was consistently unaltered across different treatments and averaged 3.27 ± 0.08 and 3.25 ± 0.12 μm for isolates ESALQ1432 and GHA, respectively. Subsequently, the cell size was indirectly inferred by the volume of an ellipsoid given by: V (μm3) = 4/3*(π*a*b 2), where a is the length and b is the width. When cells assumed ovoid or spherical shape, then length and width were considered equivalent.
Interaction between glucose concentration and agitation rate
To evaluate the effects of glucose concentration and agitation rate on DO levels and blastospore formation, both isolates of B. bassiana were grown in media containing 40 or 200 g glucose L−1 and incubated at 350 or 175 rpm agitation speeds. These glucose concentrations were known to significantly affect blastospore yield as well as the osmotic pressure of the culture medium. Using this full factorial experiment, DO levels were continuously monitored with blastospore yield and biomass accumulation measured after 2 and 3 days growth. The time of fermentation required to reach 50 % DO (DO50) was also recorded.
Analyses of growth parameters
Blastospore yield, biomass accumulation, glucose utilization, and pH were determined in all experiments as described in Mascarin et al. (2015). Biomass accumulation was expressed as dry weight per milliliter by filtering duplicate 1-mL culture samples onto a dried and pre-weighed 2.4-cm glass filter paper (G6, Fisher Scientific, Pittsburgh, PA, USA). The filter paper with biomass was dried at 80 °C until a constant weight was achieved. Blastospore concentrations were enumerated microscopically using a hemacytometer as previously described. Glucose utilization was computed as the difference between initial and final glucose concentrations in each medium measured using a glucose meter (GlucCell®, CESCO, Atlanta, GA, USA).
Harvesting and drying blastospores
After 3 days culture growth, blastospores were harvested from each liquid culture and subjected to dehydration to determine desiccation tolerance. Briefly, the whole culture broth from each flask was mixed with 7.5 % (w/v) diatomaceous earth (DE; HYFLO®, Celite Corp., Lompoc, CA, USA) and dewatered by vacuum-filtration through a 12.5-cm Whatman® No. 1 filter paper (Maidstone, England) in a Buchner funnel. The resultant filter cake (fungal biomass + DE) was broken up by pulsing in a blender (Mini Prep® Plus, Cuisinart, Stamford, CT, USA), layered in a glass Petri dish (9 × 1.5 cm), and gently air-dried overnight in a horizontal air flow chamber with relative humidity controlled at ≈50–60 % (Jackson and Payne 2007). The moisture content and water activity (aw) of filter cake samples were determined with a moisture analyzer (Mark II, Denver Instruments, Arvada, CO, USA) and a water activity meter (AquaLab series 4TEV), respectively. When moisture content reached <3 %, each filter cake sample was vacuum packed in nylon polyethylene bags (15.3 × 21.8 cm, 75 μm) with a vacuum packer (Multivac Inc., Kansas City, MO, USA) and stored at refrigerated conditions (4 °C). Three days after vacuum packaging, a sample ∼0.05 g of each dried filter cake was transferred individually into 25 mL potato dextrose (PD) broth (Difco®) in 125-mL baffled Erlenmeyer flasks (Bellco Glass Inc., USA) and incubated at 28 °C with 300 rpm in a rotary shaker incubator to assess the germination of blastospores. After 7 h incubation, two subsamples per flask were transferred onto slides with coverslips to microscopically assess the germination of ≥200 blastospores. Blastospores were considered viable when a germ tube of any size was visible at ×400 magnification.
Blastospore bioassays against whitefly
The insecticidal efficacy of B. bassiana (strain ESALQ1432) blastospores produced under different glucose concentrations were evaluated against the silverleaf whitefly, Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae) biotype B, also known as Middle East-Asia Minor 1. The insect colony was established with founders originally provided by University of Florida, Apopka, FL. Insects were reared on cabbage cv. ‘Bravo’ (Brassica oleracea L.; Harris Seeds, Rochester, NY, USA) and blue lake bush bean (Phaseolus vulgaris L.; Kelly Seeds Co., Peoria, IL, USA) plants in a greenhouse. Bioassays were performed using the ESALQ1432 isolate because the efficacy of this fungus against whiteflies was tested in previous studies (Mascarin et al. 2013, 2015). Samples used in bioassays were air-dried blastospores harvested from highly aerated liquid cultures (i.e., 50 mL liquid medium and 350 rpm) grown at different initial glucose concentrations (40, 100, and 140 g L−1). Blastospores were assayed against 2nd instar B. tabaci nymphs feeding on the underside of primary leaves of bean seedlings. Uniform-aged nymphs were obtained by placing 14-day-old potted bean plants in a cage containing the insect colony for 24 h to allow oviposition by adults. Potted plants were removed from the colony and held to allow nymphs to develop to the 2nd instar. Infested bean leaves were excised from the plant and placed ventral side up on water agar (2 %, w/v) in individual, ventilated-plastic Petri dishes, as described in Mascarin et al. (2013, 2015). Groups of 60–70 nymphs per leaf were identified by marking them with blue ink. Aqueous suspensions of blastospores at 1.25 × 107, 2.5 × 106, 5 × 105, and 2.5 × 105 blastospores mL−1 were prepared in a 0.01 % (v/v) Tween 80 solution and applied to insect infested leaves to provide treatment dosages of: 2.7 × 104, 5.4 × 103, 1.1 × 103, and 2.2 × 102 blastospores cm−2, respectively. Sprays were applied using a micro-sprayer device set to 10 PSI (=68.95 kPa) using a 3-s application time. Control batch of nymphs was included in each assay and sprayed with 0.01 % Tween 80 solution. Four infested leaves (replicates) were treated with each blastospore concentration. Treated leaves were incubated in a growth chamber set to 27 ± 1 °C, 60–70 % RH and 14:10 h L/D photophase for 6 days prior to assessing nymphal mortality. Nymphs killed by B. bassiana infection were easily identified by a red pigment due to fungal infection or by a shriveled and dehydrated appearance. The entire experiment was repeated twice using different insect cohorts and fungal batches freshly produced.
In another experiment, we compared the speed of kill (median survival time (ST50)) using blastospores from ESALQ1432 also produced in media containing 40, 100, or 140 g glucose L−1. Blastospores from each glucose treatment were tested on four replicate leaves. A single-dosage of 2.7 × 104 blastospores cm−2 was used for each treatment and then applied to infested leaves and monitored daily throughout 6 days incubation for mortality. This single-dosage experiment was conducted three times using different insect cohorts and fungal batches. Viability of blastospores were assessed before spraying and resulted in 70–80 % germination in all cases, after 7 h incubation in PD broth at 28 °C and 300 rpm.
Statistical analyses
All experiments were conducted using a completely randomized design and repeated at least two times on different dates. A linear mixed model was employed to fit experimental data for the response variables: blastospore concentration and biomass dry weight. Blastospore concentration was log-transformed prior to analysis of variance to meet the normality assumptions of the linear mixed model. In the model, the fixed effects consisted of glucose concentration, speed of agitation, culture medium volume, and combinations of these factors, according to the experimental design, while the experimental repetitions and shake flasks (experimental units repeatedly measured over time) were included as random effects. Statistics for fixed effects and their interaction terms were also determined by Wald type III F test. Post-hoc pair-wise multiple comparisons were performed using Tukey’s honestly significant difference (HSD) test at P < 0.05 for fixed effects and their interaction terms, while Student’s t test was used to compare only two groups of means when necessary. These statistical analyses were performed using the procedure MIXED in the Statistical Analysis System v.9.2 (SAS Institute Inc., Cary, NC). To explain the impact of glucose concentration on blastospore yield, empirical data were fitted to a logistic growth model (sigmoidal with four parameters) according to the equation: y = y 0 + (a/(1 + exp(−(x – x 0)/b))), where y is the concentration (blastospore mL−1), x is the glucose concentration (g L−1), and a, b, y 0, and x 0 are constants estimated by the interactive analysis performed with the software SigmaPlot 11.0 (Systat Software Inc., San Jose, CA, USA). Proportion mortality data for whitefly nymphs challenged with strain ESALQ1432 were fitted to a logistic model in PROBIT procedure of SAS to compute the median lethal concentration (LC50) for each production media treatment. Mortalities for fungal treatments were automatically corrected for control mortality in this analysis. The potency ratio test was used to statistically compare the LC50 values at a 5 % probability (Wheeler et al. 2007). Concentration-mortality nonlinear regression curves from different glucose concentrations were statistically compared through the sum-of-square reduction test, in which comparisons of nested models were performed by contrasting their residual sums of squares at P < 0.05 (PROC NLIN in SAS 9.2). ST50 (time in which 50 % of the insect population survives) values for blastospores produced with different glucose concentrations were calculated from survival curves of whitefly nymphs exposed to single-dosage treatments using the Kaplan-Meier method (Bewick et al. 2004). Survival curves were compared by log-rank test at P < 0.05, while ST50 values were considered significantly different based on nonoverlapping of 95 % confidence limits.
Results
Effect of dissolved oxygen on dimorphic growth
Higher aeration (increased DO level) was maintained with lower culture volume (50 mL) or by using a higher agitation speed (350 rpm) when compared with cultures grown in higher volumes (100 mL) or at slower (175 rpm) agitation speed (Table 1). Culture volume and agitation speed affected the dissolved oxygen profile of B. bassiana cultures as well as their growth parameters. Higher aeration rates were achieved by lowering culture volume, increasing agitation speed and by their combination, resulting in extended oxygen availability as noted by the dissolved oxygen curves (Fig. 1). Apart from the B. bassiana isolates, the fermentation time where cultures reached 50 % dissolved oxygen (DO50) were 4.5–7.3 h higher in cultures grown at 50 mL compared with cultures grown at 100 mL, and 6.8–9.6 h in cultures grown at 350 rpm compared with cultures grown at 175 rpm (Table S1 in the Electronic supplementary material; Fig. 1). Furthermore, B. bassiana cultures of both isolates showed similar DO50 values. Dissolved oxygen levels remained low (<10 % oxygen) after 24 h growth and throughout the remaining 3 days of fermentation when B. bassiana cultures were incubated at 350 rpm, suggesting that these conditions promoted maximum oxygen consumption.
Greater oxygen availability increased blastospore yield when isolates of B. bassiana were grown under higher DO levels (350 rpm and 50-mL volume). The main effects represented by agitation speed (ESALQ1432: F 1, 24 = 185.65, P < 0.0001; GHA: F 1, 24 = 75.68, P < 0.0001) and culture volume (ESALQ1432: F 1, 24 = 39.19, P < 0.0001; GHA: F 1, 24 = 32.88, P < 0.0001) supported a significant increase in the blastospore concentrations for both isolates (Table 1). Blastospore production increased over time for the fermentation of ESALQ1432 (F 1, 24 = 5.08, P = 0.034), although no significant change between days 2 and 3 was observed in GHA cultures (F 1, 24 = 0.97, P = 0.334). No significant interaction of agitation speed and culture volume was observed for blastospore yields in cultures of ESALQ1432 (P > 0.05), while this interaction played a significant role in blastospore production by cultures of GHA (F 1, 24 = 33.46, P < 0.0001).
Biomass accumulation was variable depending on the agitation rate and culture volume tested (Table 1). Faster agitation speed (ESALQ1432: F 1, 24 = 146.27, P < 0.0001; GHA: F 1, 24 = 17.08, P < 0.0001) and lower culture volume (ESALQ1432: F 1, 24 = 62.29, P < 0.0001; GHA: F 1, 24 = 13.31, P < 0.0001) together significantly increased biomass accumulation by both isolates. The lowest blastospore yields and biomass accumulations occurred in oxygen-limited grown cultures (i.e., 175 rpm and 100 mL) (Table 1). Isolate ESALQ1432 was more responsive than GHA to oxygen availability showing greater biomass accumulation when cultivated in oxygen-rich media. During early growth times (<24 h), the oxygen level was saturated (100 %). Thereafter, DO levels declined as biomass increased after 24 h fermentation. Although GHA cultures apparently consumed more glucose than ESALQ1432 cultures (F 1, 24 = 17.07, P = 0.0004), it was noticed that glucose consumption was significantly greater for both isolates grown with higher oxygen availability as in lower culture volume (F 1, 24 = 9.21, P = 0.006) and at faster agitation for GHA cultures only (F 1, 24 = 8.86, P = 0.007) (Table 1). The final pH in the culture broth was characterized as moderately acidic for both strains with a range of 4.5–5.1. The highest speed of agitation (350 rpm) combined with the lowest culture volume (50 mL) resulted in the least viscous culture of B. bassiana compared with poorly aerated cultures. The faster DO levels decreased the more viscous cultures became accompanied by fewer blastospores and increased mycelial growth.
Effect of glucose concentration on blastospore yields
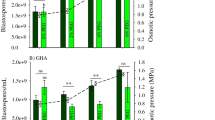
The impact of increased initial glucose concentration on liquid cultures revealed a progressive, concentration-dependent enhancement of blastospore density over time (Fig. 2). Water availability in liquid media was reduced as osmotic pressure increased, which was attributed to an increase in the glucose concentration (Fig. S1 in the Electronic supplementary material; Fig. 2). Initial glucose concentrations greater than or equal to 140 g glucose L−1 for cultures of ESALQ1432 and equal to 200 g glucose L−1 for cultures of GHA boosted blastospore yields up to 2.93 × 109 and 2.94 × 109 cells mL−1, respectively. The relationship between blastospore production and glucose concentration was significantly explained by a sigmoidal growth model (R 2 ≥ 0.85, P < 0.001) for days 2 and 3 of fermentation, with higher blastospore densities being achieved with 3 days of fermentation (Table S2 in the Electronic supplementary material; Fig. 2).
Effect of initial glucose concentration on osmotic pressure and on blastospore production by Beauveria bassiana isolates ESALQ1432 (a) and GHA (b) cultured in 50 mL media volume at 28 °C and 350 rpm. Solid lines (blue and green) represent the fitted exponential growth model (Table S3 in the Electronic supplementary material), while circles designate averages (±SE) of observed values
Total fungal biomass dry weight increased as initial glucose concentration raised up to 60 g glucose L−1 for cultures of GHA and up to 180 g glucose L−1 for cultures of ESALQ1432 (F 9, 117 = 12.78, P < 0.0001). The initial glucose availability in liquid media affected glucose consumption patterns, which in turn resulted in differing biomass accumulation by each isolate of B. bassiana (Fig. 3). In general, GHA cultures consumed more glucose while accumulating less biomass as compared with cultures of ESALQ1432. For instance, GHA utilized 50–60 g glucose L−1 compared with ESALQ1432 using 30–50 g glucose L−1 when cultured for 3 days in media amended with ≥60 g glucose L−1.
Initial glucose concentrations did not affect the desiccation tolerance of blastospores produced by cultures of ESALQ1432 (F 9, 48 = 2.09, P = 0.05) with survival rates that ranged from 64.2 % (±0.6) to 73.3 % (±1.0). Conversely, air-dried GHA blastospores from cultures in media with either 60 or 80 g glucose L−1 were significantly less desiccation tolerant as compared with blastospores grown in media containing 140–200 g glucose L−1 (F 10, 59 = 4.34, P = 0.0001) (Table 2). Air-dried blastospores from cultures of ESALQ1432 exhibited better desiccation tolerance when compared with blastospores of GHA (F 1, 113 = 8.85, P = 0.0036), regardless of the glucose concentration. The a w of air-dried blastospores for both isolates of B. bassiana fell within the range of 0.122–0.246 corresponding to a moisture content of 1.70–2.54 %. Reduction in blastospore volume of B. bassiana isolates occurred in a glucose concentration-dependent manner. Blastospores of both isolates of B. bassiana produced in media with glucose concentrations greater than 140 g L−1 were as much as 53 % smaller volume-wise and with conspicuous round or ovoid shape in comparison to blastospores produced in media with glucose concentrations of 40–100 g L−1, which in turn were longer cells and oblong in shape (ESALQ1432: F 4, 342 = 42.66, P < 0.0001; GHA: F 4, 415 = 68.11, P < 0.0001) (Fig. 4).
Effect of initial glucose concentration in culture media on blastospore size (a) and morphology (b, c) for cultures of Beauveria bassiana isolates ESALQ1432 and GHA after 3 days growth. Photomicrographs of blastospores decreasing in size as the glucose concentration increased: a 40, b 80, c 140, and d 200 g L−1. Scale bar = 20 μm. Means (±SE) followed by noncorresponding letters, within each fungal strain, are significantly different (Tukey’s test, P < 0.05)
Influence of glucose concentration and aeration on dimorphic growth
Agitation speed and glucose concentration both affected the dissolved oxygen profile in cultures of B. bassiana grown in baffled shake flasks. Regardless of the fungal isolate, cultures with an initial glucose concentration of 200 g L−1 (osmotic pressure, 2.82 MPa) and agitated at 350 rpm maintained dissolved oxygen level above 50 % (DO50) for 12.9–19.5 h longer when compared with cultures grown in media with a lower glucose concentration of 40 g L−1 (0.13 MPa) and at slower agitation rate (175 rpm) (Table S3 in the Electronic supplementary material; Fig. 5). Greater DO50 values were attributed to increased agitation speed followed by increased glucose concentration (Table S3 in the Electronic supplementary material). Increasing agitation speed by itself enhanced dissolved oxygen levels as well as blastospore production by day 3, irrespective of the fungal isolate (F 1, 76 = 94.94, P < 0.0001) (Table 3). Interestingly, cultures in media with higher glucose concentrations also maintained higher DO levels and supported better yeast-like growth even under low agitation rates for both isolates (F 1, 76 = 437.15, P < 0.0001). There was a positive interaction between glucose concentration and agitation speed on blastospore yields (F 1, 76 = 7.58, P = 0.0074), which resulted in significantly higher cell densities from highly aerated and high glucose-amended cultures. Furthermore, it was also noticeable that more blastospores were produced after 3 days growth when compared with 2-day-old cultures (F 1, 76 = 84.29, P < 0.0001). Significantly greater biomass accumulation was attributed to higher agitation rates (F 1, 78 = 39.58, P < 0.0001), whereas glucose concentration alone did not significantly affect biomass accumulation (F 1, 78 = 0.76, P = 0.3849) (Table 3). The final pH in the culture broth was moderately acidic (4.86 ± 0.2 for ESALQ1432 and 5.22 ± 0.14 for GHA) and was similar for all cultures (data not shown).
Effect of initial glucose concentrations (40 and 200 g L−1) and agitation rates (350 and 175 rpm) on dissolved oxygen profiles for cultures of Beauveria bassiana isolates ESALQ1432 (a) and GHA (b) grown in 50 mL liquid media using 250-mL baffled Erlenmeyer flasks incubated at 28 °C in a rotary shaker incubator
Effect of glucose concentration on blastospore virulence against whiteflies
Bioassays performed against second-instar whiteflies revealed that ESALQ1432 blastospores produced in media with higher glucose concentrations (140 g L−1) resulted in higher virulence when compared with blastospores produced in media with 40 g glucose L−1 (Table 4). The ratio test comparing LC50 values revealed that blastospores harvested from media supplemented with 140 g glucose L−1 exhibited twofold-improved insecticidal activity compared with blastospores produced in media with 40 g glucose L−1. Virulence (LC50) of blastospores produced in media containing 100 g glucose L−1 was intermediate. Consistent with LC50 results, the concentration-mortality response curve for blastospores was significantly different when produced in media with 140 g glucose L−1 as compared with 40 g glucose L−1 (F (3, 77) = 3.09, P = 0.032) suggesting a higher insecticidal performance for blastospores produced in medium containing higher concentrations of glucose (Fig. 6). The proportion of whiteflies surviving all blastospore treatments was significantly lower when compared with the control group (χ 2 (3) = 990.59, P < 0.0001). Moreover, the survival analysis suggested a faster insecticidal activity for blastospores produced in medium with 140 g glucose L−1 in relation to blastospores produced in media with either 40 g glucose L−1 (χ 2 (1) = 16.16, P < 0.0001) or 100 g glucose L−1 (χ 2 (1) = 5.99, P = 0.0143); however, there was no significant difference in survival rates for whiteflies treated with blastospores produced in media containing 100 or 40 g glucose L−1 (χ 2 (1) = 2.64, P = 0.104) (Fig. 6). Based on the survival time (ST50), blastospores produced in media with a high glucose concentration (100 and 140 g glucose L−1) incited 50 % infection in whitefly nymphs one day earlier (25 % faster) than blastospores produced in media containing 40 g glucose L−1 (Table 4). Concentrations of 2 × 104 blastospores cm−2 (eq. 1.25 × 107 blastospores mL−1) inflicted >95 % mortality in 2nd-instar nymphs by day 6 post-application, regardless of the glucose concentration used for blastospore production.
Discussion
The present investigation focused on determining the impact of glucose concentration and oxygen availability on blastospore formation in liquid culture and on the post-production aspects of these blastospores including desiccation tolerance and biocontrol efficacy against B. tabaci nymphs. We have shown for the first time that the combination of high glucose concentration along with increased dissolved oxygen availability enhanced yeast-like growth resulting in the rapid production of high blastospore concentrations by submerged cultures of B. bassiana. Despite some variations in the response between GHA and ESALQ1432 cultures, oxygen availability and high glucose concentrations consistently improved blastospore yields for both isolates.
Previous liquid culture studies on blastospore production by B. bassiana were generally conducted using lower glucose concentrations (≤50 g L−1) and slower agitation speeds (≤300 rpm) resulting in oxygen- and carbon-limited growing conditions. Under these culture conditions, blastospore yields were lower, fermentation times were longer (≥5 days), and the resultant blastospores were less desiccation tolerant with poor storage stability (Chong-Rodriguez et al. 2011; Humphreys et al. 1989; Lane et al. 1991; Inch and Trinci 1987; Kleespies and Zimmermann 1992; Lohse et al. 2014; Pham et al. 2009; Vidal et al. 1998; Vega et al. 2003; Ypsilos and Magan 2004).
The ability to continuously monitor dissolved oxygen levels in our shake flask cultures demonstrated the critical requirement for low culture volume (50 mL) and high shaking frequency (350 rpm) to ensure oxygen availability which increased rates of culture growth and blastospore yields (Table 1; Fig. 1). Oxygen availability is dependent on the oxygen transfer into the liquid, which is increased through the use of baffled flasks and high agitation speeds. More interestingly, we have shown the unprecedented evidence for high glucose concentrations sustaining higher dissolved oxygen levels in the culture broth of a filamentous entomopathogenic fungus (Table 3; Fig. 5). Corroborating with our findings, a previous study has demonstrated that high viscosity media tend to adhere to the flask wall, thus increasing oxygen transfer by increasing the surface area of the medium (Giese et al. 2013). We also noticed that high glucose concentrations with high aeration rates led to a less filamentous growth accompanied by lower medium viscosity and higher blastospore yields than glucose/oxygen-limited cultures (viscosity data not shown). Previous studies have linked yeast-like rather than hyphal growth to improved oxygen transfer capacity in fungal cultures (Issaly et al. 2005; Jackson 2012; Peter et al. 2004). Our results demonstrated that enhanced dissolved oxygen levels induced by high glucose concentrations facilitate the rapid production of blastospores by cultures of B. bassiana.
The high concentrations of glucose (140–200 g glucose L−1) in liquid cultures of B. bassiana not only enhanced blastospore production but also reduced the size of the blastospores and changed their shape from oblong to ovoid (Fig. 4). The hyperosmotic environment in the high glucose media (Fig. 2) may be affecting the shape of these blastospores. Previous studies have shown that hyperosmotic conditions decreased the cell volume of true yeasts, and such phenomenon is considered an osmoadaptation attributed to the concentration of solutes in the cytoplasm (Babazadeh et al. 2013; Hohmann 2002). Also, it is worth noting that the insect hemolymph is a solute-rich environment characterized by a high osmotic pressure (0.7 to 1.2 MPa) that supports yeast-like growth by B. bassiana as well as other insect fungal pathogens (Chapman 2013; Wang et al. 2008). Studies are underway to better understand the possible role of osmotic pressure in inducing yeast-like growth and in the formation of blastospores in liquid cultures of B. bassiana.
Media with 140 g glucose L−1 not only rapidly produced high yields of smaller blastospores but also produced blastospores that were more effective in killing the silverleaf whitefly when compared with blastospores produced in more conventional media containing 40 g glucose L−1 (Table 4; Fig. 6). Although not proven here that GHA and other isolates of B. bassiana could possibly display enhanced insecticidal activity when produced with high glucose concentration, it is worth noting that this increased virulence observed was specific to ESALQ1432. Previous studies have shown that blastospores of B. bassiana are significantly more efficacious in infecting and killing the silverleaf whitefly when compared with conidia, yet the latter propagule continues to be widely used for commercial mycoinsecticide products (Mascarin et al. 2015). The improved biocontrol efficacy of blastospores over conidia is presumed to result from their ability to germinate more rapidly than conidia. The small ovoid blastospores produced in the glucose rich medium germinated in less than 10 h. The enhanced biological performance we have observed for blastospores grown in a high glucose medium, coupled with high yields of desiccation tolerant blastospores, supports the commercial potential of this liquid culture production system for blastospores of B. bassiana. Nevertheless, shelf life of fungal propagules is considered a critical component for the development of a mycopesticide prior to adopting a production method. Therefore, studies on long-term viability of blastospores under nonrefrigerated conditions warrant further investigation to give support to our liquid fermentation technology.
This study highlights the importance of using high glucose concentrations coupled with high aeration rates for rapid and improved mass production of efficacious, desiccation tolerant blastospores of B. bassiana by liquid culture fermentation. The use of low-cost carbon and nitrogen substrates such as glucose and cottonseed flour further enhances the commercial potential of this production process. An improvement in our basic understanding of how environmental and nutritional factors such as glucose concentration, osmotic pressure, and dissolved oxygen levels affect dimorphic growth in B. bassiana and other filamentous entomopathogenic fungi continues to be the focus of our current research and will guide the development of optimized production methods for these biological control agents for use in agriculture.
References
Babazadeh R, Adiels CB, Smedh M, Petelenz-Kurdziel E, Goksör M, Hohmann S (2013) Osmostress-induced cell volume loss delays yeast Hog1 signaling by limiting diffusion processes and by Hog1-specific effects. PLoS ONE 8(11), e80901. doi:10.1371/journal.pone.0080901
Bewick V, Cheek L, Ball J (2004) Statistics review 12: survival analysis. Crit Care 8:389–394. doi:10.1186/cc2955
Bidochka MJ, Pfeifer TA, Khachatourians GG (1987) Development of the entomopathogenic fungus Beauveria bassiana in liquid cultures. Mycopathologia 4:77–83. doi:10.1007/BF00436909
Chapman RF (2013) The insects: structure and function, 5th edn. Cambridge University Press, Cambridge
Chong-Rodriguez MJ, Maldonado-Blanco MG, Hernandez-Escareno JJ, Galan-Wong LJ, Sandoval-Coronado CF (2011) Study of Beauveria bassiana growth, blastospore yield, desiccation-tolerance, viability and toxic activity using different liquid media. Afr J Biotechnol 10:5736–5742. doi:10.5897/AJB10.2264
Duran R, Cary JW, Calvo AM (2011) Role of the osmotic stress regulatory pathway in morphogenesis and secondary metabolism in filamentous fungi. Toxins 2:367–381. doi:10.3390/toxins2040367
Faria MR, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43:237–256. doi:10.1016/j.biocontrol.2007.08.001
Feng MG, Poprawski TJ, Khachatourians GG (1994) Production, formulation and application of the entomopathogenic fungus Beauveria bassiana for insect control: current status. Biocontrol Sci Techn 4:3–34. doi:10.1080/09583159409355309
Giese H, Azizan A, Kümmel A, Liao A, Peter CP, Fonseca JA, Hermann R, Duarte TM, Büches J (2013) Liquid films on shake flask walls explain increasing maximum oxygen transfer capacities with elevating viscosity. Biotechnol Bioeng 111:295–308. doi:10.1002/bit.25015
Hohmann S (2002) Osmotic stress signaling and osmoadaptation in yeasts. Microbiol Mol Biol R 66:300–372. doi:10.1128/MMBR.66.2.300-372.2002
Humber RA (2008) Evolution of entomopathogenicity in fungi. J Invertebr Pathol 98:262–266. doi:10.1016/j.jip.2008.02.017
Humphreys AM, Matewele P, Trinci APJ (1989) Effects of water activity on morphology, growth and blastospore production of Metarhizium anisopliae, Beauveria bassiana and Paecilomyces farinosus in batch and fed-batch culture. Mycol Res 92:257–264. doi:10.1080/09583150400016910
Inch JM, Trinci APJ (1987) Effects of water activity on growth and sporulation of Paecilomyces farinosus in liquid and solid media. J Gen Microbiol 113:247–252. doi:10.1099/00221287-133-2-247
Issaly N, Chauveau H, Aglevor F, Fargues J, Durand A (2005) Influence of nutrient, pH and dissolved oxygen on the production of Metarhizium flavoviridae Mf189 blastospores in submerged batch culture. Process Biochem 40:1425–1431. doi:10.1016/j.procbio.2004.06.029
Jackson MA (1997) Optimizing nutritional conditions for the liquid culture production of effective fungal biological control agents. J Ind Microbiol Biotechnol 19:180–187. doi:10.1038/sj.jim.2900426
Jackson MA (2012) Dissolved oxygen levels affect dimorphic growth by the entomopathogenic fungus Isaria fumosorosea. Biocontrol Sci Technol 22:67–79. doi:10.1080/09583157.2011.642339
Jackson MA, Payne AR (2007) Evaluation of the desiccation tolerance of blastospores of Paecilomyces fumosoroseus (Deuteromycotina: Hyphomycetes) using a lab-scale, air-drying chamber with controlled relative humidity. Biocontrol Sci Technol 17:709–719. doi:10.1080/09583150701527235
Jackson MA, McGuire MR, Lacey LA, Wraight SP (1997) Liquid culture production of desiccation tolerant blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. Mycol Res 101:35–41. doi:10.1017/S0953756296002067
Jackson MA, Cliquet S, Iten LB (2003) Media and fermentation process for the rapid production of high concentrations of stable blastospores of the bioinsecticidal fungus Paecilomyces fumosoroseus. Biocontrol Sci Technol 13:23–33. doi:10.1080/0958315021000054368
Jackson MA, Dunlap CA, Jaronski ST (2010) Ecological considerations in producing and formulating fungal entomopathogens for use in insect biocontrol. BioControl 55:129–145. doi:10.1007/s10526-009-9240-y
Kleespies RG, Zimmermann G (1992) Production of blastospores by three strains of Metarhizium anisopliae (Metch.) Sorokin in submerged culture. Biocontrol Sci Technol 2:127–135. doi:10.1080/09583159209355226
Lane BL, Trinci PJ, Gillespie AT (1991) Endogenous reserves and survival of blastospores of Beauveria bassiana harvested from carbon- and nitrogen-limited batch cultures. Mycol Res 95:821–828. doi:10.1016/S0953-7562(09)80045-2
Li Q, Bai Z, O’Donnell A, Harvey LM, Hoskisson PA, McNeil (2011) Oxidative stress in fungal fermentation processes: the roles of alternative respiration. Biotechnol Lett 33:457–467. doi:10.1007/s10529-010-0471-x
Lohse R, Jakobs-Schönwandt D, Patel AV (2014) Screening of liquid media and fermentation of an endophytic Beauveria bassiana strain in a bioreactor. AMB Express 4:47. doi:10.1186/s13568-014-0047-6
Mascarin GM, Kobori NN, Quintela ED, Delalibera I Jr (2013) The virulence of entomopathogenic fungi against Bemisia tabaci biotype B (Hemiptera: Aleyrodidae) and their conidial production using solid substrate fermentation. Biol Control 66:209–218. doi:10.1016/j.biocontrol.2013.05.001
Mascarin GM, Jackson MA, Kobori NN, Behle RW, Delalibera I Jr (2015) Liquid culture fermentation for rapid production of desiccation tolerant blastospores of Beauveria bassiana and Isaria fumosorosea strains. J Invertebr Pathol 127:11–20. doi:10.1016/j.jip.2014.12.001
Norrish RS (1966) An equation for the activity and equilibrium relative humidities of water in confectionary syrups. J Food Technol 1:476–484. doi:10.1111/j.1365-2621.1966.tb01027.x
Peter CP, Lotter S, Maier U, Büchs J (2004) Impact of out-of-phase conditions on screening results in shaking flask experiments. Biochem Eng J 17:205–215. doi:10.1016/S1369-703X(03)00179-7
Pham TA, Kim JJ, Kim K (2009) Production of blastospore of entomopathogenic Beauveria bassiana in a submerged batch culture. Mycobiology 37:218–224. doi:10.4489/MYCO.2009.37.3.218
Ruiz-Herrera J (1985) Dimorphism in Mucor species with emphasis on M. rouxii and M. bacilliformis. In: Szaniszlo PJ, Harris JL (eds) Fungal dimorphism with emphasis on fungi pathogenic for humans. Springer, New York, USA, pp 361–384
Samsinakova A (1966) Growth and sporulation of submerged cultures of the fungus Beauveria bassiana in various media. J Invertebr Pathol 8:395–400. doi:10.1016/0022-2011(66)90056-5
Vega FE, Jackson MA, Mercadier M, Poprawski TJ (2003) The impact of nutrition on spore yields for various fungal entomopathogens in liquid culture. World J Microbiol Biotechnol 19:363–368. doi:10.1023/A:1023924304456
Vidal C, Fargues J, Lacey LA, Jackson MA (1998) Effect of various liquid culture media on morphology, growth, propagule production, and pathogenic activity to Bemisia argentifolii of the entomopathogenic hyphomycete, Paecilomyces fumosoroseus. Mycopathologia 143:33–46. doi:10.1023/A:1006962808712
Wanchoo A, Lewis MW, Keyhani NO (2009) Lactin-mapping reveals stage-specific display of surface carbohydrates in in vitro and hemolymph-derived cells of the entomopathogenic fungus Beauveria bassiana. Microbiology 155:3121–3133. doi:10.1099/mic.0.029157-0
Wang C, Duan Z, St Leger RJ (2008) MOS1 osmosensor of Metarhizium anisopliae is required for adaptation to insect host hemolymph. Eukaryot Cell 7:302–309. doi:10.1128/EC.00310-07
Wang J, Liu J, Hu Y, Ying SH, Feng MG (2013) Cytokinesis-required Cdc14 is a signaling hub of asexual development and multi-stress tolerance in Beauveria bassiana. Sci Rep 3:3086. doi:10.1038/srep03086
Wheeler MW, Fadel J, Robertson JK, Bailer J (2007) Confidence interval construction for relative toxicity endpoints such as LD50 or LD90 ratios. J Econ Entomol 100:1945–1949. doi:10.1603/0022-0493(2007)100[1945:CICFRT]2.0.CO;2
Ypsilos IK, Magan N (2004) Impact of water-stress and washing treatments on production, synthesis and retention of endogenous sugar alcohols and germinability of Metarhizium anisopliae blastospores. Mycol Res 108:1337–1345. doi:10.1017/S0953756204001030
Acknowledgments
We thank Brazilian Agricultural Research Corporation (EMBRAPA) for the scholarship to the first author. Special thanks to Dr. Steven P. Arthurs for providing the whitefly founders. We express our sincere gratitude to two anonymous reviewers for their insightful comments on an earlier draft of this article.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding authors
Additional information
“Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by EMBRAPA or the U.S. Department of Agriculture. USDA is an equal opportunity provider and Employer”
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 156 kb)
Rights and permissions
About this article
Cite this article
Mascarin, G.M., Jackson, M.A., Kobori, N.N. et al. Glucose concentration alters dissolved oxygen levels in liquid cultures of Beauveria bassiana and affects formation and bioefficacy of blastospores. Appl Microbiol Biotechnol 99, 6653–6665 (2015). https://doi.org/10.1007/s00253-015-6620-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6620-3