Abstract
The objective of this study is to improve the viability after freeze-drying and during storage of delicate or recalcitrant strains safeguarded at biological resource centers. To achieve this objective, a joint experimental strategy was established among the different involved partner collections of the EMbaRC project (www.embarc.eu). Five bacterial strains considered as recalcitrant to freeze-drying were subjected to a standardized freeze-drying protocol and to seven agreed protocol variants. Viability of these strains was determined before and after freeze-drying (within 1 week, after 6 and 12 months, and after accelerated storage) for each of the protocols. Furthermore, strains were exchanged between partners to perform experiments with different freeze-dryer-dependent parameters. Of all tested variables, choice of the lyoprotectant had the biggest impact on viability after freeze-drying and during storage. For nearly all tested strains, skim milk as lyoprotectant resulted in lowest viability after freeze-drying and storage. On the other hand, best freeze-drying and storage conditions were strain and device dependent. For Aeromonas salmonicida CECT 894T, best survival was obtained when horse serum supplemented with trehalose was used as lyoprotectant, while Aliivibrio fischeri LMG 4414T should be freeze-dried in skim milk supplemented with marine broth in a 1:1 ratio. Freeze-drying Campylobacter fetus CIP 53.96T using skim milk supplemented with trehalose as lyoprotectant resulted in best recovery. Xanthomonas fragariae DSM 3587T expressed high viability after freeze-drying and storage for all tested lyoprotectants and could not be considered as recalcitrant. In contrary, Flavobacterium columnare LMG 10406T did not survive the freeze-drying process under all tested conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For biological resource centers (BRCs) maintaining and providing prokaryotes, freeze-drying or lyophilization is generally preferred over cryopreservation as long-term preservation method. Although cryopreservation of bacterial strains at cryogenic temperatures (<−150 °C) generally results in higher survival compared to freeze-drying (Heylen et al. 2012; Hoefman et al. 2012), a continuous supply of liquid nitrogen or a permanent cooling is mandatory. A properly freeze-dried bacterial strain can be stored for 30 years and even beyond that (personal observations at participating collections), without any high cooling expenses during storage or transport. Moreover, freeze-dried bacterial cells can immediately be used after rehydration without any wash steps, in contrary to cryopreservation. However, freeze-drying is a very complex physical process affected by many parameters and variables such as growth medium, cell concentration, freezing rate, lyoprotectant, reconstitution medium, and time (Carvalho et al. 2003a, b, 2004; Hoefman et al. 2012; Morgan et al. 2006). Therefore, bacteria are generally more sensitive to freeze-drying compared to cryopreservation, where possible damage is mainly related to freezing and thawing injuries (Prakash et al. 2013). Long-term experience with freeze-drying of a wide variety of strains within BRCs reveals that certain bacterial species are vulnerable to freeze-drying and are often not viable after rehydration. As BRCs are reference institutions for microbial diversity, fundamental aspects of freeze-drying should be well documented and experience should be shared. Despite there are many studies on freeze-drying of bacteria, the focus lies mainly on the optimization of industrially important micro-organisms such as probiotic strains, which are not considered recalcitrant to freeze-drying at BRCs (Broadbent and Lin 1999; Carvalho et al. 2002, 2004; Conrad et al. 2000; de Valdez et al. 1985a, b; Miao et al. 2008; Ming et al. 2009; Pehkonen et al. 2008; Saarela et al. 2005; Schoug et al. 2006; Shao et al. 2014; Tymczyszyn et al. 2007; Zavaglia et al. 2003; Zhao and Zhang 2005). Currently, the amount of bacterial strains recalcitrant to freeze-drying at BRCs is not well mapped. The few reported bacterial strains recalcitrant to freeze-drying are often associated with slow growth or the need of microaerophilic or anaerobic growth conditions and include species from the genera Campylobacter (Malik and Lang 1996; Portner et al. 2007) and Helicobacter (Spengler et al. 1992). Motile genera, producing peritrichous flagella such as Vibrio and Aeromonas show as well low survival rates after freeze-drying (Miyamoto-Shinohara et al. 2008). However, a single freeze-drying protocol is used in most studies excluding the effect of different freeze-dryer-dependent parameters such as drying temperature and drying pressure on survival of bacteria. The main aim of this study is to develop an optimal freeze-drying protocol for five selected bacterial strains considered recalcitrant to freeze-drying at participant BRCs, in order to enhance their viability after freeze-drying as well as during storage. Variables during growth and variations in lyoprotectant are considered as well as freeze-dryer-dependent parameters. Bacterial strains used in this study belong on one hand to genera reported to be recalcitrant to freeze-drying, such as Campylobacter, Aeromonas, and Vibrio. On the other hand, due to few reported studies, a preliminary aim of this work is to map bacterial strains recalcitrant to freeze-drying among participant BRCs.
Materials and methods
Biological material
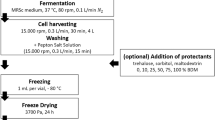
A first objective of this study is to map all bacterial strains considered as recalcitrant to freeze-drying among partner culture collections. Therefore, strains were listed based on consideration of being recalcitrant by three or more culture collections. The amount of recalcitrant species and strains per reported genus is represented by Fig. 1a. Because of limitations in time and funding, five genera were chosen from different physiological origins. The distribution of species of these genera is shown in Fig. 1b. Out of these mapped species, five representative type strains were chosen. Bacterial strains used in this study, their optimal growth media, and growth time as well as their physiological origin are represented in Table 1. Bacterial cells were harvested from solid agar media with an inoculation loop at three different growth times: optimal growth time (OGT), elongated growth time (OGT + 1/3 × OGT) and shortened growth time (OGT – 1/3 × OGT). For most effective recovery after freeze-drying, start cell concentrations should be at least 1×108 cfu/ml (Morgan et al. 2006; Palmfeldt et al. 2003). Accordingly, after harvesting, cells were weighed on a balance (Mettler-Toledo) and suspended in 1 ml of the respective lyoprotectant (Table 2) to become a mother suspension of 10 mg cells/ml: corresponding to at least 1×108 cfu/ml. Start cell concentrations of each investigated strain prior to freeze-drying are shown in Supplementary Table S1 and S2. Another variable during growth was the application of a cold shock: the mother suspension is placed for 2 h at 7 °C. A cold shock triggers production of small compatible compounds (Broadbent and Lin 1999; Morgan et al. 2006; Shao et al. 2014). In this way, cells were prepared to the upcoming aggressive dehydration.
Mapping of bacterial strains recalcitrant to freeze-drying according to five participant biological resource centers. (a) Amount of strains (light gray bars) and species (dark gray bars) per recalcitrant genus. Asterisk refers to chosen genus for species selection (b) Distribution of species of five chosen recalcitrant genera. The number of recalcitrant strains per species is shown inside the cake part. The chosen species are shown in bold
Product formulation
Four different lyoprotectants were formulated in this study (Table 2): 20 % skim milk (Sigma-Aldrich), 20 % skim milk supplemented with 10 % trehalose (final concentration, Sigma-Aldrich), 20 % skim milk + liquid growth medium (1:1) and horse serum + 10 % trehalose (final concentration, Sigma-Aldrich). Skim milk formulations were autoclaved for 13 min at 115 °C, horse serum + trehalose was filter sterilized. All lyoprotectants were checked for sterility, by plating a few drops on a trypticase soy agar plate, followed by incubation at 28 °C. Viable cells in mother suspensions of 10 mg cells/ml lyoprotectant were counted (see “Viable cell counting”) and expressed as colony-forming units per milliliter (Supplementary Table S1 and S2). The suspension was aliquoted in sterile glass ampules with a filling volume of 200 μl.
Freeze-drying process
During freeze-drying, three main phases can be distinguished: freezing phase, primary drying phase, and secondary drying phase. During the freezing phase, water is converted into ice, usually entrapped in the lyoprotectant. Filled ampules were frozen for 2 h at −80 °C in a freezer and transferred to the freeze-dryer. During primary drying, ice is removed through sublimation by lowering the pressure and adding heat to the product, leaving channels in the dried product, thereby creating a porous structure. During secondary drying, residual unfrozen water entrapped in the glassy matrix of the lyoprotectant is removed by isothermal desorption. After completion of secondary drying, glass ampules were constricted and placed on a manifold freeze-dryer under vacuum. After at least 18 min on the manifold freeze-dryer (Table 3), ampules were gas-tight sealed with a hand torch. Gas tightness of the seal was checked with a high-frequency Tesla coil spark tester (Vander Heyden, Belgium). Storage under vacuum was performed to limit any diffusion and oxidative degeneration of bacterial cells.
In this study, four freeze-dryers with different parameter settings were used to freeze-dry bacterial cell suspensions (see Table 3). A typical freeze-drying plot, with indicated parameters of Table 3 is shown in Fig. 2.
Typical freeze-drying plot at BCCM/LMG with indication of different freeze-drying parameters mentioned in Table 2: 1 freezing shelf temperature; 2 primary drying shelf temperature; 3 primary drying pressure; 4 secondary drying shelf temperature; 5 secondary drying pressure
Viable cell counting
Viable cell concentrations, expressed as colony-forming units per milliliter, were determined before and after freeze-drying, using the plate count method. Serial dilutions were made ranging from 10−1 to 10−8: 200 μl mother suspension was transferred to 1.8 ml of fresh growth medium to obtain a dilution of 10−1. Further dilutions were made by transferring 500 μl of the previous dilution to 4.5 ml growth medium. Of each dilution, 100 μl was transferred and spread to solid growth media in triplicate. Inoculated dishes were incubated according to their optimal growth temperature and time (see Table 1).
Storage and viability assay
Heat-sealed ampules were stored in the dark at 4 °C. After 1 week and 6 and 12 months storage, three ampules of each variable (Table 2) were opened and reconstituted with 200 μl liquid growth medium. The rehydrated cells were allowed to equilibrate for 30 min at room temperature. Viability was determined as described in “Viable cell counting”. Additionally, an accelerated storage test was performed by placing heat-sealed ampules at 37 °C for 14 days, corresponding to a storage time of 20 years at room temperature (Sakane 1997). The change in viability after freeze-drying and storage is expressed as “Survival factor,” according to the following formula:
Process survival (PS) is defined as survival factor within first week of storage. Death rate is defined as the reduction of viable cells during storage and is expressed as
Death rate = LOG10 (cfu/ml 1st week) – LOG10 (cfu/ml base); base = 6 months, 12 months or accelerated storage.
All viability data are mean values of three viability counts.
Residual moisture content determination
Determination of residual moisture content (RMC) was performed with a coulometric Karl-Fischer titrator (831 KF Coulometer, Metrohm) with oven (832 KF Thermoprep, Metrohm). The oven was used at a temperature of 150 °C to evaporate residual water from the product without decomposition. Generated water vapor was transferred to the titration vessel by a dried air flow. The titration cell contained an anolyte solution (Hydranal coulomat A for oven, Sigma-Aldrich). Background titration drifts stayed below 10 μg water/min. Ampules were opened and the freeze-dried cake was transferred to a 6-ml vial (Metrohm). The mass of the cake was weighed using an analytical balance (Mettler-Toledo). Vials were capped with an aluminum-encapsulated rubber septum (Metrohm) and placed in to the oven of the Karl-Fischer system. End-point was determined automatically when titration drift dropped below 10 μg water/min. The RMC is expressed as mass fraction: \( \left[\frac{\mathrm{mass}\ \mathrm{water}}{\mathrm{mass}\ \mathrm{freeze}\hbox{-} \mathrm{dried}\ \mathrm{product}}\right]\kern0.37em \times 100\ \left(\%\mathrm{m}/\mathrm{m}\right) \). Blanks (vials filled with air) were inserted into the oven to measure background moisture content, which was subtracted from sample measurements. The system was calibrated using a water standard (Hydranal water standard KF oven 140−160 °C, Sigma-Aldrich).
Results
For freeze-drying bacterial strains, eight variables were included, which are shown in Table 2. Condition V1 is considered as a reference condition, while V2 till V8 are single variants of V1: conditions V2–V5 are variations during growth (supplementation of growth medium, growth time, cold shock) while V6–V8 are variations of lyoprotectant. Viability results are displayed graphically in Fig. 3. Numeric values are given in Supplementary Table S1.
Aeromonas salmonicida CECT 894T
A. salmonicida was freeze-dried according to LP3. Changing lyoprotectant composition had the biggest effect on process survival and death rate during storage. Reference lyoprotectant skim milk (V1) was not suitable since process survival was low (55 %) and no single growth variation with skim milk as lyoprotectant surpassed the accelerated storage test. However, by adding trehalose (V6), process survival raised to 75 % and death rate after accelerated storage slowed down to 4.22. Best process survival (83 %) was achieved by using horse serum supplemented with trehalose (V8). This condition resulted also in lowest death rate after accelerated storage (2.00) and could be considered as the optimal condition. Shifting up or down the growth time resulted in lower process survival compared to the reference growth condition. When older cells were used (OGT + 1/3, V5), no recovery was seen after 1 week storage. Adding trehalose to the growth medium (V2) resulted in low process survival (24 %) and even no recovery after 6 months storage. Cold shock (V3) had no beneficial impact on process and storage survival compared to the reference condition.
Aliivibrio fischeri LMG 4414T
A. fischeri was freeze-dried according to LP1. Skim milk (V1) yielded lowest process survival (48 %) and a high death rate (3.81), showing no recovery after accelerated storage. However, by adding trehalose to a protein formulation (V6, V8), process survival raised to 73 % for skim milk and 77 % for horse serum, respectively, and death rate after accelerated storage slowed down (1.52 and 1.89, respectively). Adding growth medium to skim milk (V7) resulted in best process survival (81 %) and lowest death rate after accelerated storage (0.83) and was considered as the optimal condition. Cold shock (V3) seemed effective for better survival of the freeze-drying process compared to the reference condition, resulting in 77 % process survival and a death rate of 2.81 after accelerated storage. Altering growth time resulted in higher process survival, resp. 59 and 57 % for OGT − 1/3 (V4) and OGT + 1/3 (V5). Nevertheless, no recovery was observed after the accelerated storage test. Adding trehalose to the growth medium (V2) did not result in better process survival and yielded highest death rate of all conditions (4.10).
Flavobacterium columnare LMG 10406T
F. columnare was freeze-dried according to LP1 and did not survive freeze-drying for all conditions tested, except for the longer incubation time OGT + 1/3 (V5) (no graphical data shown). However, no recovery was seen after accelerated storage. None of the lyoprotectants used seem to be useful. Combinations of longer growth time (V5) with addition of lyoprotectant (conditions V6, V7, and V8) gave no recovery after 1 week and after accelerated storage (data not shown).
Campylobacter fetus CIP 53.96T
C. fetus was freeze-dried according to LP2. Skim milk (V1) is not a good lyoprotectant since resulting process survival was low (54 %) and no single growth variation with skim milk as lyoprotectant passed the accelerated storage test, except the longer incubation time (V5). However, when adding trehalose to skim milk (V6), process survival reached its highest value (84 %) and death rate after accelerated storage decreased to 0.49. Low death rates after accelerated storage were also observed for skim milk + growth medium (V7) and horse serum + trehalose (V8): 2.20 and 1.27, respectively. High process survival (77 %) was also obtained when shortening growth time to 2 days (OGT − 1/3, V4). However, death rate after accelerated storage was high (6.74) resulting in no recovery. On the other hand, extended growth (V5) resulted in an acceptable process survival (69 %) and overall lowest death rate after accelerated storage (0.00). However, absolute recovery after accelerated storage is lower than condition V6. Cold shock (V3) and adding trehalose to growth medium (V2) did not clearly result in any advantage compared to the reference condition.
Xanthomonas fragariae DSM 3587 T
X. fragariae was freeze-dried according to LP4. In contrast to other examined strains, X. fragariae yielded very good process survival values with skim milk as lyoprotectant (V1, V2, and V3). However, death rate after accelerated storage was high compared to other lyoprotectants (V6, V7, V8) used. Changing growth time affected death rate after accelerated storage positively (2.42 for OGT − 1/3 and 1.67 for OGT + 1/3), although a loss of more viable cells during freeze-drying was also observed (96 and 89 % survival) compared to the reference condition. Addition of growth medium to skim milk (V7) resulted in lowest death rate after accelerated storage (1.25), but also impaired loss of viability during freeze-drying (82 %). Skim milk supplemented with trehalose (V6) and horse serum + trehalose (V8), performed very well yielding survival values of 96 and 97 %, respectively, and low death rates after accelerated storage (2.74 and 2.24, respectively). Cold shock (V3) resulted in highest death rate (4.79) and did not seem favorable for preserving X. fragariae.
Residual moisture content
Residual moisture after freeze-drying is mainly dependent on freeze-drying parameters (drying temperature, pressure, and time) of the secondary drying. Apart from these parameters, RMC is also dependent on the type of lyoprotectant used and even the kind of micro-organism. Therefore, for all lyoprotectants and bacterial strains used in this study, RMC was determined. As predicted, the RMC of freeze-dried material varied with the freeze-dried strain as well as lyoprotectant. For skim milk, lowest value is 3.51 % and highest value 5.65 % (Fig. 4, Table 4). Highest averaged RMC is observed when formulations without trehalose are used as lyoprotectant: 4.64 % for skim milk and 4.47 % for skim milk + growth medium. However, when adding trehalose to skim milk, RMC is lowered to 2.19 %. Horse serum + trehalose had also a low average RMC value after freeze-drying (2.53 %). Moreover, variability of RMC values, represented by the standard deviation, are higher for skim milk and skim milk + growth medium (resp. 1.01 and 1.87 %) compared to skim milk + trehalose and horse serum + trehalose (resp. 0.61 and 0.56 %).
Influence of the freeze-drying process on viability after freeze-drying
As freeze-drying consists of a freezing, primary and secondary drying phase, settings of temperature, time, and pressure is operator and device dependent. In this study, four different freeze-dryers were used, each with different process parameters and named as LP1 to LP4 (Table 3). As a consequence, freezing and drying damage to bacterial cells can differ dependent on the used freeze-drying procedure. In order to investigate this dependency, strains were exchanged among partner BRCs (Table 5) to be freeze-dried using the reference condition (V1) and the best condition for each strain as shown in Supplementary Table S1. Due to import restrictions of quarantine organisms, X. fragariae DSM 3587T was not exchanged and instead strain CECT 549T was used with LP3. Results are represented graphically in Fig. 5. Numeric values are shown in Supplementary Table S2.
Survival factor after 1 week storage (= process survival) and after accelerated storage of exchanged strains as function of the reference (V1) condition and the best condition(s) according to Table 5. An additional variable is the freeze-drying process
A. salmonicida CECT 894T
For all three freeze-drying protocols used (LP1, LP3, and LP4) conditions with highest and lowest process survival could be reproduced, although absolute values are different. Lowest death rate could not be reproduced by all freeze-drying protocols since LP4 resulted in overall lowest death rate (1.48) for skim milk (V1). However, highest process survival (92 %) and survival factor after accelerated storage (75 %) is retained with LP1 for horse serum + trehalose (V8).
A. fischeri LMG 4414T
In contrary to LP1, survival factors for conditions V1 and V7 with LP2 are similar. In accordance with LP1, death rate after accelerated storage is lowest for skim milk + growth medium (V7). However, it should be emphasized that process survival and survival factor after accelerated storage for V7 is remarkably lower when LP2 (resp. 68 and 49 %) is used compared to LP1 (resp 81 and 71 %).
F. columnare LMG 10406T
No reproducible process survival values could be obtained by two freeze-drying protocols (LP1 and LP2). Death rate was reproducible since no recovery was seen for three variables used in two freeze-drying protocols after accelerated storage.
C. fetus CIP 53.96T
For all three freeze-drying protocols used (LP1, LP2, and LP3), conditions with highest and lowest process survival could be reproduced except with LP1 where all conditions yielded similar and highest process survival factor values (V1, 94 %; V5, 96 %; V6, 96 %). Lowest death rate could not be reproduced by all three freeze-drying protocols since LP2 resulted in overall lowest death rate (no starvation) for longer incubated cells (OGT + 1/3, V5). However, highest absolute survival (78 %) after accelerated storage is retained with LP2 for skim milk + trehalose (V6).
X. fragariae DSM 3587T, CECT 549T
Best and worst conditions could be reproduced by two tested freeze-drying protocols (LP3 and LP4), but absolute survival factor within first week of storage and after accelerated storage were obviously higher with LP4. Highest process survival and lowest death rate are obtained with LP4, using skim milk (V1, 109 %) and skim milk + growth medium (V7, 1.25), respectively.
Discussion
Bacterial strains recalcitrant to freeze-drying were first subjected to different device-independent variables of the freeze-drying process in order to enhance survival after freeze-drying and during storage. In a second aspect, freeze-dryer-dependent parameters were examined to determine their role in improving and reproducing viability after freeze-drying and during storage.
Altering growth conditions to improve survival after freeze-drying did not seem to be the major factor influencing cell survival. Accordingly, no beneficial effects could be observed when growth time was altered for A. salmonicida (CECT 894T), A. fischeri (LMG 4414T), and X. fragariae (DSM 3587T). Better process survival was seen for C. fetus (CIP 53.96T) for both OGT + 1/3 and OGT − 1/3 and F. columnare (LMG 10406T) for OGT + 1/3. However, this beneficial effect was lost during storage in the dried state. Best survival rates were obtained when cells are grown to the late exponential phase. These results are in accordance with the theory that older cells grown to early stationary phase trigger starvation responses protecting them also during desiccation (Morgan et al. 2006).
Supplementation of growth media with sugars has been shown to be beneficial for survival after freeze-drying for Enterococcus faecalis, Enterococcus durans, and Lactobacillus delbrueckii ssp. bulgaricus (Carvalho et al. 2003a, 2004). Trehalose has been described as a sugar with very good lyoprotective properties towards membranes and proteins in bacteria (Leslie et al. 1995). When cells have the ability to take up trehalose intracellulary, critical intracellular macromolecules (DNA, ribosomes,…) could possibly be protected against freezing and drying injuries from the inside, while the cell wall is protected by the lyoprotectant used in the freeze-drying formulation. Moreover, addition of trehalose to the growth medium provides a better protection against desiccation for Bradyrhizobium japonicum (Streeter 2003). The use of 1 % trehalose in trypticase soy broth for cultivation of methane oxidizing bacteria was shown to be very effective for recovery after freeze-drying (Hoefman et al. 2012). Therefore, bacteria were grown on their optimal growth medium supplemented with trehalose to a final concentration of 10 %. This study shows that for none of the strains, adding trehalose to the growth medium had a beneficial effect on process survival and survival rate during storage. In contrary, death rate increased in all cases. Trehalose could either not be imported intracellularly or was possibly used as carbon source, resulting in a more acidic growth medium and starvation of cells during growth.
Some studies suggest a cold shock to trigger production of small compatible compounds before freeze-drying (Broadbent and Lin 1999; Morgan et al. 2006; Shao et al. 2014). In this way, cells are prepared to the upcoming aggressive dehydration. In our study, cold shock was only effective for A. fischeri (LMG 4414T), resulting in higher survival after freeze-drying and even after accelerated storage compared to the reference condition.
This study clearly demonstrates that process and storage survival are mainly dependent on the lyoprotectant used during freeze-drying. Usage of skim milk without any additives was deleterious for survival during storage for nearly all strains tested in this study, except X. fragariae (DSM 3587T). Particularly, trehalose had a remarkable beneficial effect: a mixture of 10 % trehalose-skim milk raised process and storage survival compared to skim milk alone for all strains included in this study, except F. columnare (LMG 10406T). For C. fetus (CIP 53.96T) and X. fragariae (DSM 3587T), using trehalose-skim milk resulted in best storage survival. These results confirm previous research (Portner et al. 2007). Moreover, using trehalose-horse serum as lyoprotectant resulted in best process and storage survival for A. salmonicida (CECT 894T). The general beneficial effect of trehalose in this study is in accordance with other studies (Conrad et al. 2000; Costa et al. 2000; Miao et al. 2008; Ming et al. 2009; Pehkonen et al. 2008; Portner et al. 2007; Tymczyszyn et al. 2007; Zavaglia et al. 2003; Zayed and Roos 2004).
Besides trehalose, addition of growth medium to skim milk had also an advantageous effect on process and storage survival for all strains except X. fragariae (DSM 3587T). Moreover, this condition resulted in best process and storage survival for A. fischeri (LMG 4414T). This finding is surprising because the high salt concentration in the growth media will crystallize during freezing, possibly damaging the cell wall. The inhibitory effect of sugar mixtures present in the product formulation on crystallization could be an explanation for our results (Morgan et al. 2006).
The residual moisture content (RMC) present in the freeze-dried material strongly affects viability or activity during storage. Several studies showed that for freeze-dried bacterial solutions, a high residual water content negatively affects viability during storage (de Valdez et al. 1985a; Santivarangkna et al. 2011; Zayed and Roos 2004). In our study, RMC is mainly dependent on the used lyoprotectant, particularly on the presence of trehalose. Skim milk and skim milk + growth medium contained highest RMC compared to trehalose formulations, although the bulking agent of the latter can either be skim milk or horse serum. These results show that a small concentration of trehalose can alter the complete physical properties of the lyoprotectant towards water, resulting in less residual water content after freeze-drying. Moreover, RMC values of lyoprotectants containing trehalose show a lower variability among the freeze-dried bacterial strains compared to lyoprotectants without trehalose. The higher RMC for skim milk and skim milk + growth medium could explain the high death rate of all tested bacteria during storage after freeze-drying. The higher variability of RMC values of skim milk and skim milk +growth medium could be explained by the variations in the freeze-drying process parameters between partner collections. For instance, a RMC value of 2.08 % is obtained for A. fischeri (LMG 4414T) when freeze-dried in skim milk + growth medium with LP1, which is far below the average value of 4.47 %. This could explain the low death rate of A. fischeri (LMG 4414T) during storage when skim milk + growth medium is used. However, this hypothesis assumes that trehalose formulations are possibly less sensitive towards fluctuating RMC values caused by variations in the freeze-drying process parameters.
In order to investigate the impact of freeze-dryer-dependent parameters, strains were exchanged among partner collections and subjected to freeze-drying with different freeze-drying settings. In most cases, the reference condition (V1) and best condition (Table 5) could be reproduced, although absolute recovery values differ clearly among different freeze-drying protocols. This indicates that freeze-dryer-dependent parameters have a major influence on the survival after freeze-drying and during storage. More stringently controlled processes like LP1 and LP4 give better results regarding viability after freeze-drying and during storage compared to less controlled processes like LP2 and LP3. A separate primary and secondary drying is favorable for product integrity since collapse events can be prevented. The collapse temperature (Tc) is generally defined as the maximum temperature where dried products can support their own weight during primary drying. As a consequence, the product temperature should stay below Tc during primary drying to avoid collapse. Collapsed products lose their stable amorphous glassy state by viscous flow and are considered to have a higher moisture content, a longer reconstitution time, and a possible loss of functionality (Fonseca et al. 2004). Further research on freeze-drying process parameters should reveal their importance on viability of freeze-dried strains after reconstitution.
Considering all tested freeze-drying protocols and variables, best recovery after freeze-drying for the bacteria A. salmonicida (CECT 894T) and A. fischeri (LMG 4414T) was obtained when LP1 was used with horse serum + trehalose and skim milk + growth medium as respective lyoprotective agents. C. fetus (CIP 53.96T) should be freeze-dried according to LP2 with skim milk + trehalose as lyoprotective agent. For X. fragariae (DSM 3587T), skim milk + growth medium seems to be an acceptable lyoprotectant when LP4 is used as freeze-drying protocol. F. columnare (LMG 10406T) did not survive the freeze-drying process for all tested conditions and protocols. This result is contradictional to previously published work (Desolme and Bernardet 1996), where survival was observed one week after freeze-drying when horse serum + Brucella broth was used as lyoprotectant. Further effort should find a way to properly freeze-dry F. columnare, for instance by figuring out what part of the process is most critical for survival of this species (start cell concentration, growth condition, freezing, drying, and reconstitution).
A joint experimental setup between bacterial culture collections resulted in development of successful freeze-drying protocols for four out of five recalcitrant strains. In this study, choice of the lyoprotectant had a major impact on process and storage survival. Moreover, lyoprotectants yielding high recovery rates shortly after freeze-drying are often not suitable for protection during long-term storage in the dried state. Therefore, the lyoprotectant should be chosen carefully. This study clearly shows that optimal freeze-drying conditions are strain specific: a single optimal protocol suitable for all strains in a culture collection seems therefore utopian. On the other hand, for institutions safeguarding thousands of micro-organisms, strain specific protocols are practically and economically impossible. Based on the mapping of recalcitrant strains in this study, the investigated strains are considered as representatives of their genus. Therefore, culture collections could adapt their preservation protocols to certain genera or groups of micro-organisms. Furthermore, BRCs should optimize their freeze-drying protocols to evolve to better controlled processes, resulting in freeze-dried products of reproducible viability during long-term storage.
References
Broadbent JR, Lin C (1999) Effect of heat shock or cold shock treatment on the resistance of Lactococcus lactis to freezing and lyophilization. Cryobiology 39(1):88–102
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P (2002) Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol Lett 24(19):1587–1591
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P (2003a) Effect of various growth media upon survival during storage of freeze-dried Enterococcus faecalis and Enterococcus durans. J Appl Microbiol 94(6):947–952
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P (2003b) Effects of addition of sucrose and salt, and of starvation upon thermotolerance and survival during storage of freeze-dried Lactobacillus delbrueckii ssp bulgaricus. J Food Sci 68(8):2538–2541
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P (2004) Effects of various sugars added to growth and drying media upon thermotolerance and survival throughout storage of freeze-dried Lactobacillus delbrueckii ssp bulgaricus. Biotechnol Prog 20(1):248–254
Conrad PB, Miller DP, Cielenski PR, de Pablo JJ (2000) Stabilization and preservation of Lactobacillus acidophilus in saccharide matrices. Cryobiology 41(1):17–24
Costa E, Usall J, Teixido N, Garcia N, Vinas I (2000) Effect of protective agents, rehydration media and initial cell concentration on viability of Pantoea agglomerans strain CPA-2 subjected to freeze-drying. J Appl Microbiol 89(5):793–800
de Valdez GF, de Giori GS, de Ruiz Holgado AP, Oliver G (1985a) Effect of drying medium on residual moisture content and viability of freeze-dried lactic acid bacteria. Appl Environ Microbiol 49(2):413–415
de Valdez GF, de Giori GS, de Ruiz Holgado AP, Oliver G (1985b) Effect of the rehydration medium on the recovery of freeze-dried lactic acid bacteria. Appl Environ Microbiol 50(5):1339–1341
Desolme B, Bernardet JF (1996) Freeze-drying of Flavobacterium columnare, Flavobacterium psychrophilum and Flexibacter maritimus. Dis Aquat Org 27(1):77–80
Fonseca F, Passot S, Cunin O, Marin M (2004) Collapse temperature of freeze-dried Lactobacillus bulgaricus suspensions and protective media. Biotechnol Prog 20(1):229–238
Heylen K, Hoefman S, Vekeman B, Peiren J, De Vos P (2012) Safeguarding bacterial resources promotes biotechnological innovation. Appl Microbiol Biotechnol 94(3):565–574. doi:10.1007/s00253-011-3797-y
Hoefman S, Van Hoorde K, Boon N, Vandamme P, De Vos P, Heylen K (2012) Survival or revival: Long-term preservation induces a reversible viable but non-culturable state in methane-oxidizing bacteria. Plos One 7(4). doi:10.1371/journal.pone.0034196
Leslie SB, Israeli E, Lighthart B, Crowe JH, Crowe LM (1995) Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl Environ Microbiol 61(10):3592–3597
Malik KA, Lang E (1996) Successful preservation of Campylobacteraceae and related bacteria by liquid-drying under anaerobic conditions. J Microbiol Methods 25(1):37–42
Miao S, Mills S, Stanton C, Fitzgerald GF, Roos Y, Ross RP (2008) Effect of disaccharides on survival during storage of freeze dried probiotics. Dairy Sci Technol 88(1):19–30
Ming LC, Rahim RA, Wan HY, Ariff AB (2009) Formulation of protective agents for improvement of Lactobacillus salivarius I 24 survival rate subjected to freeze drying for production of live cells in powderized form. Food Bioprocess Technol 2(4):431–436
Miyamoto-Shinohara Y, Sukenobe J, Imaizumi T, Nakahara T (2008) Survival of freeze-dried bacteria. J Gen Appl Microbiol 54(1):9–24
Morgan CA, Herman N, White PA, Vesey G (2006) Preservation of micro-organisms by drying; a review. J Microbiol Methods 66(2):183–193
Palmfeldt J, Radstrom P, Hahn-Hagerdal B (2003) Optimisation of initial cell concentration enhances freeze-drying tolerance of Pseudomonas chlororaphis. Cryobiology 47(1):21–29. doi:10.1016/S0011-2240(03)00065-8
Pehkonen KS, Roos YH, Miao S, Ross RP, Stanton C (2008) State transitions and physicochemical aspects of cryoprotection and stabilization in freeze-drying of Lactobacillus rhamnosus GG (LGG). J Appl Microbiol 104(6):1732–1743
Portner DC, Leuschner RGK, Murray BS (2007) Optimising the viability during storage of freeze-dried cell preparations of Campylobacter jejuni. Cryobiology 54(3):265–270
Prakash O, Nimonkar Y, Shouche YS (2013) Practice and prospects of microbial preservation. FEMS Microbiol Lett 339(1):1–9. doi:10.1111/1574-6968.12034
Saarela M, Virkajarvi I, Alakomi HL, Mattila-Sandholm T, Vaari A, Suomalainen T, Matto J (2005) Influence of fermentation time, cryoprotectant and neutralization of cell concentrate on freeze-drying survival, storage stability, and acid and bile exposure of Bifidobacterium animalis ssp. lactis cells produced without milk-based ingredients. J Appl Microbiol 99(6):1330–1339
Sakane T (1997) Viabilities of dried cultures of various bacteria after preservation for over 20 years and their prediction by the accelerated storage test. Microbiol Cult Coll 13(1):1–7
Santivarangkna C, Aschenbrenner M, Kulozik U, Foerst P (2011) Role of glassy state on stabilities of freeze-dried probiotics. J Food Sci 76(8):R152–R156. doi:10.1111/j.1750-3841.2011.02347.x
Schoug A, Olsson J, Carlfors J, Schnurer J, Hakansson S (2006) Freeze-drying of Lactobacillus coryniformis Si3—effects of sucrose concentration, cell density, and freezing rate on cell survival and thermophysical properties. Cryobiology 53(1):119–127
Shao YY, Gao SR, Guo HL, Zhang HP (2014) Influence of culture conditions and preconditioning on survival of Lactobacillus delbrueckii subspecies bulgaricus ND02 during lyophilization. J Dairy Sci 97(3):1270–1280. doi:10.3168/jds. 2013-7536
Spengler A, Gross A, Kaltwasser H (1992) Successful freeze storage and lyophilization for preservation of Helicobacter pylori. J Clin Pathol 45(8):737–737
Streeter JG (2003) Effect of trehalose on survival of Bradyrhizobium japonicum during desiccation. J Appl Microbiol 95(3):484–491. doi:10.1046/j.1365-2672.2003.02017.x
Tymczyszyn EE, Gomez-Zavaglia A, Disalvo EA (2007) Effect of sugars and growth media on the dehydration of Lactobacillus delbrueckii ssp. bulgaricus. J Appl Microbiol 102(3):845–851
Zavaglia AG, Tymczyszyn E, De Antoni G, Disalvo EA (2003) Action of trehalose on the preservation of Lactobacillus delbrueckii ssp bulgaricus by heat and osmotic dehydration. J Appl Microbiol 95(6):1315–1320
Zayed G, Roos YH (2004) Influence of trehalose and moisture content on survival of Lactobacillus salivarius subjected to freeze-drying and storage. Process Biochem 39(9):1081–1086
Zhao G, Zhang G (2005) Effect of protective agents, freezing temperature, rehydration media on viability of malolactic bacteria subjected to freeze-drying. J Appl Microbiol 99(2):333–338
Acknowledgments
The research leading to these results has received funding from the European Community’s Seventh Framework Programme (FP7, 2007–2013), Research Infrastructures action, under the grant agreement No. FP7-228310 (EMbaRC project).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 214 kb)
Rights and permissions
About this article
Cite this article
Peiren, J., Buyse, J., De Vos, P. et al. Improving survival and storage stability of bacteria recalcitrant to freeze-drying: a coordinated study by European culture collections. Appl Microbiol Biotechnol 99, 3559–3571 (2015). https://doi.org/10.1007/s00253-015-6476-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6476-6