Abstract
Biodiesel production by microalgae with photosynthetic CO2 biofixation is thought to be a feasible way in the field of bioenergy and carbon emission reduction. Knowledge of the carbon-concentrating mechanism plays an important role in improving microalgae carbon fixation efficiency. However, little information is available regarding the dramatic changes of cells suffered upon different environmental factors, such as CO2 concentration. The aim of this study was to investigate the growth, lipid accumulation, carbon fixation rate, and carbon metabolism gene expression under different CO2 concentrations in oleaginous Chlorella. It was found that Chlorella pyrenoidosa grew well under CO2 concentrations ranging from 1 to 20 %. The highest biomass and lipid productivity were 4.3 g/L and 107 mg/L/day under 5 % CO2 condition. Switch from high (5 %) to low (0.03 %, air) CO2 concentration showed significant inhibitory effect on growth and CO2 fixation rate. The amount of the saturated fatty acids was increased obviously along with the transition. Low CO2 concentration (0.03 %) was suitable for the accumulation of saturated fatty acids. Reducing the CO2 concentration could significantly decrease the polyunsaturated degree in fatty acids. Moreover, the carbon-concentrating mechanism-related gene expression revealed that most of them, especially CAH2, LCIB, and HLA3, had remarkable change after 1, 4, and 24 h of the transition, which suggests that Chlorella has similar carbon-concentrating mechanism with Chlamydomonas reinhardtii. The findings of the present study revealed that C. pyrenoidosa is an ideal candidate for mitigating CO2 and biodiesel production and is appropriate as a model for mechanism research of carbon sequestration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Greenhouse effect has been recognized as a major factor of the global warming. Among which, carbon dioxide (CO2) is the most important cause of greenhouse gases, thus making the CO2 mitigation attracting much attention from both environmental and economical societies. In nature, aquatic photosynthetic microorganisms account for almost 50 % of the world’s photosynthesis by acquiring CO2 from the environment. Microalgae are expected to have strong CO2 biofixation efficiency by dozens of times higher than terrestrial plants owing to their fast growth rate and short generation time (Tredici 2010). Therefore, using photoautotrophic microalgae for biological CO2 mitigation has become a popular topic in this research area (Lam and Lee 2013; Zheng et al. 2014).
In fact, through photosynthesis, certain microalgae biofix CO2 in the form of carbohydrate, protein, and lipids inside their cells, making the whole algal biomass a treasure, whereas only parts are valuable in the land plants. Microalgae can fix CO2 from different sources, including CO2 from the atmosphere and industrial exhaust gas and also in the form of soluble carbonates (Lam and Lee 2013). Nevertheless, there are several challenges existing in acquiring CO2 by microalgae from the environment, especially the low solubility of CO2 in water. Based on the fact that the diffusion of CO2 in an aqueous solution is 104 times slower than the diffusion of CO2 in air (Spalding 2008), the first challenge these organisms face is the ability to capture CO2 as quickly as possible. The second challenge is rendered by the properties of ribulose bisphosphate carboxylase-oxygenase (Rubisco). However, Rubisco has a poor apparent affinity for CO2 because the concentration of dissolved CO2 is less than the Km (CO2) of Rubisco and due to the relatively high concentration of O2 which competes with CO2. So, the relative rate of oxidation and carboxylation is the key factor to decide the efficiency of photosynthesis as well as CO2 fixation rate. Third, dissolved inorganic carbon (DIC) has significant fluctuations in three forms: CO2, HCO3 −, and CO3 2−. Inorganic carbon (Ci) in the form of CO2 has an acidic pH value, while the overwhelming majority of Ci is in the form of HCO3 − at an alkaline pH, which makes the microalgae suffer from dramatic changes in the supply of DIC (Kim 2014).
In order to overcome these challenges, a number of algae, including both the prokaryotic and eukaryotic algae, have developed a CO2-concentrating mechanism (CCM) to maximize photosynthesis under limiting CO2 conditions (Singh et al. 2014). The CCM increases the CO2 concentration at the site of Rubisco which supplies sufficient Ci to augment photosynthetic productivity in algal cells. Chlamydomonas reinhardtii, a unicellular green alga, has served as a model system to study the CCM, including the energy-coupled Ci uptake and transport systems and various carbonic anhydrases (CAs) which catalyze interconversion of CO2 and HCO3 − (Jungnick et al. 2014; Spalding 2008). All of them play important roles in the CCM. Interestingly, the CCM can only be induced in low-CO2 (LC, ~0.04 % CO2) or very-low-CO2 (VLC, <0.02 % CO2) conditions. Maybe the high CO2 (HC, >1 % CO2) significantly suppresses the activity of CAs. Various CAs are induced when cells grown under HC are transferred to LC. To date, nine carbonic anhydrase genes (CAH1–CAH9) and putative transporters or other low CO2-inducible genes have been discovered and have been hypothesized to relate to the CCM in C. reinhardtii (Duanmu et al. 2009b; Ohnishi et al. 2010). In addition, several transcription regulators have also been identified and characterized for induction of the CCM, e.g., Cia5 or Ccm1 (Fang et al. 2012). Although some progress has been achieved in this model organism, the detail of the CCM remains unclear, not to mention the understanding in other algae. Simple mechanisms for carbon concentration and carbon assimilation have been described in several algae; these mechanisms typically rely on protein function prediction with next-generation genome and transcriptome sequencing (Brueggeman et al. 2012; Fang et al. 2012; Radakovits et al. 2012). However, an actual mechanism for a CCM in single-cell alga has not been fully characterized, and further studies are needed to biochemically verify the proposed carbon-concentrating mechanisms in algae (Eckardt 2012; Jungnick et al. 2014).
Similar to C. reinhardtii, Chlorella spp. were used to be a model species for the study of photosynthesis for a long time. In recent years, they were found to have a fast growth rate both by heterotrophic and phototrophic cultivation (Liang et al. 2009). They are also considered to be one of the most promising commercial algae candidates due to the cells rich in nutrients. Moreover, Chlorella can accumulate large amounts of lipids from trophic transition or environmental stress factors, such as nutrient deficiency, so they were known as one of the potential strains for biodiesel production (Fan et al. 2014a). In the application for removal of CO2, Chlorella have been studied for CO2 reduction using industrial flue gas or pure CO2 (Lam and Lee 2013). Despite these interesting studies to understanding the performance of Chlorella, large-scale production of biomass has still turned out to be problematic. One reason for that is most of the previous researches were mainly focused on the cultivation process level and little information is available regarding the dramatic changes of cells suffered upon different environmental factors, such as CO2 concentration. Therefore, knowledge of physical and biochemical signs as well as metabolic changes and nutritional needs plays a central role in process development (Sydney et al. 2010).
Chlorella pyrenoidosa, a robust industrial strain that is already cultivated widely, mainly for food, supplement, and biofuel production, was selected as a research object in the present study. C. pyrenoidosa was firstly cultivated under different CO2 concentrations ranging from 0.03 (air) to 20 % (v/v), and then, switch from 5 to 0.03 % was also conducted. The impacts of different CO2 concentrations on the growth characteristics, lipid content, fatty acid profiles, and CO2 biofixation rate, as well as CCM metabolism-related gene expression were fully investigated.
Materials and methods
Strain, medium, and culture conditions
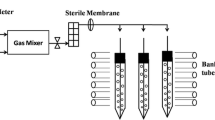
C. pyrenoidosa (FACHB 9) was obtained from the Institute of Hydrobiology, Chinese Academy of Sciences (Wuhan, China), and purified aseptically for further study. Seed cells were grown in Endo medium and maintained at 30 °C for 3.5 days with a reciprocating shaker (150 rpm) under low-intensity light (50 μmol/m2/s). Two percent of cells were inoculated into air-lift column photobioreactors (60 cm high and 5 cm in diameter) containing 800 mL Bold’s basal medium (BBM) and cultured at 30 °C under continuous illumination using white fluorescent lamps (approximately 150 μmol/m2/s) with one-side illumination. Photobioreactors were aerated with various concentrations of CO2: filtered ambient air (LC) and HC ranging from 1 to 20 % CO2 (v/v) at a flow rate of 0.3 L/min for comparison. After that, four group experiments supplemented with 20 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer solution (pH 7.2) were cultured with the same conditions but with bubbling of 5 % CO2 for the first 5 days, and then, two groups were shifted from HC to LC conditions until the end of cultivation. Two biological replicates of each induction condition were processed.
Measurements of biomass density
The cell density was measured by spectrophotometer (optical density 680) and dry cell weight (DCW). Whatman GF/A glass microfiber filters (47-mm Ф circles, Whatman, England) were dried in an electric oven at 80 °C overnight and then cooled in a desiccator and weighed. Three to ten milliliters of the algal sample was filtered by vacuum filtration and was dried and cooled before weighing again. Biomass concentration was determined by the difference between the two weights.
Lipid extraction and content analysis
Total lipid extraction followed the method reported by Bligh and Dyer with some modifications. The microalgal cells were collected by centrifugation at 4000 g for 10 min and washed with deionized water twice. Freeze-dried cells were obtained by lyophilization and weighed (W). Each sample (approximately 300 mg) was extracted with a mixture of methanol/chloroform (2:1, v/v) for 40 min by ultrasonication and then centrifuged at 6000 g for 10 min after precipitation. After that, the extracts (supernatant) were transferred into a preweighed dry glass tube (G1) and evaporated by Termovap Sample Concentrator. The procedure was repeated until the organic solvent had no color, which meant the total lipids were entirely extracted. Finally, the residue and glass tube were dried in an electric oven at 80 °C for 12 h and cooled in a desiccator until the weight (G2) was constant. The total lipid content of the sample was calculated as follows:
Chemical and physiological parameter analysis
pH in each sample was measured immediately after collection with an ISFET pH meter which was calibrated daily using standard solutions of pH 4 and 7. The method of total chlorophyll contents was referred to Dere et al. (1998) with some modifications. The cells were centrifuged, and the residue was dealt with 96 % methanol (v/v, fresh) in an ultrasonic cleaner (dark and cool environment) for 30 min to extract pigments. After that, cellular debris was pelleted by centrifugation at 6000 g for 10 min and chlorophyll a and b levels in the supernatant were estimated spectrophotometrically by recording optical absorbance at 653 and 666 nm.
Measurements of CO2 fixation rate
The CO2 biofixation rate \( {R}_{C{O}_z} \) was calculated by using the following equation:
where C C is the carbon content of the microalgal cell (%, w/w), which was measured by an element analyzer (Elementar Vario ELIII, Germany); M C is the molecular weight of carbon; \( {M}_{\mathrm{C}{\mathrm{O}}_z} \) is the molecular weight of CO2; and P is the biomass productivity (g/L/day).
RNA preparation and isolation
During the whole experiment, the pH values of the four groups were maintained with 20 mM HEPES buffer solution in case of the pH fluctuation. On the fifth day, these samples were collected from the bioreactors at 0, 1, 4, 8, 24, and 48 h after shifting from HC to LC conditions and then centrifuged at 5000 g for 5 min at 4 °C immediately. The supernatant was discarded, and the centrifuge tube was immersed in liquid nitrogen to freeze the cellular pellet. Samples were stored at −80 °C until RNA extraction.
Total RNA was isolated using the TRIzol Reagent (Invitrogen, USA). The previous sample was grinded in liquid nitrogen and transferred rapidly to a microcentrifuge tube with 1 mL TRIzol Reagent. The cellular pellet was resuspended by Mixplus and incubated for 10 min at room temperature and centrifuged at 12,000 g for 10 min at 4 °C. The sample was added into 0.2 mL chloroform and shaken vigorously by hand for 15 s and incubated for 3 min at room temperature. Next, samples were centrifuged at 12,000 g for 15 min at 4 °C. The aqueous phase was transferred to a fresh tube. Isopropanol was added to the aqueous phase, and the resulting solution was incubated for 10 min at room temperature and centrifuged at 12,000 g for 10 min at 4 °C to precipitate RNA. Isopropanol was removed, and the RNA pellet was washed with 75 % ethanol. Following further centrifugation (7500 g for 5 min at 4 °C), the supernatant was removed and the RNA pellets were dried briefly for 5 min. Finally, the air-dried RNA pellets were dissolved in 20–40 mL of RNase-free water. The integrity of RNA was examined by agarose gel electrophoresis, and the concentration was analyzed by a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific) to verify RNA quantity and purity. The RNA solutions were stored at −80 °C for further experiments.
Quantitative real-time RT-PCR analysis
The first-strand complementary DNA (cDNA) synthesis and real-time quantitative PCR were performed using the ReverTra Ace qPCR RT Master Mix with gDNA Remover and SYBR Green Real-Time PCR Master Mix (Toyobo, Japan), respectively, according to the manufacturer’s instruction. Using gene sequences retrieved from NCBI genome sequencing databases, primers of CCM-related key genes (Table 1) were designed and used for expression-level quantitative detection. The actin gene from C. pyrenoidosa was used as internal control for normalization of qPCR data. Each reaction has three repeats for fidelity assay and was employed in a final volume of 20 μL containing 1 μL of cDNA template, 1 μL of each primer (10 mM), 10 μL of SYBR Mix, and 7 μL distilled water. All PCR reactions were performed on a C1000 Thermal Cycler Real-Time PCR Detection System (Bio-Rad, USA) with a program of initial denaturation at 95 °C for 30 s, 39 cycles of 95 °C denaturation for 10 s, 65 °C annealing for 15 s, and 72 °C extension for 25 s. The 2−ΔΔCT method was used to analyze gene expression relative to control based on the average fold change.
Results
Growth characteristics and lipid accumulation of C. pyrenoidosa under different CO2 concentrations
To investigate the effect of different CO2 concentrations on the growth of C. pyrenoidosa in the process of photoautotrophy, algal cells were firstly cultured 3.5 days until the depletion of glucose and then inoculated into 1-L photobioreactors using BBM. In Fig. 1a, the initial pH was 6.5 and changed immediately when different CO2 were aerated into the medium. Meanwhile, the curve of cell density showed no lag phase, and the growth was kept strong except for pure CO2 and air, which suggested that in the process of growth, the algal cells adapted to the environment and adjusted the external environment to be suitable for growing at the same time (Richmond 2008). The difference of concentration of CO2 has a significant bearing on pH value which decreased from approximately 9 to 5 with the increase of CO2 concentrations from 0.03 (air) to 100 %. However, four treatment groups (1, 5, 10, and 20 % CO2) showed less change in pH (between 6.5 and 7.5, approaching to neutral) over the whole cultivation period, whereas in the control group, the pH value of the broth aerated with air increased gradually during the first 4 days (from 6.5 to 9). In contrast, pH fell to 4.8 at once after 1 day and was too acidic in the presence of 100 % CO2, and the growth of the cells was completely inhibited.
The difference between the biomass concentrations of the four HC conditions (1–20 % CO2) was little especially during the first 7 days. From the eighth day, the biomass of cells under the four concentrations began to appear different, of which the biomass concentrations of 1 and 5 % CO2 continued to increase and attained to over 4.3 g/L finally, while for 10 and 20 % CO2, they had a tendency of declining and leveling off, and the biomass concentration was about 3.5 g/L.
As shown in Fig. 1c, the chlorophyll content of heterotrophic cells as seed was very low (about 15 mg/g DCW) for the lack of light. However, chlorophyll in cells had a sudden increase after 24-h photoautotrophy; several groups of CO2 concentrations (except for 100 % CO2) increased from 15 to about 40 mg/g DCW during 24 h. During the following 4 days, the chlorophyll contents of the four groups’ CO2 concentration (1–20 % CO2) were in a linearly decreasing trend and down to less than 15 mg/g DCW in the fifth day which basically closed to normal heterotrophic levels. This is due to the high concentration of CO2 which promoted the growth of algal cells; most of the carbon sources were used for the increase of cell biomass, which resulted in the decrease of chlorophyll content. After that, chlorophyll content had been maintaining at a low level (nearly 10 mg/g DCW) by the end of cultivation.
The initial total lipid content of the algal cells was about 12 % and showed sustained increase along the whole period of cultivation expected for pure CO2, and finally reached to 25–35 % of the dry cell weight (Fig. 1d), which increased more than the double amount. In this study, the total lipid content exhibited the most marked increase under the 1 % condition, whereas the worst result was achieved by 20 % CO2, even below that of air condition. The results suggested that the 1 and 5 % CO2 strategy could result in the most favorable lipid productivity (about 107 mg/L/day), while in the LC way, the lipid productivity was only 16 mg/L/day, which suggested an efficient microalga lipid production mode by C. pyrenoidosa using CO2 as the carbon source.
Effects of high CO2 to low CO2 transition under pH control on C. pyrenoidosa growth
As shown in Fig. 2a, after shifting to the air cultivation, the cell density of the experimental group increased slowly, which was lesser than that of the control group with continuous CO2 due to the sudden reduction of carbon source. Finally, the dry weight of the control group reached 4.18 g/L, while that of experimental group was only 3.15 g/L. The chlorophyll content (Fig. 2e) showed an increasing trend at first and descending later as a whole; this trend was also consistent with the change of chlorophyll under different CO2 concentrations. Comparing the two groups, there was little difference in the chlorophyll content after the switch.
In order to further explore the intracellular growth state, the carbon and nitrogen contents of the dry algal powder were measured by an elemental analyzer. As shown in Fig. 2c, the intracellular nitrogen content fell with the increase of culture time, especially in the first 5 days, with a sharp drop from 8.5 to 4.5 %. The nitrogen content decreased much slower from the fifth day which basically maintained at a constant level. By contrast, the change of intracellular carbon content was not obvious; it was kept at about 45 % over the whole culture period. Even though the cells were shifted to the air condition, the carbon content changed a little. It may be because the early high-CO2 concentration made the carbon content saturated, and the fixed carbon content would not have a huge variation in a short time even after the switch to the air.
In Fig. 2d, the carbon fixation rate showed an early upregulation and then a downward trend overall. The carbon fixation rate of the switched group was lower than that of continuous 5 % CO2 after the shift because there was no extraneous inorganic carbon source to be fixed which resulted in the carbon fixation rate being reduced correspondingly.
Fatty acid composition of C. pyrenoidosa grown under high CO2 (5 %) to low CO2 (0.03 %) transition
The fatty acid composition of C. pyrenoidosa grown under high-CO2 (5 %) to low CO2 (0.03 %) transition was analyzed using gas chromatography (Table 2). The results showed that the main components of this alga were fatty acids with C16–C18 by photoautotrophy, accounting for about 72–92 % of the total fatty acid content in the whole cultivation process. The fatty acid profiles were favorable for biodiesel production. The amount of the saturated fatty acids was increased obviously along the time course cultivation, especially after the switch from 5 % CO2 to air. Low CO2 concentration (0.03 %) was suitable for the accumulation of saturated fatty acids, such as palmitic acid (C16:0) and short-chain fatty acid (C12:0).
Effects of CO2 concentration changes on CCM-related gene expression
As shown in Fig. 3, the expression abundance of CAH2 revealed a normal distribution. It began to be induced after 1 h and then increased gradually over time, showing a trend toward a higher peak at 8 h. Its expression quantity at 8 h was ten times greater than that of the control group. On the whole, CAH2 was a moderately upregulated gene with severe changeable points at 4 and 8 h in C. pyrenoidosa. The expression amount of CAH4 was remarkably raised at 1 h which was also the peak value, and the expression quantity was about four times as much as that of the control group and then fell to a basic stable state which was similar to that of the control group. It is obvious that HLA3 is a strong-expression gene in C. pyrenoidosa, reaching the peak value (about 110 times of that of the control group) at 1 h after the switch, and then, the expression abundance fell, but it still maintained at a high level, about 40 times at 4 h and 34 times at 8 h, which were significantly higher than those of the CAH2 and CAH4 genes.
In this study, CO2 concentration decreased dramatically after sudden deprivation of CO2, and this may be because LCIA encodes bicarbonate transporters, in order to make up for the limiting CO2; the LCIA gene raised quickly within 1 h which promoted the accumulation of bicarbonate. This result is consistent with the report that its expression is the most intense within 1 h in C. reinhardtii. The strange thing is that the expression abundance of LCIA was not very high, and the highest point was just increased by about 3.4 times; after that, it even decreased a little compared to the control. It is likely to serve as an auxiliary synergy in the CCM regulation. LCIB is an upregulated gene in C. pyrenoidosa, and the highest expression abundance was at 4 h. Interestingly, it maintained at a high continuous expression level after 4 h when the expression abundance was about 210 times, 130 times, 160 times, and 120 times greater than the control group, respectively.
The transcript level of the Rubisco small subunit under a limiting CO2 condition was relatively constant, and the variation between the treatment and the control was minor, indicating that the rbcS might be a constitutive expression in C. pyrenoidosa. In contrast, the expression abundance of rbcL showed a downward trend overall during the whole period, but the variability of the population was modest except when it was at 8 h. The expression abundance at 8 h in the continuous-CO2 condition was about 20 times higher than that in the CO2-limiting condition.
In Fig. 3, the variation of the expression level of CIA5 was not obvious, suggesting that the constitutive expression of the gene led to the stable control of the putative Ci transporters and induced CAs. With regard to LCR1, the overall tendency of its expression level was upregulated and showed the highest change for 24 h, which was about 5.7 times higher than that of the control group. Compared to CIA5, both of them revealed the most expression quantity in a 24-h time point after the switch.
Discussion
Biodiesel production by microalgae with photosynthetic CO2 biofixation is thought to be a feasible way in the field of bioenergy and carbon emission reduction. The results obtained in the present study demonstrated that the cultures of C. pyrenoidosa using CO2-enriched air as the sole carbon source exhibited high biomass concentrations (4.3 g/L in 5 % CO2). CO2 concentration influenced pH, growth, and lipid content. The pH value of the broth aerated with air increased gradually in the first few days; however, the pH did not increase without limit, and cells would gradually die when the nutrition is not enough to reproduce again; then, the dead cells would release reserve substances which made the medium acidic, slowed down the increase of pH, and prevented algal cells from absorbing and utilizing CO2 (Cheng et al. 2004). So, that was why pH showed less change and maintained a certain range of 9 basically. On the whole, in either HC or LC conditions, pH did not change all the time in the period of cultivation.
Since CO2 was used as the sole carbon source in the medium, the higher the CO2 concentration is, the higher the DIC concentration becomes; in theory, that meant the more carbon source would be used for the increase of biomass concentration. Quite the opposite has been the case; cells in the presence of 10 and 20 % CO2 did not obtain a better cell density than those with 1 and 5 % CO2 (Fig. 1b) because higher CO2 concentration could result in lower pH as shown in Fig. 1a. This was especially true for 100 % CO2 level; the Chlorella cells ceased growing basically and approached to death after 4 days. Another control of air showed a low biomass due to the deficient in carbon source compared to the HC conditions. Maybe early cells were hungrier and could consume the increasing DIC. As time went on, intracellular carbon was gradually saturated and the rate of growth tended to be relatively stable.
The contents of chlorophyll in the whole cultivation showed a downward trend overall. It just had a surge response process in the early stage because of the switch of heterotrophic cells for light environment and reboot their photosynthesis ability. Because, on the one hand, the light can activate light energy utilization-related enzyme systems which were closed in the stage of heterotrophy, especially for the restart of photosynthetic carbon sequestration reaction, making algal cells from the heterotrophic nutrition by using organic carbon source and releasing CO2 into the photosynthetic nutrition by intake CO2. On the other hand, the optimal light intensity for algal cells under the normal growth condition is about 100–200 μmol/m2/s; in general, high light has a strong chemical and thermal damage effect on algal cells. Chlorophyll, as the main biochemical component converted by absorbing sun energy, has a direct protection on the algal cells which are exposed to the strong high lights. So, this sharp increase maybe associated with the self-protection mechanism of algal cells (Fan et al. 2012a), and the sudden light was a strong factor for algal cells in the long time of darkness or even an intimidation factor, so the algal cells need to synthesize relevant protective substances, such as pigment for protecting cells. Compared to the two ways of air and pure CO2, it was found that algal cells bubbled with air grew slowly and had low cell density because of the low CO2 concentration; so, a similar light intensity of the stimulus to algal cells under the above situation was obviously higher than that of high density under higher CO2 concentrations. Thus, the high light had much more intense entrainment to the synthesis of chlorophyll under this condition, and algal cells suffered from the physiological pressure and the chlorophyll content was significantly higher. The condition of pure CO2 (may be due to low pH value) harmed the growth of algal cells at all and the further synthesis of chlorophyll.
Lipid productivity is of particular importance in large-scale microalgal lipid production processes since it takes into account both lipid content and biomass production rate. The trophic switch from heterotrophy to photoautotrophy might be a critical reason that contributes to lipid overproduction. It was also a protective mechanism by the synthesis of total lipid when algal cells were under light pressure. Previous studies revealed that the heterotrophic Chlorella seeds exhibited a superiority on both biomass growth and lipid accumulation in the subsequent photoautotrophic cultivation (Han et al. 2012). Consistent with this, it was found that the transition from heterotrophy to photoinduction culture can stimulate a dramatic increase of lipid in three Chlorella species (Fan et al. 2014b; 2012b).
Effects of switch from high CO2 to low CO2 in buffered BBM on C. pyrenoidosa growth and CO2 biofixation were studied in this section. From the above experimental results, it was concluded that the growth of cells under 1 and 5 % CO2 concentrations was better than that under 10 and 20 % CO2 concentrations. Furthermore, considering that the objective of the experiment was to investigate the CCM-related response when cells were switched from high CO2 concentration to low CO2 concentration, so finally, we chose the higher concentration (5 % CO2) as the research model of culture process and carbon sequestration mechanism.
In this new experiment, the experimental groups were firstly aerated with 5 % CO2 (HC), and then, the cells were shifted from HC to LC (air), as for the control group was aerated with continuous 5 % CO2, both of them were conducted in buffered BBM by HEPES buffer. A preliminary experiment about the effect of HEPES buffer on algae cell growth was done to determine the concentration and the potential toxicity before this formal experiment (data not shown). In the previous results (Fig. 1a), the pH rose sharply under the air condition without adding HEPES buffer. However, as shown in Fig. 2a, HEPES buffer had a very good control of the pH value of the culture after the switch from 5 % CO2 to the air; the pH was maintained at around 7.2 till the end of the switch. There was little difference of pH value between continuous 5 % CO2 and 5 % CO2 to air transition under HEPES buffer. This approximate neutral pH was conducive to the growth of algal cells. In addition, the control of pH eliminated the effect of pH change on CCM-related gene expression.
In the early stage of cultivation, the carbon fixation rate had a small amplitude increase, because the early algal cells were physiologically vigorous, and at this time, they were in a state of hunger because the algal cells were transferred from a heterotrophic organic carbon source to a autotrophic inorganic carbon source, so they began to fix a large number of inorganic carbon. Subsequently, the physiological and biological activities of the cells went down which affected the ability of carbon sequestration and the intracellular carbon content approached to saturated value in the late state of growth, so the carbon fixation rate declined gradually.
It is worth noting that reducing the CO2 concentration could significantly decrease the polyunsaturated degree in fatty acids, dropped by more than 22 % (absolute value) compared to the control group. It seems that little influence was found upon monounsaturated fatty acids, as shown in Table 2; the contents of this part were only around 3–4 % along the process. It was reported that at low CO2 concentrations, the desaturation rate was slower than the elongation rate, making the production of less polyunsaturated fatty acids (Ota et al. 2009). The regular patterns of fatty acid profiles in this study were consistent with those in the study of Ota et al. (2009) and Tang et al. (2011).
To the best of our knowledge, nine different α- and β-CA genes have been identified in C. reinhardtii by far, but it is not entirely clear what roles are played by these CAs; some of these proteins are implicated to have possible functions in the CCM (Jungnick et al. 2014). In this study, we just focused on the CAH2 and CAH4 genes in C. pyrenoidosa. CAH2 is a periplasmic soluble α-CA in C. reinhardtii. Tachiki et al. isolated a carbonic anhydrase from high CO2 (5 % CO2) grown C. reinhardtii which was identified as the isozyme from CAH2. They found that CAH1 and CAH2 shared high sequence similarity, and both of them were present in the wild-type cells of C. reinhardtii (Tachiki et al. 1992). Surprisingly, they are differentially regulated by the CO2 concentration. CAH1 is induced by limiting CO2 and light, while CAH2 is poorly expressed and downregulated under limiting CO2 conditions (Moroney and Ynalvez 2007). However, the results were opposite to those in the present study.
CAH4 is a mitochondrial β-CA (mtCA) which is induced under limiting CO2 conditions, but it is not very clear how it plays a role in CCM, and multiple hypotheses have been advanced regarding the potential function of the protein. One assumed that mtCAs buffer the mitochondrial matrix due to the complete induction of the CCM, and photorespiratory glycine decarboxylation produces equivalent amounts of NH3 and CO2. The mtCA might serve to catalyze the hydration of CO2, producing H+, which would prevent alkalinization in the mitochondrial matrix as a result of the generation of NH3 by glycine decarboxylation (Moroney and Ynalvez 2007). Alternatively, the mtCAs might play a role in converting the CO2 generated by respiration and photorespiration to HCO3 − (Raven 2001). The change of CAH4 in C. pyrenoidosa was consistent with the reports in the literature in C. reinhardtii.
High light-induced gene 3 (HLA3) was identified when C. reinhardtii cells were exposed to high light, by encoding a putative ATP-binding cassette-type transporter of the multidrug resistance-related protein subfamily (Im and Grossman 2001). Unlike other low CO2-induced genes, its expression is activated by both high light and low CO2, for which it was shown to be under the control of zinc-finger transcription factor Ci accumulation 5 (CIA5), a master regulator for the response of C. reinhardtii to limiting CO2 conditions (Miura et al. 2004). High expression of this gene may be because it is related to its function of activating the ATP, and it can be induced under low CO2.
Low CO2-induced gene A (LCIA) is an attractive candidate transporter which encodes a chloroplast membrane protein reported to transport bicarbonate in C. reinhardtii (Moroney and Ynalvez 2007). According to some findings which have been reported, it is no surprise that the expression of LCIA is induced under limiting CO2 conditions; the point is that it is also partially under the control of CIA5, a transcription factor required for induction of most other CCM genes. Moreover, simultaneous knockdown of LCIA and HLA3 in wild-type Chlamydomonas cells showed high pH-sensitive phenotype and low photosynthetic Ci affinity, which had more severe phenotypic effect than HLA3 knockdown alone, indicating that LCIA and HLA3 are key synergistic or complementary components of the active Ci transport pathway in limiting Ci-acclimated cells (Duanmu et al. 2009a).
Low CO2-induced gene B (LCIB) encodes a soluble protein which localized in the vicinity of pyrenoid, a prominent structure in the chloroplast, and it is responsible for the normal Ci uptake or accumulation in low-CO2 conditions. This gene belongs to a novel gene family which has three homologous genes in Chlamydomonas, LCIC, LCID, and LCIE. All of them are responsive to limiting CO2, but LCIB and LCIC are the most abundant transcripts upon the limiting CO2 induction (Wang et al. 2011). Recent report found that LCIB interacts with LCIC and forms a hexamer complex of approximately 350 kDa which gathered around the pyrenoid from the matrix under light illumination and LC conditions during active operation of the CCM. In contrast, in the dark or under high-CO2 conditions when the CCM was inactive, the related proteins immediately diffused away from the pyrenoid to the matrix (Yamano et al. 2010); it seems that the pyrenoid related to location is very important for the function of LCIB. Combining the HLA3 knockdown with both the off-target LCIA knockdown and an LCIB mutation confirmed the overlapping functions of HLA3, LCIA, and LCIB (Duanmu et al. 2009a). LCIB is suggested to have two functional possibilities (Yamano et al. 2010): one is that it mainly traps CO2 leaking from the pyrenoid matrix and transfers the captured CO2 to stromal carbonic anhydrase CAH6; the other is that LCIB combined with LCIC to form a complex as a structural barrier to prevent the leakage of CO2 and maintain CO2 concentration in the pyrenoid matrix. So, in either case, the continuous and abundant expression at a later stage was likely to maintain cell growth and photosynthesis under LC. In Chlamydomonas, overexpression of LCIA and LCIB could enhance the growth rate and biomass density by about 40–80 % comparing with the wild type under normal CO2 conditions (Spalding 2012).
Rubisco is short for ribulose 1,5-bisphosphate carboxylase/oxygenase, a hexadecamer consisting of eight 55-kDa large subunits and eight 12-kDa small subunits. It catalyzes the first major step of carbon fixation in the Calvin cycle, a process by which the carbon dioxide in the atmosphere is converted to energy-rich molecules. So, it is a key enzyme deciding the rate of carbon assimilation in photosynthesis. It is also involved in the photorespiratory pathway when it reacts with CO2 or O2. The Rubisco large subunit (RBCL) is encoded by the single-copy rbcL gene of the chloroplast genome and is translated by chloroplast ribosomes, while the Rubisco small subunit (RBCS) is encoded by a multigene family of rbcS on the nuclear genome and is posttranslationally transported to the chloroplast. The different conclusions were obtained by Winder et al. (1992) that the expression level of rbcL in C. reinhardtii did not vary during the time course of adaptation to limiting CO2, while the rbcS2 messenger RNA (mRNA) which contributed most of the mRNA to the rbcS mRNA pool remained essentially unchanged in abundance, but a more distinct shift in abundance was observed for rbcS1 mRNA in cell wall-deficient mutant cells (but not in the wild-type strain). The obvious difference between these two studies maybe the light; the algae cells were cultured under illumination in our study, but they were not in Winder’s study. It should not be surprising that the illumination has a similar effect on the biosynthesis of Rubisco as well as CO2. In view of the evidence that synthesis of the large subunit of Rubisco dropped remarkably within 15 min and only gradually recovered at about 6 h when C. reinhardtii cells were transferred from low light to higher light intensities, the Rubisco small subunit was not dramatically affected, and the further research revealed that downregulation of large subunit synthesis was not related with a decline in the steady-state level of the rbcL transcript. So, we conclude that maybe the relatively stable expression abundance of rbcL and rbcS in the present study is a function of the presence of light, if at all; the results of the transcripts of rbcL and rbcS were insufficient to overlook the importance of Rubisco in the control over CCM. Besides, the low catalytic activity of Rubisco raises the possibility of the results in this study.
CIA5 and low CO2-induced gene1 (LCR1) have been confirmed as the most two important transcription regulators in C. reinhardtii CCM regulatory mechanisms (Fang et al. 2012). CIA5 is a zinc-finger-type transcription regulator. It was found that the expression of most putative Ci transporters and induced CAs relies on CIA5. However, the transcription of CIA5 itself does not depend on the CO2 level, whereas posttranslational activation in low CO2 apparently is required for regulation. As to LCR1, it has a Myb domain and appears to regulate the expression of several low CO2-induced genes. In addition, LCR1 itself is also regulated by limiting CO2, and this induction requires CIA5. The results also supported the conclusion that the expression of the CIA5 gene is not influenced by CO2 concentration (Miura et al. 2004). The transition from high CO2 concentration to a low-CO2 condition showed significant impacts on the CCM-related gene expression. In short, C. pyrenoidosa could be successfully exploited to simultaneously mitigate CO2 and to produce biofuels as well as to study the carbon sequestration mechanism.
References
Brueggeman AJ, Gangadharaiah DS, Cserhati MF, Casero D, Weeks DP, Ladunga I (2012) Activation of the carbon concentrating mechanism by CO2 deprivation coincides with massive transcriptional restructuring in Chlamydomonas reinhardtii. Plant Cell 24(5):1860–1875
Cheng LH, Chen HL, Zhang L, Gao CY, Gao CJ (2004) Study on medium composition of microalgae optimization for CO2 removal from air by a membrane-photobioreactor. SAE Technical Paper
Dere S, Günes T, Sivaci R (1998) Spectrophotometric determination of chlorophyll-A, B and total carotenoid contents of some algae species using different solvents. Turk J Bot 22:13–17
Duanmu D, Miller AR, Horken KM, Weeks DP, Spalding MH (2009a) Knockdown of limiting-CO2-induced gene HLA3 decreases HCOFormula transport and photosynthetic Ci affinity in Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 106(14):5990–5995
Duanmu D, Wang Y, Spalding MH (2009b) Thylakoid lumen carbonic anhydrase (CAH3) mutation suppresses air-Dier phenotype of LCIB mutant in Chlamydomonas reinhardtii. Plant Physiol 149(2):929–937
Eckardt NA (2012) Gene regulatory networks of the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell 24(5):1713–1713
Fan JH, Cui YB, Huang JK, Wang WL, Yin WB, Hu ZM, Li YG (2012a) Suppression subtractive hybridization reveals transcript profiling of Chlorella under heterotrophy to photoautotrophy transition. PLoS One 7(11):e50414
Fan JH, Cui YB, Wan MX, Wang WL, Li YG (2014a) Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol Biofuels 7:17
Fan JH, Cui YB, Zhou Y, Wan MX, Wang WL, Xie JL, Li YG (2014b) The effect of nutrition pattern alteration on Chlorella pyrenoidosa growth, lipid biosynthesis-related gene transcription. Bioresour Technol 164:214–220
Fan JH, Huang JK, Li YG, Han FF, Wang J, Li XW, Wang WL, Li SL (2012b) Sequential heterotrophy-dilution-photoinduction cultivation for efficient microalgal biomass and lipid production. Bioresour Technol 112:206–211
Fang W, Si Y, Douglass S, Casero D, Merchant SS, Pellegrini M, Ladunga I, Liu P, Spalding MH (2012) Transcriptome-wide changes in Chlamydomonas reinhardtii gene expression regulated by carbon dioxide and the CO2-concentrating mechanism regulator CIA5/CCM1. Plant Cell 24(5):1876–1893
Han FF, Huang JK, Li YG, Wang WL, Wang J, Fan JH, Shen GM (2012) Enhancement of microalgal biomass and lipid productivities by a model of photoautotrophic culture with heterotrophic cells as seed. Bioresour Technol 118:431–437
Im CS, Grossman AR (2001) Identification and regulation of high light-induced genes in Chalmydomonas reinhardtii. Plant J 30(3):301–313
Jungnick N, Ma Y, Mukherjee B, Cronan JC, Speed DJ, Laborde SM, Longstreth DJ, Moroney JV (2014) The carbon concentrating mechanism in Chlamydomonas reinhardtii: finding the missing pieces. Photosynth Res:1-15
Kim J (2014) Effects of dissolved inorganic carbon, pH, and light on growth and lipid accumulation in microalgae. University of Cincinnati
Lam MK, Lee KT (2013) Effect of carbon source towards the growth of Chlorella vulgaris for CO2 bio-mitigation and biodiesel production. Int J Greenh Gas Con 14:169–176
Liang YN, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31(7):1043–1049
Miura K, Yamano T, Yoshioka S, Kohinata T, Inoue Y, Taniguchi F, Asamizu E, Nakamura Y, Tabata S, Yamato KT, Ohyama K, Fukuzawa H (2004) Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol 135(3):1595–1607
Moroney JV, Ynalvez RA (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell 6(8):1251–1259
Ohnishi N, Mukherjee B, Tsujikawa T, Yanase M, Nakano H, Moroney JV, Fukuzawa H (2010) Expression of a low CO2-inducible protein, LCI1, increases inorganic carbon uptake in the green alga Chlamydomonas reinhardtii. Plant Cell 22(9):3105–3117
Ota M, Kato Y, Watanabe H, Watanabe M, Sato Y, Smith RL Jr, Inomata H (2009) Fatty acid production from a highly CO2 tolerant alga, Chlorocuccum littorale, in the presence of inorganic carbon and nitrate. Bioresour Technol 100(21):5237–5242
Radakovits R, Jinkerson RE, Fuerstenberg SI, Tae H, Settlage RE, Boore JL, Posewitz MC (2012) Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun 3:686
Raven JA (2001) A role for mitochondrial carbonic anhydrase in limiting CO2 leakage from low CO2-grown cells of Chlamydomonas reinhardtii. Plant Cell Environ 24(2):261–265
Richmond A (2008) Handbook of microalgal culture: biotechnology and applied phycology. John Wiley & Sons
Singh SK, Sundaram S, Kishor K (2014) Carbon-concentrating mechanism of microalgae photosynthetic microorganisms. Springer, pp 63-81
Spalding M (2012) The microalgal CO2-concentrating mechanism and its manipulation to increase yields. Algae Biomass Summit USA
Spalding MH (2008) Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J Exp Bot 59(7):1463–1473
Sydney EB, Sturm W, de Carvalho JC, Thomaz-Soccol V, Larroche C, Pandey A, Soccol CR (2010) Potential carbon dioxide fixation by industrially important microalgae. Bioresour Technol 101(15):5892–5896
Tachiki A, Fukuzawa H, Miyachi S (1992) Characterization of carbonic anhydrase isozyme CA2, which is the CAH2 gene product, in Chlamydomonas reinhardtii. Biosci Biotechnol Biochem 56(5):794–798
Tang DH, Han W, Li PL, Miao XL, Zhong JJ (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102(3):3071–3076
Tredici MR (2010) Photobiology of microalgae mass cultures: understanding the tools for the next green revolution. Biofuels 1(1):143–162
Wang Y, Duanmu D, Spalding MH (2011) Carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii: inorganic carbon transport and CO2 recapture. Photosynth Res 109(1–3):115–122
Winder TL, Anderson JC, Spalding MH (1992) Translational regulation of the large and small subunits of ribulose bisphosphate carboxylase/oxygenase during induction of the CO2-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol 98(4):1409–1414
Yamano T, Tsujikawa T, Hatano K, Ozawa S, Takahashi Y, Fukuzawa H (2010) Light and low-CO2-dependent LCIB-LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol 51(9):1453–1468
Zheng YL, Yuan C, Liu JH, Hu GR, Li FL (2014) Lipid production by a CO2-tolerant green microalgae Chlorella sp. MRA-1. J Microbiol Biotechnol 24(5):683–689
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31300295), China Postdoctoral Science Foundation (2013 M541483), National Special Fund for State Key Laboratory of Bioreactor Engineering (2060204), National Basic Research Program of China (2011CB200904), the Fundamental Research Funds for the Central Universities, and Open Funding Project of the State Key Laboratory of Bioreactor Engineering.
Conflict of interest
The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, J., Xu, H., Luo, Y. et al. Impacts of CO2 concentration on growth, lipid accumulation, and carbon-concentrating-mechanism-related gene expression in oleaginous Chlorella . Appl Microbiol Biotechnol 99, 2451–2462 (2015). https://doi.org/10.1007/s00253-015-6397-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-015-6397-4