Abstract
Polyhydroxyalkanoate (PHA) synthase from Bacillus cereus YB-4 (PhaRCYB4) catalyzes not only PHA polymerization but also alcoholytic cleavage of PHA chains. The alcoholysis activity of PhaRCYB4 is expressed when a hydroxyacyl-CoA monomer is absent but an alcohol compound is present. In this study, we performed alanine mutagenesis of the putative catalytic triad (Cys151, Asp306, and His335) in the PhaCYB4 subunit to identify the active site residues for polymerization and alcoholysis activities. Individual substitution of each triad residue with alanine resulted in loss of both polymerization and alcoholysis activities, suggesting that these residues are commonly shared between polymerization and alcoholysis reactions. The loss of activity was also observed following mutagenesis of the triad to other amino acids, except for one PhaRCYB4 mutant with a C151S substitution, which lost polymerization activity but still possessed cleavage activity towards PHA chains. The low-molecular-weight PHA isolated from the PhaRCYB4(C151S)-expressing strain showed a lower ratio of alcohol capping at the P(3HB) carboxy terminus than did that from the wild-type-expressing strain. This observation implies that hydrolysis activity of PhaRCYB4 might be elicited by the C151S mutation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polyhydroxyalkanoates (PHAs) are polyesters produced by a wide variety of bacteria and archaea for intracellular storage of carbon and energy. PHAs have many attractive properties such as biocompatibility, biodegradability, and thermoplasticity, and can be produced from renewable biomass (Sudesh et al. 2000). The most commonly seen PHA in nature is poly[(R)-3-hydroxybutyrate] [P(3HB)], which shows thermal properties similar to those of polypropylene, one of the petroleum-derived commodity plastics. In the biosynthesis of P(3HB) from acetyl-coenzyme A (acetyl-CoA), only three enzymes are required: 3-ketothiolase (PhaA); NADPH-dependent acetoacetyl-CoA reductase (PhaB); and PHA synthase (PhaC), which are encoded by the phaA, B and C genes, respectively (Stubbe and Tian 2003). Transformation with these three genes drives P(3HB)-negative bacteria such as Escherichia coli to accumulate P(3HB) (Slater et al. 1988).
PhaC catalyzes the polymerization reaction of the hydroxyacyl (HA) moiety in HA-CoA to PHAs, with the concomitant release of CoA (Kawaguchi and Doi 1990; Sudesh et al. 2000; Stubbe and Tian 2003). The polymerization reaction by PhaC proceeds in an aqueous solution at ambient temperature without using a template, organic solvents, or metal cofactors (Ushimaru et al. 2013). In the PhaC enzyme, the cysteine residue in the lipase box-like sequence (Gly-X-Cys-X-Gly) plays a role as the active center for the polymerization reaction (Rehm 2003). PHA depolymerases, on the other hand, which cleave the ester bond of the PHA chain, have a serine residue in the lipase box sequence (Gly-X-Ser-X-Gly) as the active center (Jendrossek and Handrick 2002).
PHA synthases are currently grouped into four classes (classes I–IV) based on subunit composition and substrate specificity; the detailed classification of PHA synthases is reviewed by Rehm (2003). Class I and class II synthases are composed of a single PhaC subunit. The synthase from Ralstonia eutropha (PhaCRe), one of the most well-studied synthases, is grouped as a class I synthase, and it has been reported that the Cys319 in PhaCRe plays the role of an active center (Rehm 2003). The synthase from Delftia acidovorans also belongs to class I and is capable of synthesizing high-molecular-weight P(3HB)s in E. coli (Tsuge et al. 2004; Hiroe et al. 2013). Class III and class IV PhaCs require an additional subunit, PhaE and PhaR, respectively. The class III PhaCAv from Allochromatium vinosum has a catalytic triad consisting of Cys149, Asp302, and His331, in which Cys149 functions as the catalytic center for the PHA polymerization reaction (Jia et al. 2000). Class IV is the most recent classification to be proposed, owing to the discovery of the Bacillus megaterium synthase (McCool and Cannon 1999). We have characterized two additional class IV synthases, from Bacillus cereus YB-4 (PhaRCYB4) and B. megaterium NBRC15308T (PhaRCBm), defining their ability to produce PHA and their substrate specificity (Tomizawa et al. 2010; Mizuno et al. 2010; Tomizawa et al. 2011; Hyakutake et al. 2011).
In our previous studies (Tomizawa et al. 2011; Hyakutake et al. 2014), it was demonstrated that PhaRCYB4 manifests alcoholysis activity towards the P(3HB) polymer chain when the HA-CoA monomer is absent but alcohol is present (Scheme 1). The alcoholysis activity of PhaRCYB4 was confirmed by both in vivo and in vitro analyses (Hyakutake et al. 2014). Similarly, the class IV synthase from Bacillus sp. INT005 is presumed to have alcoholysis activity because of an unusual reduction in P(3HB) molecular weight observed when it was used to synthesize P(3HB) in PHA-accumulating E. coli (Agus et al. 2010). Furthermore, PhaRCBm from B. megaterium also showed weak alcoholysis activity (Hyakutake et al. 2014). These observations suggest that alcoholysis activity is shared in common between class IV synthases. However, little is known about the PhaRC alcoholysis activity itself; the catalytic residues involved and the reaction mechanism remain unknown.
The aim of this study was to identify the amino acid residues involved in the alcoholysis activity of PhaRCYB4. To this end, PhaRCYB4 mutants were constructed by site-directed mutagenesis and subjected to an in vivo assay system to test their alcoholysis activities. The results obtained showed that a common active site of PhaRCYB4 is involved in both PHA polymerization and alcoholysis reactions.
Materials and methods
Bacterial strain, plasmids, and culture media
The bacterial strain and plasmids used in this study are listed in Table 1. E. coli JM109 was used as a host strain for P(3HB) biosynthesis throughout the study. For pre-culturing, the recombinant E. coli were grown in Lysogeny-Broth (LB) medium (10 g/L tryptone, 5 g/L yeast extract, 10 g/L NaCl). For maintenance of the plasmid within the cell, ampicillin (100 mg/L) and/or kanamycin (50 mg/L) was added to the media as appropriate.
Plasmid construction
To construct mutated gene variants of phaC YB4, site-directed point mutagenesis was performed on pGEM-phaRC YB4 AB using the QuikChange Multi Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA). The PCR primers utilized for mutagenesis were as follows: 5′-CTT TAC TTG GTT ATG CCA TGG GGG GAA CGC-3′ for construction of pGEM-phaRC YB4 AB(C151A); 5′-CTT TAC TTG GTT ATA GCA TGG GGG GAA CGC-3′ for pGEM-phaRC YB4 AB(C151S); 5′-TTT CCG GGA AAC GTG CCC ATA TCG CTT TGC-3′ for pGEM-phaRC YB4 AB(D306A); 5′-TTT CCG GGA AAC GTA ACC ATA TCG CTT TGC-3′ for pGEM-phaRC YB4 AB(D306N); 5′-TAT GTT TAC CGA CAG GGG CCA TGT CTA TCG-3′ for pGEM-phaRC YB4 AB(H335A); and 5′-TAT GTT TAC CGA CAG GGA ACA TGT CTA TCG-3′ for pGEM-phaRC YB4 AB(H335N). The sites of introduced mutation sequence are underlined.
Mutagenesis of phaC Re and phaC Bm was carried out in the same manner as above. The PCR primers (5′-AAC GTG CTC GGC TTC AGC GTG GGC GGC ACC-3′and 5′-GTT CTT GGT TAC AGC ATG GGC GGA AC-3′) were utilized for construction of pGEM-phaC Re AB(C319S) and pGEM-phaRC Bm AB(C152S), respectively.
To remove the phaAB Re gene from pGEM-phaRC YB4 AB wild-type plasmid or its variants (C151A/S, D306A/N or H335A/N), the plasmid was digested with SalI and NruI and self-ligated using a Blunting Kination Ligation (BKL) kit (Takara Bio Inc., Otsu, Japan) to yield pGEM-phaRC YB4(wild type, C151A/S, D306A/N or H335A/N). pGEM-phaRC Bm(C152S) was constructed from pGEM-phaRC Bm AB(C152S) by deleting the phaAB Re gene in the same manner. To construct pGEM-phaC Re(C319S), the phaRC YB4 gene in pGEM-phaRC YB4 was replaced with the phaC Re(C319S) gene derived from cleaving the vector pGEM-phaC Re AB(C319S) at the restriction sites Csp45I and Sse8387I. All constructed plasmids were sequenced to confirm the presence and accuracy of the mutation.
Amino acid sequence alignment
Amino acid sequence alignment was performed using ClustalW (version 2.1) on the server at DDBJ (the DNA Data Bank of Japan). The PHA synthase sequences used for alignment were from: B. cereus YB-4 (accession no. BAI68395); B. megaterium NBRC15308T (BAI68396); Bacillus azotoformans LMG9581 (EKN68379); Bacillus sp. INT005 (BAC45232); Bacillus weihenstephanensis KBAB4 (ABY42479); Bacillus cytotoxicus NVH391-98 (ABS21370); A. vinosum DSM180 (BAE20055); and R. eutropha H16 (CAJ92572).
P(3HB) biosynthesis and isolation
Recombinant E. coli JM109 was cultivated in 500 mL shake flasks containing 100 mL LB medium with glucose (20 g/L), on a reciprocal shaker (130 rpm) at 37 °C for 72 h. After cultivation, cells were harvested, washed once with deionized water, and lyophilized. The polymers that had accumulated in the cells were extracted with chloroform for 72 h at room temperature and then purified with methanol.
Ethanol assay
The ethanol concentration in the culture liquid was measured by the enzymatic method using an F-kit (Roche Diagnostics, Basel, Switzerland). Culture liquid was centrifuged at 13,040g for 2 min, and supernatant was diluted as appropriate and used for the assay.
Gas chromatography and gel permeation chromatography
The P(3HB) content of the dried cells was determined by gas chromatography (GC). Samples for GC analysis were prepared from lyophilized cells by methanolysis using 15 % v/v sulfuric acid (Kato et al. 1996).
The number average molecular weight (M n) and the weight average molecular weight (M w) of P(3HB)s synthesized by the recombinant strains were determined by gel permeation chromatography (GPC). GPC measurements were performed at 40 °C, using a Shimadzu 10A GPC system (Shimadzu, Kyoto, Japan) equipped with two Shodex K-806M joint columns (Showa Denko KK, Tokyo, Japan). Chloroform was used as the eluent at a flow rate of 0.8 mL/min. Samples for GPC analysis were prepared at a P(3HB) concentration of 1.0 mg/mL and passed through a 0.45 μm filter. Molecular weights were determined using a standard curve calibrated with low polydispersity polystyrenes (M p = 1.3 × 103–7.5 × 106).
For reference, as determined previously (Kusaka et al. 1998), the relationship between absolute molecular weight M w(Abs) and relative molecular weight M w(GPC) determined by GPC is as follows for P(3HB):
Therefore, the following equation was used to estimate the degree of polymerization (P n) from GPC data:
where PDI(GPC) is the polydispersity index (M w/M n) determined by GPC and 86 is the molecular weight of the 3HB repeating unit.
In vivo cleavage activity assay
E. coli JM109 harboring pJRD-phaC Da AB, which carries the PHA synthase gene (phaC Da) from D. acidovorans and phaAB Re genes from R. eutropha H16, was used as the host strain for the in vivo cleavage activity assay. This strain is capable of producing high-molecular-weight P(3HB) from glucose; the P(3HB) provides a scissile substrate for the synthases under assessment. The cleavage activity was monitored by measuring P(3HB) molecular weight. The number of P(3HB) chains (N) in 1 L culture was calculated as follows (Tomizawa et al. 2013):
where W is the weight (g/L) of P(3HB) synthesized by the recombinant strain. The relative P(3HB) chain number is defined as the number of chains in the strain expressing PhaCDa and the assayed synthase (PhaRC or PhaC), divided by that of the strain expressing PhaCDa alone (N ref):
NMR analysis
The end-group structures of the isolated P(3HB) samples were analyzed by NMR spectroscopy. Methanol-purified polymers were dissolved in chloroform, further purified with hexane three times and dried completely. Each purified polymer (30 mg) was dissolved in CDCl3 (0.7 mL) and subjected to 1H-nuclear magnetic resonance (NMR) analysis. NMR spectra were recorded using a JEOL LA500 spectrometer (JEOL Co. Ltd., Tokyo, Japan). For 1H-NMR analysis, data were collected at 45 °C with a 7.2-ms pulse width (90° pulse angle), a 5-s pulse repetition, a 6000-Hz spectra width, and 16 K data points. Tetramethylsilane (Me4Si) was used as an internal standard for calculating chemical shift.
Results
Putative catalytic triad for the polymerization reaction in class IV synthases
Alcoholysis activity has been reported for certain lipases that have a lipase-box sequence (Gly-X-Ser-X-Gly) in their active site; in these, the central serine acts as a catalytic center (Gupta et al. 2004). In PhaRCYB4, the PhaCYB4 subunit is responsible for the alcoholysis reaction (Tomizawa et al. 2011); however, it does not have lipase-box sequence. We presumed that the lipase box-like sequence (Gly-X-Cys-X-Gly), which is generally responsible for the polymerization reaction of PhaC, is also utilized for the alcoholysis reaction. Catalytic residues for the polymerization reaction remain unidentified in class IV synthases; thus, our first aim was to identify these.
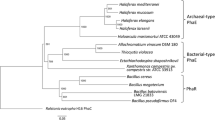
Figure 1 shows the amino acid sequence alignment of class IV synthases from six Bacillus strains, together with class III synthases from A. vinosum (PhaCAv), which has a catalytic triad consisting of Cys149, Asp302, and His331 (Jia et al. 2000). Cys149 in PhaCAv, which acts as a nucleophile, corresponds to Cys151 in PhaCYB4. Asp302 and His331 in PhaCAv, which act as the catalytic acid and base, correspond to Asp306 and His335 in PhaCYB4, respectively. These three residues also correspond to catalytic residues in PhaCRe, Cys319, Asp480, and His508. Therefore, Cys151, Asp306, and His335 in PhaCYB4 were thought to form the catalytic triad on the basis of homology.
ClustalW sequence alignment of PHA synthase (PhaC) active domains from B. cereus YB-4, other class IV synthases, synthases from Allochromatium vinosum (class III), and Ralstonia eutropha (class I). The accession numbers of sequences used for this alignment are provided in the “Materials and methods” section. Residues conserved across all eight synthases are highlighted and marked with an asterisk. The lipase box-like sequence is indicated with an open box. Amino acid residues shown in bold are putative amino acid residues for polymerization activity and were substituted to other residues in this study
Mutagenesis and the polymerization activity assay
Alanine mutagenesis of the putative catalytic triad (Cys151, Asp306, and His335) in PhaCYB4 was performed to identify active site residues for the polymerization reaction. The three PhaRCYB4 mutants with individually substituted sites (PhaC_C151A, D306A, and H335A) were expressed in E. coli, together with PhaABRe, to examine their abilities to produce P(3HB). None of the strains expressing PhaRCYB4 mutants accumulated any measurable P(3HB) (data not shown), suggesting that the catalytic function of PhaRCYB4 was significantly impaired by alanine mutagenesis. To exclude the possibility of a higher-order structural change of PhaCYB4 concomitant with alanine mutagenesis, other mutants in which the triad residues were replaced with structurally similar amino acids were generated. The PhaRCYB4 mutants with PhaC_C151S, D306N, or H335N mutations were also examined for their abilities to produce P(3HB) in E. coli, with the result that these mutants were also inactive for PHA polymerization (data not shown). These observations strongly suggest that Cys151, Asp306, and His335 in PhaCYB4 form a catalytic triad for the polymerization reaction.
Cleavage activity assay of C151A/S mutants
To test the hypothesis that a common active site of PhaCYB4 is involved in both polymerization and alcoholysis reactions, PhaRCYB4 mutants with a PhaC_C151A/S mutation, which are inactive for polymerization, were subjected to the in vivo cleavage activity assay using E. coli JM109/pJRD-phaC Da AB as the host strain. This assay method was based on the molecular weight change of P(3HB) that was provided by the use of D. acidovorans PHA synthase (PhaCDa) as the scissile substrate, due to alcoholysis of the P(3HB) chain by PhaRCYB4 using host-produced ethanol. Time-dependent changes in P(3HB) concentration and molecular weight during the in vivo assay are shown in Fig. 2, and the results are tabulated in Table 2.
PhaCDa is capable of synthesizing high molecular weight P(3HB) (M n = 1440 × 103) in E. coli JM109, while co-expression of PhaRCYB4 (wild type) led to significant reduction of P(3HB) molecular weight (M n = 14 × 103) due to alcoholysis activity as reported previously (Hyakutake et al. 2014). Such alcoholysis would be induced by host-produced ethanol, which was present in our system at 500 mg/L in the culture medium after 24 h of cultivation and gradually decreased to 300 mg/L by 72 h due to assimilation by E. coli and/or evaporation from the medium. The molecular weight of P(3HB) (M n = 1030 × 103) produced by the PhaRCYB(C151A)-expressing strain was almost the same as that from PhaCDa alone. As ethanol was present at 380 mg/L (24 h) in the culture medium, which seemed to be sufficient to promote alcoholysis induction, this result suggested that the C151A mutation caused loss of alcoholysis activity.
On the other hand, the co-expression of PhaRCYB4(C151S) led to a significant reduction of P(3HB) molecular weight (M n = 21 × 103), unlike the C151A mutant but similar to wild type. C151S and C151A mutants are both polymerization inactive; however, their behaviors with respect to cleavage activity were not the same. As shown in Fig. 2, the amount of P(3HB) from both mutants was almost unchanged from 24 to 72 h of cultivation while the P(3HB) molecular weight markedly decreased for C151S. Figure 3 shows the molecular weight distribution of P(3HB) isolated from the PhaRCYB4(C151S)-expressing strain. The high-molecular-weight fraction of the polymer was decreased with increasing culture time, while the low-molecular-weight fraction was increased. These observations suggest that the high-molecular-weight P(3HB) chains were cleaved by the action of PhaRCYB4(C151S). Without the PhaRYB4 subunit, the C151S mutant did not express any cleavage activity (Table 2).
Cleavage activity assay of other synthase mutants
Because PhaRCYB4(C151S) unexpectedly showed cleavage activity towards the P(3HB) chain, we further examined whether other synthase mutants with serine substitution of the cysteine at the active center express the cleavage activity. To this end, mutants of synthases belonging to class I [PhaCRe(C319S)] and class IV [PhaRCBm(C152S)] were constructed from R. eutropha and B. megaterium synthases, respectively. It is noteworthy that both Cys319 in PhaCRe and Cys152 in PhaCBm correspond to Cys151 in PhaCYB4 in multiple sequence alignments (Fig. 1). The resulting synthase mutants were confirmed to be polymerization inactive in recombinant E. coli JM109 (data not shown). These mutants were next subjected to the in vivo assay in the same manner as described above. The results are listed in Table 2. PhaCRe(C319S) did not show any cleavage activity towards the P(3HB) chain. On the other hand, PhaRCBm(C152S) showed significant cleavage activity, as did PhaRCYB4(C151S). Thus, the cleavage activity was commonly observed in serine-substituted mutants belonging to class IV synthases.
End structure analysis of low-molecular-weight P(3HB)
Our previous study showed that the P(3HB) carboxy terminus was capped by ethanol as a consequence of PhaRCYB4-catalyzed alcoholysis. 1H-NMR analysis was carried out to identify the terminal structure on low-molecular-weight P(3HB) produced by strains co-expressing PhaCDa and PhaRCYB4(C151S). As shown in Fig. 4, the quartet peak at 4.15 ppm (i) assigned to the methylene of the ethanol-capped carboxy terminus was observed. This analysis also suggested the existence of the ethanol-capped carboxy terminus together with a minor carboxy terminus capped with longer chain alcohols than ethanol (i*). These two peaks were also observed for the P(3HB) produced by the strain co-expressing PhaCDa together with PhaRCYB4; therefore, the PhaRCYB4(C151S) mutant proved to have alcoholysis activity.
A 500-MHz 1H-NMR spectra of P(3HB) isolated from 72-h cultures of E. coli JM109 expressing PhaCDa, and PhaRCYB4 wild type (Hyakutake et al. 2014) or PhaRCYB4(C151S). i*, the methylene resonance for alcohols longer than ethanol
However, as listed in Table 3, the molar ratio of the alcohol-capped carboxy terminus to the hydroxy terminus, estimated from the peak intensities of the signals i plus i* and the signal bH, was 0.62, which value is significantly lower than that of PhaRCYB4 alone (1.07) or PhaCDa plus PhaRCYB4 (1.03). These results implied that PhaRCYB4(C151S) catalyzes not only alcoholysis but also hydrolysis reactions, unlike wild-type PhaRCYB4. Hydrolysis of the P(3HB) chain generates free carboxy and hydroxy termini, but the free carboxy terminus is undetectable by 1H-NMR analysis. Therefore, the ratio of the alcohol-capped carboxy terminus to the hydroxy terminus is an important clue for supporting the occurrence of hydrolysis reactions. These results are further supported by the good agreement observed between the degrees of polymerization obtained by NMR analysis, with those determined by GPC (Table 3).
Similarly, the PhaRCBm(C152S) mutant was suggested to have both alcoholysis and hydrolysis activities because an ethyl-esterified carboxy terminus was observed; however, the molar ratio of the alcohol-capped carboxy termini to the hydroxy terminus was significantly lower than 1 (Table 3). From these observations, we propose that the hydrolysis activities of PhaRCYB4 and PhaRCBm might be elicited by serine substitution.
Cleavage activity assay of D306A/N and H335A/N mutants
To investigate whether catalytic resides other than cysteine are involved in cleavage activity, PhaRCYB4(D306A/N) and PhaRCYB4(H335A/N) were subjected to the in vivo cleavage activity assay. The results are also listed in Table 2. The molecular weight (M n) of P(3HB) isolated from the strains co-expressing PhaCDa and a PhaRCYB4 mutant (D306A/N or H335A/N) was in the range of (1190–1520) × 103. These values were as high as that of the strain expressing PhaCDa alone; therefore, the introduced mutations suppressed the P(3HB) molecular weight decrease due to loss of cleavage activity. From these results, it was indicated that Asp306 and His335 also play an important role in alcoholysis reaction by PhaRCYB4.
Discussion
A variety of PHA synthases are widespread in bacteria and archaea. PHA-producing Bacillus strains possess the class IV synthase (PhaRC), which is the most recent classification noted among PHA synthases (Rehm 2003). In previous studies, it was found that the molecular weight of P(3HB) synthesized by PhaRCYB4 decreased with increasing culture time when using E. coli as a host for P(3HB) production (Tomizawa et al. 2011). As there are no genes for P(3HB) polymerization and depolymerization in the E. coli genome, it was thought that the molecular weight decrease was caused by the exogenous PhaRCYB4. Further study revealed that the molecular weight decrease was caused by PhaRCYB4 via an alcoholysis reaction using host-produced ethanol (Hyakutake et al. 2014). However, little is known about the alcoholysis activity of PhaRCYB4 itself. In this study, we attempted to identity the catalytic residues involved in the alcoholysis activity of PhaRCYB4 and investigated the reaction mechanism as well.
PhaCYB4 has a lipase box-like sequence (Gly-X-Cys151-X-Gly), in which Cys151 is an active center for polymerization as demonstrated in this study. The in vivo cleavage activity assay revealed that the cleavage activity of PhaRCYB4(C151A) was significantly suppressed together with the polymerization activity, indicating that Cys151 plays an important role not only in polymerization but also in P(3HB) chain scission. Another polymerization-inactive mutant, PhaRCYB4(C151S), was also tested using the in vivo assay, and cleavage activity was unexpectedly detected. This may be because the introduced serine residue (Ser151) functions as a nucleophile, as in the case of lipases and other serine hydrolases (Hide et al. 1992; Ekici et al. 2008). In this instance, the substituted serine may act in place of the cysteine as the catalytic residue for the P(3HB) scission reaction. PhaRCYB4(C151S) is able to catalyze alcoholysis, as confirmed by NMR analysis, but may acquire hydrolysis activity as well since the molar ratio of the alcohol-capped terminus to the hydroxy terminus was significantly decreased (Table 3).
Similar observation has been obtained from studies with β-peptidyl aminopeptidase from Pseudomonas aeruginosa PAO1 (Arima et al. 2014). This enzyme has serine as the catalytic center and possesses both aminolysis and hydrolysis activities. However, by replacement of the active site serine with cysteine, this enzyme lost hydrolysis activity but still maintained aminolysis activity. This demonstrates an interesting example of change in an acyl acceptor for enzymatic acylation reactions by serine/cysteine switching.
The cysteine residue in the lipase box-like sequence (Gly-X-Cys-X-Gly) of PHA synthases, together with the histidine and aspartic acid residues, forms a catalytic triad for the polymerization reaction (Stubbe and Tian 2003; Rehm 2003). The function of these residues is proposed as follows (Stubbe and Tian 2003): in the P(3HB) elongation reaction mediated by synthase, the histidine residue activates cysteine for a nucleophilic attack on (R)-3HB-CoA monomer, forming a thiol ester bond (Cys-3HB). Another cysteine (Cys*), in another PhaC (PhaC*), has already been acylated by the growing P(3HB) chain [Cys*-(3HB)n]. The hydroxy group of Cys-3HB is activated by aspartic acid, and then a nucleophilic attack on the carbonyl carbon of the thiol ester in the Cys*-(3HB)n results in elongation by a monomer unit [Cys-(3HB)n+1]. After the elongation reaction repeats, the aspartic acid at the active site activates a hydroxy compound (chain transfer agent) and facilitates deacylation of the PhaC. This deacylation process is called a chain transfer reaction (Kawaguchi and Doi 1992). Another elongation model has also been proposed, in which cysteine contributes to the polymerization reaction, whereupon the P(3HB) chain instantly disengages from the residue (Stubbe and Tian 2003).
Based on the functional role of the catalytic residues in the elongation and chain transfer reactions, a molecular mechanism for P(3HB) alcoholysis reaction by PhaRCYB4 is proposed as illustrated in Fig. 5. First, Cys151 is ready for a nucleophilic attack by forming a hydrogen bond with the adjacent His335. Asp306 makes hydrogen bond with His335 and fixes the orientation of the imidazole ring of His335 to enhance the nucleophilicity of the thiol group of Cys151. Based on the 3D structure prediction, Tariq et al. also proposed that Asp306 and His335 in PhaC from B. cereus FA11, corresponding to Asp306 and His335 in PhaCYB4, form hydrogen bond (Tariq et al. 2014). When a P(3HB) chain is bound to the substrate-binding pocket of PhaRCYB4, a nucleophilic attack by Cys151 on the carbonyl carbon involved in the scissile bond results in the formation of a covalent acyl-enzyme intermediate, and the release of an initial product. The Cys151-3HB bond is subsequently cleaved by a hydroxy compound that is activated by a basic residue (shown as “B” in Fig. 5) resulting in the deacylation of the enzyme. Asp306 could be considered as one of the possible basic residues, because it has been proposed that the Asp302 residue in A. vinosum synthase, corresponding to Asp306 in PhaCYB4, directly activate a hydroxy group of a chain transfer agent (Jia et al. 2001). His335 is another possibility of the basic residue, because it was reported that His forming a hydrogen bond with Asp in the PHA depolymerases (Hisano et al. 2006) and cysteine/serine proteases (Ekici et al. 2008), corresponding to His335 and Asp306 in PhaCYB4, activate a hydroxy group of a substrate such as water molecule. Cys151 plays the most important role in the alcoholysis reaction, but it can be substituted with serine, as demonstrated in this study. On the other hand, alcoholytic cleavage was not observed in serine-substituted PhaCRe which has the same three conserved residues (Fig. 1 and Table 2). There might be other key residues involved in the alcoholysis reaction by PhaRCYB4.
In conclusion, the present study identified the amino acid residues responsible for the alcoholysis activity of PhaRCYB4. It was found that Cys151, Asp306, and His335 in PhaCYB4 are involved in not only PHA polymerization but also alcoholysis activities. In particular, Cys151, which acts as an active center for polymerization activity, was also suggested to be an active center for alcoholysis activity. Furthermore, the serine mutants PhaRCYB4(C151S) and PhaRCBm(C152S) were thought to have hydrolysis activity along with alcoholysis activity towards P(3HB) chains. This study provides new insight into the mechanisms of the alcoholysis reaction catalyzed by PhaRCs.
References
Agus J, Kahar P, Hyakutake M, Tomizawa S, Abe H, Tsuge T, Satoh Y, Tajima K (2010) Unusual change in molecular weight of polyhydroxyalkanoate (PHA) during cultivation of PHA-accumulating Escherichia coli. Polym Degrad Stab 95:2250–2254
Arima J, Tanaka A, Morimoto M, Mori N (2014) Mutation of active site serine residue with cysteine displays change in acyl-acceptor preference of β-peptidyl aminopeptidase from Pseudomonas aeruginosa PAO1. Appl Microbiol Biotechnol 98:1631–1640
Ekici ÖD, Paetzel M, Dalbey RE (2008) Unconventional serine proteases: variations on the catalytic Ser/His/Asp triad configuration. Protein Sci 17:2023–2037
Gupta R, Gupta N, Rathi P (2004) Bacterial lipases: an overview of production, purification and biochemical properties. Appl Microbiol Biotechnol 64:763–781
Hide WA, Chan L, Li WH (1992) Structure and evolution of the lipase superfamily. J Lipid Res 33:167–178
Hiroe A, Ushimaru K, Tsuge T (2013) Characterization of polyhydroxyalkanoate (PHA) synthase derived from Delftia acidovorans DS-17 and the influence of PHA production in Escherichia coli. J Biosci Bioeng 115:633–638
Hisano T, Kasuya K, Tezuka Y, Ishii N, Kobayashi T, Shiraki M, Oroudjevd E, Hansmad H, Iwata T, Doi Y, Saito T, Miki K (2006) The crystal structure of polyhydroxybutyrate depolymerase from Penicillium funiculosum provides insights into the recognition and degradation of biopolyesters. J Mol Biol 356:993–1004
Hyakutake M, Saito Y, Tomizawa S, Mizuno K, Tsuge T (2011) Polyhydroxyalkanoate (PHA) synthesis by class IV PHA synthases employing Ralstonia eutropha PHB−4 as host strain. Biosci Biotechnol Biochem 75:1615–1617
Hyakutake M, Tomizawa S, Mizuno K, Abe H, Tsuge T (2014) Alcoholytic cleavage of polyhydroxyalkanoate chains by class IV synthases induced by endogenous and exogenous ethanol. Appl Environ Microbiol 80:1421–1429
Jendrossek D, Handrick R (2002) Microbial degradation of polyhydroxyalkanoates. Annu Rev Microbiol 56:403–432
Jia Y, Kappock TJ, Frick T, Sinskey AJ, Stubbe JA (2000) Lipases provide a new mechanistic model for polyhydroxybutyrate (PHB) synthases: characterization of the functional residues in Chromatium vinosum PHB synthase. Biochem 39:3927–3936
Jia Y, Yuan W, Wodzinska J, Park C, Sinskey AJ, Stubbe JA (2001) Mechanistic studies on class I polyhydroxybutyrate (PHB) synthase from Ralstonia eutropha: class I and III synthases share a similar catalytic mechanism. Biochem 40:1011–1019
Kato M, Bao HJ, Kang CK, Fukui T, Doi Y (1996) Production of a novel copolyester of 3-hydroxybutyric acid and medium-chain-length 3-hydroxyalkanoic acids by Pseudomonas sp. 61-3 from sugars. Appl Microbiol Biotechnol 45:363–370
Kawaguchi Y, Doi Y (1990) Structure of native poly(3-hydroxybutyrate) granules characterized by X-ray diffraction. FEMS Microbiol Lett 70:151–155
Kawaguchi Y, Doi Y (1992) Kinetics and mechanism of synthesis and degradation of poly(3-hydroxybutyrate) in Alcaligenes eutrophus. Macromol 25:2324–2329
Kusaka S, Iwata T, Doi Y (1998) Microbial synthesis and physical properties of ultra-high-molecular-weight poly[(R)-3-Hydroxybutyrate]. Macromol Sci Part A 35:319–335
Matsusaki H, Abe H, Taguchi K, Fukui T, Doi Y (2000) Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant bacteria expressing the PHA synthase gene phaC1 from Pseudomonas sp. 61-3. Appl Microbiol Biotechnol 53:401–409
McCool GJ, Cannon MC (1999) Polyhydroxyalkanoate inclusion body-associated proteins and coding region in Bacillus megaterium. J Bacteriol 181:585–592
Mizuno K, Ohta A, Hyakutake M, Ichinomiya Y, Tsuge T (2010) Isolation of polyhydroxyalkanoate-producing bacteria from a polluted soil and characterization of the isolated strain Bacillus cereus YB-4. Polym Degrad Stab 95:1335–1339
Rehm BH (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Slater SC, Voige WH, Dennis DE (1988) Cloning and expression in Escherichia coli of the Alcaligenes eutrophus H16 poly-β-hydroxybutyrate biosynthetic pathway. J Bacteriol 170:4431–4436
Stubbe J, Tian J (2003) Polyhydroxyalkanoate (PHA) homeostasis: the role of the PHA synthase. Nat Prod Rep 20:445–457
Sudesh K, Abe H, Doi Y (2000) Synthesis, structure and properties of polyhydroxyalkanoates: biological polyesters. Prog Polym Sci 25:1053–1555
Tariq A, Hameed A, Bokhari H, Masood F (2014) Is atomic rearrangement of type IV PHA synthases responsible for increased PHA production? J Biomol Struc Dynam
Tomizawa S, Saito Y, Hyakutake M, Nakamura Y, Abe H, Tsuge T (2010) Chain transfer reaction catalyzed by various polyhydroxyalkanoate synthases with poly(ethylene glycol) as an exogenous chain transfer agent. Appl Microbiol Biotechnol 87:1427–1435
Tomizawa S, Hyakutake M, Saito Y, Agus J, Mizuno K, Abe H, Tsuge T (2011) Molecular weight change of polyhydroxyalkanoate (PHA) caused by the PhaC subunit of PHA synthase from Bacillus cereus YB-4 in recombinant Escherichia coli. Biomacromolecules 12:2660–2666
Tomizawa S, Sato S, Lan JCW, Nakamura Y, Abe H, Tsuge T (2013) In vitro evidence of chain transfer to tetraethylene glycols in enzymatic polymerization of polyhydroxyalkanoate. Appl Microbiol Biotechnol 97:4821–4829
Tsuge T, Imazu S, Takase K, Taguchi S, Doi Y (2004) An extra large insertion in the polyhydroxyalkanoate synthase from Delftia acidovorans DS-17: its deletion effects and relation to cellular proteolysis. FEMS Microbiol Lett 231:77–83
Ushimaru K, Sangiambut S, Thomson N, Sivaniah E, Tsuge T (2013) New insights into activation and substrate recognition of polyhydroxyalkanoate synthase from Ralstonia eutropha. Appl Microbiol Biotechnol 97:1175–1182
Acknowledgments
We thank Dr. Y. Nakamura (Tokyo Institute of Technology) for NMR analysis. This work was supported by a Grant-in-Aid for Scientific Research (KAKENHI 23310060) to T. Tsuge. M. Hyakutake was a recipient of a JSPS Young Scientist Fellowship (12J07940).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hyakutake, M., Tomizawa, S., Mizuno, K. et al. A common active site of polyhydroxyalkanoate synthase from Bacillus cereus YB-4 is involved in polymerization and alcoholysis reactions. Appl Microbiol Biotechnol 99, 4701–4711 (2015). https://doi.org/10.1007/s00253-014-6276-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6276-4