Abstract
In these studies, butanol (acetone butanol ethanol or ABE) was produced from concentrated lactose/whey permeate containing 211 g L−1 lactose. Fermentation of such a highly concentrated lactose solution was possible due to simultaneous product removal using a pervaporation membrane. In this system, a productivity of 0.43 g L−1 h−1 was obtained which is 307 % of that achieved in a non-product removal batch reactor (0.14 g L−1 h−1) where approximately 60 g L−1 whey permeate lactose was fermented. The productivity obtained in this system is much higher than that achieved in other product removal systems (perstraction 0.21 g L−1 h−1 and gas stripping 0.32 g L−1 h−1). This membrane was also used to concentrate butanol from approximately 2.50 g L−1 in the reactor to 755 g L−1. Using this membrane, ABE selectivities and fluxes of 24.4–44.3 and 0.57–4.05 g m−2 h−1 were obtained, respectively. Pervaporation restricts removal of water from the reaction mixture thus requiring significantly less energy for product recovery when compared to gas stripping.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In order to achieve sustainable production of biofuels, it is essential that they be produced from renewable and economically available feedstocks/or substrates including agricultural residues (straws, stover, bagasse, grasses, leaves, and hay) and industrial and municipal wastes. The use of industrial by-products and municipal wastes not only would reduce cost of production of biofuels considerably but also would reduce environmental pollution. One such valuable industrial by-product is whey permeate (a dairy industry by-product). It is produced in significant amounts in countries or states such as New Zealand, Denmark, and the USA (in California, Minnesota, and Wisconsin states). In previous decades, whey permeate used to be disposed in water streams or sprayed on farms which caused environmental pollution. Whey permeate contains approximately 45–50 g L−1 lactose that can be converted to ethanol and/or butanol. Although this lactose concentration is low for ethanol-producing microorganisms, it is ideal for butanol batch fermentation. However, if desired, lactose concentration can be increased using reverse osmosis. Production of ethanol from whey permeate is an established technology in New Zealand (Mawson 1987; Wongso 1993; Domingues et al. 2001).
Butanol is superior to ethanol as a biofuel and contains about 30 % more energy. Butanol production from renewable biomass (Jones and Woods 1986; Dürre 1998; Zverlov et al. 2006) and other useful industrial by-products such as whey permeate is plagued by problems such as its toxicity to the microbial cultures that produce it. Due to product toxicity, butanol concentration in the fermentation broth in excess of 13–20 g L−1 is rarely achieved which results in low productivity in batch reactors and high product removal costs. This also necessitates the utilization of dilute sugar solution thus increasing process volumes and affecting process economics adversely. In order to address toxicity problem, a number of novel product recovery techniques have been developed including gas stripping (Maddox 1989; Groot et al. 1992), pervaporation (Maddox 1989; Groot et al. 1992; Friedl et al. 1991; Qureshi et al. 1999), adsorption (Qureshi et al. 2005), and vacuum fermentation (Mariano et al. 2011). In situ use of these techniques results in the production of significantly more butanol or ABE as compared to the batch process. Also, simultaneous removal of butanol results in increased utilization of acids such as acetic and butyric (reaction intermediates for this fermentation) which improves butanol or ABE yield.
Production of a low-value high-volume product including biofuel butanol requires the use of existing industrial fermentation technologies such as batch processes. In batch butanol production, approximately 50–60 g L−1 of sugars are used in feed of which approximately 5 g L−1 is left unused due to butanol toxicity as the culture cannot tolerate greater than 20 g L−1 ABE of which 13–15 g L−1 is butanol. In a couple of fermentation-product removal studies, we were able to use concentrated lactose/whey permeate to produce ABE (Maddox et al. 1995; Qureshi and Maddox 2005); however, in these systems, low reactor productivities were obtained. Another disadvantage of these systems was that the product could not be concentrated easily. The objectives of the present studies were to produce ABE from concentrated whey permeate/lactose solution and recover and concentrate these using a pervaporation membrane. Another objective was to compare the performance of the other reactor systems studied previously (Maddox et al. 1995; Qureshi and Maddox 2005). The substrate of choice was whey permeate as it is a commercial dairy industry by-product and requires less upstream processing.
Materials and methods
Organism and media
Clostridium saccharobutylicum P262 (formerly C. acetobutylicum P262: American Type Culture Collection Number ATCC® BAA-117™ and German Collection of Microorganisms and Cell Cultures Number DSMZ 13864) was a gift from Professor David Woods (University of Cape Town, South Africa). Since 1985, when the strain was obtained by one of us (ISM), this strain has been used in a number of laboratories around the globe (Ennis and Maddox 1985; Parekh et al. 1988; Ezeji and Blaschek 2008). Spores of the culture were maintained in sterile distilled water at 4 °C until needed. Cooked meat medium (2.5 g) (CMM; Difco Laboratories, Detroit, MI, USA) and lactose (0.25 g) were transferred into a 35-mL screw-capped bottle containing 25 mL distilled water. The CMM was soaked for 15 min before autoclaving the medium at 121 °C for 15 min. Once autoclaved, the bottle was placed in a 75 °C water bath until bottle temperature had decreased to 75 °C. At that time, 100 μL spores of C. sacharobutylicum P262 were transferred to the bottle aseptically. Then, the bottle was allowed to stand in the water bath for 2 min followed by cooling it in ice-cold water for 1 min. After cooling, the bottle containing CMM and spores was incubated at 35–37 °C for 14–16 h in an anaerobic jar. After 14–16 h of spore germination and cell growth, highly motile cells were observed under a microscope. This culture (stage I inoculum) was ready for inoculation in a 100-mL medium (contained in a 125-mL screw-capped bottle) containing 20–30 g L−1 lactose/or whey permeate and 5 g L−1 yeast extract (Difco Laboratories). Five to seven milliliters of stage I culture was inoculated into a 100-mL bottle. The inoculated bottle was allowed to incubate at 36 ± 1 °C for 12–16 h. This was called stage II inoculum and was used to inoculate a (70 mL culture) bioreactor containing 1.0 L spray-dried sulfuric acid casein whey permeate medium.
Spores of C. saccharobutylicum can tolerate temperatures up to 75 °C for 2 min prior to germination. Spore survival at a higher temperature (80 °C) and longer period of time (up to 10 min) is strain specific and has been demonstrated for C. beijerinckii BA101 (Ezeji et al. 2004).
Bioreactor, membrane, and the cooling system
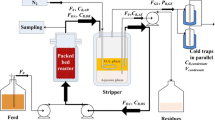
Spray-dried sulfuric acid casein whey permeate was obtained from the New Zealand Dairy Research Institute (Palmerston North, New Zealand). The whey permeate contained approximately 84 % (w/w) lactose. For control experiment, 60 g L−1 whey permeate lactose (approximately 70 g L−1 whey permeate) and 5 g L−1 yeast extract were combined followed by autoclaving the bioreactor (New Brunswick, New Jersey, USA) at 121 °C for 15 min. After autoclaving, the bioreactor was cooled to 35 °C under oxygen-free nitrogen gas environment by sweeping gas across the surface of the medium. This 2.0-L reactor contained 1.0 L medium, and the bioreactor was inoculated with 70 mL stage II culture. The culture was allowed to grow and produce ABE at 35 °C, and 2-mL samples were taken to measure ABE and lactose.
Following the above experiment, another fermentation was run in which 70 g L−1 whey permeate, 152 g L−1 lactose, and 5 g L−1 yeast extract were mixed in a 2-L bioreactor (New Brunswick) and the medium was autoclaved at 121 °C for 15 min followed by cooling the medium under oxygen-free nitrogen gas. This reactor was inoculated with 70 mL stage II culture, and fermentation was allowed at 35 °C and pH 5.0. Sweeping nitrogen gas was continued to keep it anaerobic until the culture started producing its own gases. In order to control pH, 50 % (v/v) ammonia solution was used. From the bioreactor, ABE was recovered by pervaporation using a silicone membrane which was previously used to recover ABE by perstraction (Qureshi and Maddox 2005). The membrane’s surface area based on the internal diameter was 0.21 m2. A peristaltic pump was used to circulate fermentation broth through the pervaporation membrane at a flow rate of 30.6 L h−1. The diffused ABE from the membrane surface was swept using nitrogen gas at a recycle rate of 40 to 42 L h−1 and condensed in a condenser (Qureshi et al. 1992). The sweep gas was recycled using a gas recycle pump (Gast MFG Corp, Benton Harbor, MI, USA) and a flow measuring device (Electronic Flo-meters Ltd., Hounslow, England). Ethylene glycol (25–30 % v/v in water) was circulated through the condenser at a flow rate of 4.0 L min−1 to condense vapors, and the coolant temperature was controlled at −2.0 to −2.5 °C. The cooling/chilling machine was obtained from Grant Instruments Ltd. (Barrington, Cambridge, England). More details on the recovery of ABE from fermentation broth by pervaporation have been provided elsewhere (Qureshi et al. 1992). To concentrate ABE to 755 g L−1 (organic phase), the same membrane and cooling system was used. During ABE concentration, reactor temperature was maintained at 35 °C and lost water through the membrane was replaced by adding distilled water to keep liquid level constant in the reactor.
Continuous production of ABE in free cell reactors
To improve productivity, ABE was produced in a suspended cell continuous reactor (New Brunswick) with total and working volumes 2000 and 1300 mL, respectively. The reactor containing medium (60 g L−1 whey permeate and 5 g L−1 yeast extract) was autoclaved at 121 °C for 15 min followed by cooling under oxygen-free nitrogen gas to keep anaerobic as mentioned above. Then, the reactor was inoculated with 65 mL stage II inoculum, and cell growth and fermentation was allowed at 35 °C in a batch mode for 95 h before starting with continuous feed and broth removal at a flow rate 36 mL h−1. After a steady state was reached, the dilution rate was changed. At each dilution rate, samples were collected and analyzed for ABE and residual lactose.
Attempts were also made to produce ABE in two series reactors. The capacity of the first reactor was 2000 mL with a working volume of 1300 mL. The second reactor’s capacity was 5000 mL with a working volume of 2000 mL. Both the reactors were started as described above, and batch fermentation was continued for 95 h followed by feeding the first reactor at a flow rate of 65 mL h−1. The effluent from the first reactor was fed to the second reactor. The rate of broth removal from the second reactor was 65 mL h−1. The dilution rate of the first reactor was 0.05 h−1 and of the second reactor, it was 0.033 h−1. The two series reactors were operated at two feed flow rates including 65 and 40 mL h−1. The feed medium for the series reactors was the same as for the first reactor.
Analyses
ABE, acetic acid, and butyric acid were determined using gas chromatography (Model GC-8A; Shimadzu Corporation, Kyoto, Japan) using a flame ionization detector and a column of Porapak Q. Lactose was measured using high-performance liquid chromatography (HPLC) as described by Ennis and Maddox (1985). Reactor productivity was determined as the total ABE produced in grams per liter divided by the fermentation time. Fermentation time was the time period between inoculation and time when fermentation ceased and was noted by no increase in ABE concentration. ABE yield was calculated as total ABE produced in grams per liter divided by total lactose utilized (g L−1). ABE selectivity α was calculated by the following relationship [{y / (1 − y)} / {x / (1 − x)}], where x is the weight fraction of ABE in retentate and y is the weight fraction of ABE in condensate. ABE flux was calculated as the amount of ABE diffused through the membrane in grams divided by membrane area (m2) and amount of time during which diffusion occurred (h). Total flux was calculated as the amount of ABE plus water (g) diffused through the membrane per square meter membrane area per hour. Cell concentration was measured by a relationship between optical density (at λ = 540 nm) and dry weight cell concentration. For the optical density and dry weight cell concentration relationship, a graph was plotted between these two parameters. Dry cell weight was measured after drying at 100 °C until constant weight was obtained. Where applicable, specific productivity (h−1) was measured by dividing productivity with dry weight cell concentration. For continuous fermentations, productivity was measured as ABE concentration (g L−1) multiplied by dilution rate (h−1). Dilution rate was defined as feed flow rate (mL h−1) divided by liquid volume in the reactor. The overall dilution rate of the two reactors was calculated by dividing feed flow rate with the total working volume (3300 mL). The results presented here are an average of two replicates with error margins of ±3.0–8.5 %.
Results
Control batch reactor
In order to compare results of the integrated process, a batch reactor was operated with 61.3 g L−1 whey permeate lactose (approximately 70 g L−1 whey permeate) concentration. In this system, over the course of 92 h, 12.43 g L−1 ABE was produced, resulting in a productivity of 0.14 g L−1 h−1. The concentrations of individual ABE components were acetone 2.78 g L−1, butanol 9.26 g L−1, and ethanol 0.39 g L−1. In the reactor, 3.73 g L−1 acetic acid and 3.69 g L−1 butyric acid were also produced. The culture used 40.3 g L−1 lactose with a yield of 0.31. The fermentation profile of this batch reactor is presented in Fig. 1a. The culture was very active and within 8 h of inoculation, it initiated producing ABE vigorously. The highest cell concentration was 2.08 g L−1 at 52 h, after which it started declining (Fig. 1b). Based on the maximum cell concentration in the reactor, a specific productivity of 0.07 h−1 was calculated.
Concentrated lactose fermentation with simultaneous product removal
Next, an experiment with 211 g L−1 initial lactose was started. At such a high lactose concentration, the culture showed a slow fermentation as compared to the control reactor. At 22 h, 2.57 g L−1 ABE accumulated and at this time, product recovery by pervaporation was initiated (Fig. 2a). As a result of product (butanol) recovery, butanol concentration throughout the run remained below 3.00 g L−1. The highest concentration of butanol was 2.55 g L−1 at 128 h. Between 100 and 185 h, lactose utilization was fast and the culture was able to utilize all the lactose present in the system. It was noted that acetone concentration kept increasing in the reactor until 145 h when it was 3.50 g L−1 which decreased to 2.10 g L−1 at 170 h and 1.83 g L−1 at 185 h. The possible reasons for the accumulation of acetone were its faster production and slower removal as its recovery/removal selectivity was much lower (discussed later) than butanol. Ethanol concentration in the reactor remained low at 0.06–0.54 g L−1. Acetic acid and butyric acid concentrations also remained low due to their conversion into acetone and butanol. At the end of fermentation, total acetic acid and butyric acid concentrations were low at 0.82 g L−1 as opposed to 7.42 g L−1 in the control batch reactor.
Production of ABE from concentrated lactose/whey permeate in a batch reactor assisted with product recovery by pervaporation. a Products and lactose in the reactor: the arrow at 22 h shows when product recovery was started, the arrow at 155 h shows when 25-mL sterile solution containing 1 g yeast extract was added. b Lactose utilization rate
The fermentation was completed in 185 h when all the lactose (100 %) was converted to ABE. The lactose utilization rates during the course of fermentation process are presented in Fig. 2b which ranged from 0.38 to 2.18 g L−1 h−1 as compared to the batch system in which it was 0.44 g L−1 h−1. The average rate of lactose utilization in the integrated system was 1.14 g L−1 h−1. A close look at the lactose utilization indicates that lactose utilization rates can be divided into two groups: (i) from 0.0 to 98 h when lactose utilization rate was relatively low perhaps due to lactose inhibition and (ii) from 99 to 185 h when lactose utilization rate was comparatively high as there was no lactose inhibition.
From this fermentation, ABE was recovered using a pervaporation membrane. When the concentration of ABE in the condensed liquid (also called pervaporate) reached greater than 78 g L−1, it separated into two phases: an organic phase (top layer) and an aqueous phase (bottom layer). The organic phase can be easily separated by decanting off. In our experiment, the organic phase contained 755 g L−1 ABE and the aqueous phase contained 85.96 g L−1 ABE. During the course of this fermentation and separation experiment, the organic phase was 35.2 mL and it contained 26.58 g of ABE while the aqueous phase was 557.9 mL and contained 47.96 g ABE. At the end of recovery, the fermentor contained 4.51 g of ABE in the broth. The total amount of ABE that was produced in the reactor was 79.0 g (acetone 4.78 g, butanol 72.39 g, and ethanol 1.84 g; acetic acid and butyric acid were not detected in the condensed mixture) thus resulting in an ABE yield of 0.37 and a productivity of 0.43 g L−1 h−1.
ABE selectivity and flux are two important membrane parameters that were evaluated for this membrane. During the recovery of ABE from the reactor in the pervaporation-assisted system, ABE concentration in the reactor ranged from 3.12 to 5.77 g L−1. At these concentrations, both ABE selectivity and flux were calculated which are presented in Fig. 3. An ABE selectivity of 24.4 was calculated at an ABE concentration of 3.12 g L−1 in the reactor. The highest ABE selectivity of 44.3 was measured at an ABE concentration of 3.82 g L−1. At this ABE concentration in the reactor, an ABE flux of 0.57 g m−2 h−1 was obtained. While selectivity had no clear trend, flux displayed a straight line relationship with ABE concentration in the reactor. A maximum flux of 4.05 g m−2 h−1 was obtained at an ABE concentration of 5.77 g L−1. It should be noted that the flux values presented in this article are ABE fluxes and do not include transported water.
Butanol/ABE concentration
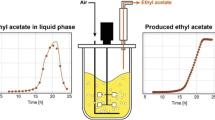
The fermentation-pervaporation experiment aqueous phase (557.9 mL) contained 85.96 g L−1 total ABE. The individual concentrations of these components were acetone 5.30 g L−1, ethanol 2.66 g L−1, and butanol 78 g L−1. Further, an attempt was made to recover these solvents using the same membrane. To this solution, 442.1 mL ABE solution was added thus making the total volume 1.00 L with the same ABE concentrations (acetone 5.30 g L−1, ethanol 2.66 g L−1, and butanol 78.0 g L−1). A solution of 1.00 L was required to concentrate ABE/butanol. The recovery experiment was run for 71 h, and during this time period butanol concentration was reduced from 78.0 g L−1 (total ABE 85.96 g L−1) to 4.00 g L−1 (Fig. 4a). The concentrations of these components on the reactor and recovery side are presented in Table 1. The diffused butanol concentration in the pervaporate ranged from 78.0 to 611.0 g L−1, depending on butanol concentration in the reactor. Figure 4a suggests that recovery was fast for the first 25–35 h after which it slowed down considerably. The effect of ABE concentration in the reactor on ABE flux and selectivity is presented in Fig 4b. This figure suggests that there was a straight line relationship between ABE concentration in the reactor and ABE flux. At an ABE concentration of 78.6 g L−1 in the reactor, an ABE flux of 25.29 g m−2 h−1 was obtained. At low ABE concentrations in the reactor, ABE selectivities showed an upward trend followed by a decrease.
During ABE separation, in this experiment, 101.36 mL of organic phase containing 755 g L−1 ABE and 109.27 mL of aqueous phase containing 85.94 g L−1 ABE were recovered. The residual concentration of ABE in the reactor was 5.03 g L−1. Material balance showed that 76.53 g of ABE was present in the organic phase, 9.39 g in the aqueous phase, and 5.03 g in the reactor thus totaling 90.95 g ABE. This suggests that the ABE recovery was 105.8 % which is within the error of measurement (approximately 6 %). The amounts recovered from this solution are presented in Table 2. Of the 76.53 g organic phase recovered ABE, 42.69 g was from 557.9 mL aqueous solution that was initially recovered from the fermentation bioreactor and was calculated using material balance.
During the recovery process, acetone and ethanol were also recovered, and their selectivities are presented in Table 3. The acetone concentrations in the reactor ranged from 0.47 to 4.10 g L−1, and in this concentration range, acetone selectivities ranged from 1.30 to 1.62 g L−1. Ethanol selectivities ranged from 1.05 to 2.04 g L−1 in the ethanol concentration range of 1.27 to 2.58 g L−1. Separation of these two coproducts is essential for economic and environmental reasons.
Continuous fermentation
In our control batch system, a productivity of 0.14 g L−1 h−1 was obtained, which is considered to be low. One of the methods to improve productivity is to run a continuous fermentation where downtime is reduced or eliminated thus resulting in increased productivity. For this reason, a continuous reactor was operated with whey permeate as a substrate. The reactor was operated at dilution rates ranging from 0.01 to 0.05 h−1. In this run, ABE and acid concentrations and lactose utilization are presented in Fig. 5a. The highest lactose utilization of 75.1 % of that available in feed was obtained which decreased with the increase in dilution rates. At a dilution rate of 0.01 h−1, a total ABE concentration of 5.75 g L−1 was achieved, which is considered to be low. The highest ABE concentration of 6.22 g L−1 was obtained at a dilution rate of 0.03 h−1, resulting in a productivity of 0.19 g L−1 h−1 (Fig. 5b). Although this productivity is 36 % higher than the control run, it was at the expense of low lactose utilization which was only 52 % of that available in feed (56.3 to 58.7 g L−1). In this run, the cell concentration of 0.79 to 1.12 g L−1 was obtained. In order to increase lactose utilization, two series reactors were operated where the first reactor was fed with fresh feed and the effluent of the first reactor was fed to the second reactor. These reactors were operated at two feed flow rates (65 and 40 mL h−1) which correspond to overall dilution rates of 0.02 and 0.01 h−1, respectively. At a dilution rate of 0.01 h−1, 4.81 g L−1 ABE was produced with a productivity of 0.05 g L−1 h−1. At a dilution rate of 0.02 h−1, 2.94 g L−1 ABE was produced thus resulting in a productivity of 0.06 g L−1 h−1. The productivities achieved in the series reactors were lower than those obtained in the control batch fermentation.
Discussion
In the control batch reactor, the culture could not produce more than 12.43 g L−1 ABE, even when 21.0 g L−1 residual sugar was available. This was due to severe product inhibition (Qureshi et al. 1988). Three possible solutions to overcome this problem are (i) development of a superior butanol tolerant strain, (ii) simultaneous product removal, and (iii) a combination of both. Although a significant progress has been made on strain development, the developed strains rarely produce greater than 20–25 g L−1 ABE. Hence, we explored the use of simultaneous product removal from the reactor using a pervaporation membrane. Unless simultaneous product is removed, the culture cannot utilize greater than 60–70 g L−1 lactose or sugar. Additional advantages of using simultaneous product removal are improvement in reactor productivity as compared to the control batch reactor and possibly achieving a higher product yield due to reduced acid production.
Following the control batch process, a concentrated lactose/whey permeate reactor was used to produce butanol. In this system, 211 g L−1 lactose was present in the beginning of the fermentation. As a result of simultaneous product recovery and keeping butanol concentration low (<3.0 g L−1) in the reactor, the culture was able to utilize all the lactose. There are numerous advantages of using such a concentrated sugar solution including (i) reduction in water usage and process streams, (ii) reduction in process equipment size such as bioreactor, and (iii) reduction in energy requirement such as for steam sterilization and cooling the reactor contents. In the present system, water usage was reduced by a factor of 3.5.
In our earlier perstraction-assisted product recovery system, we were able to ferment concentrated lactose/whey permeate medium containing 227 g L−1 lactose and achieved a productivity of 0.21 g L−1 h−1 (Qureshi and Maddox 2005; Table 4). In this system, ABE produced in the reactor diffused through the perstraction membrane and were extracted into oleyl alcohol. Further recovery of ABE from oleyl alcohol requires distillation. In another system, Maddox et al. (1995) utilized 199 g L−1 lactose and reported a productivity of 0.32 g L−1 h−1 (Table 4). The fermentation was started with a lactose concentration of 199 g L−1 which was utilized completely. In this run, ABE was recovered using gas stripping (CO2 and H2), and ABE removal selectivities in the range of 9.5 to 13 were achieved. In the present reactor product removal experiment, a productivity of 0.43 g L−1 h−1 was achieved, which is higher than that achieved in either of the above fermentation-product removal systems (Table 4). This productivity is 307 % of the productivity achieved in the control batch reactor (0.14 g L−1 h−1). The reasons for the improved reactor productivity were reduction of product inhibition as we kept product butanol concentration in the reactor below 3.0 g L−1 and increase in cell concentration (14.3 g L−1) due to significantly reduced cell growth inhibition. It was also noted that acids neither accumulated nor diffused through the membrane, and they were converted to acetone butanol resulting in high product yield (0.37). The ratios of acids to ABE in the control and integrated systems were 0.60 and 0.01, respectively. An increase in productivity and cell concentration demonstrated that pervaporation is an effective technique for product removal in butanol fermentation. It also minimizes diffusion of water through the membrane thus requiring significantly less amount of energy for product recovery. It is suggested that further improvements in productivity are possible with the application of fed-batch or continuous bioreactors assisted with product removal by pervaporation as exercised by Ezeji et al. (2004, 2013) using gas stripping technique to remove product. By employing fed-batch/continuous feeding technique, sugar inhibition can be eliminated by keeping lactose/sugar concentration in the reactor preferably below 30–50 g L−1.
During fermentation, concentrations of ABE and acids fluctuated which affect rates of product removal from the fermentation broth. It should be noted that fluctuations in acids and/or ABE concentrations occur due to conversion of cells into spores and dead cells (Qureshi et al. 1988, 2004) which is a characteristic of C. beijerinckii/C. acetobutylicum fermentation (Ennis et al. 1986; Huang et al. 2004).
The membrane that we used was effective in removing butanol from the bioreactor with high selectivity and good flux. The ABE selectivities were measured to be in the range of 24.4 to 44.3 as compared to 9.5 to 13 in the gas stripping experiment (Maddox et al. 1995). These selectivities (24.4 to 44.3) resulted in a concentrated butanol/ABE solution (755 g L−1) in the recovered stream from the fermentation mixture and ABE solution. Further increase in butanol concentration to 810 g L−1 (maximum butanol 810 g L−1 as ρ = 0.81 g mL−1) may be achieved employing a hydrophilic membrane or silicalite that adsorbs water or azeotropic distillation.
To summarize, in the fermentation-pervaporation experiment, we were able to ferment 211 g L−1 lactose to produce ABE with a high productivity of 0.43 g L−1 h−1 which is 307 % of the control experiment productivity of 0.14 g L−1 h−1. This was possible due to simultaneous recovery of the toxic product (butanol) from the culture broth. Also, we were able to concentrate ABE from low concentration (<5.0 g L−1) in the reactor to 755 g L−1. The results obtained in the control and integrated experiments are summarized and compared with the previous work and are presented in Table 4. Approximately 69 g ABE (produced from lactose/whey permeate) was recovered as organic phase (755 g L−1), and 12.56 g remained either in fermentation reactor or pervaporation retentate or in aqueous phase. Recovery of the 12.56 g ABE is possible by recycling these streams. With these results, the objectives stated in the “Introduction” section of this article have been achieved. Pervaporation is a technique which removes ABE from the reactor selectively and minimizes diffusion of water through the membrane thus requiring significantly less energy for product recovery as compared to gas stripping. Continuous fermentations using suspended or free cells of C. saccharobutylicum P262, neither in single reactor nor in two series reactors, resulted in high ABE productivity.
References
Domingues L, Lima N, Teixeira JA (2001) Alcohol production from cheese whey permeate using genetically modified flocculent yeast cells. Biotechnol Bioeng 72:507–514
Dürre P (1998) New insights and novel developments in clostridial acetone/butanol-isopropanol fermentation. Appl Microbiol Biotechnol 49:639–648
Ennis BM, Maddox IS (1985) Use of Clostridium acetobutylicum P262 for production of solvents from whey permeate. Biotechnol Lett 7:601–606
Ennis BM, Maddox IS, Schoutens GH (1986) Immobilized Clostridium acetobutylicum for continuous butanol production from whey permeate. N Z J Dairy Sci Technol 21:99–109
Ezeji TC, Blaschek HP (2008) Fermentation of dried distillers’ grains and soluble (DDGS) hydrolysates to solvents and value-added products by solventogenic clostridia. Bioresour Technol 99:5232–5242
Ezeji TC, Qureshi N, Blaschek HP (2004) Acetone-butanol-ethanol production from concentrated substrate: reduction in substrate inhibition by fed-batch technique and product inhibition by gas stripping. Appl Microbiol Biotechnol 63:653–658
Ezeji TC, Qureshi N, Blaschek HP (2013) Microbial production of a biofuel (acetone-butanol-ethanol) in a continuous bioreactor: impact of bleed and simultaneous product removal. Bioprocess Biosyst Eng 36:109–116
Friedl A, Qureshi N, Maddox IS (1991) Continuous acetone-butanol-ethanol (ABE) fermentation using immobilized cells of Clostridium acetobutylicum in a packed bed reactor and integration with product removal by pervaporation. Biotechnol Bioeng 38:518–527
Groot WJ, van der Lans RGJM, Luyben KCAM (1992) Technologies for butanol recovery integrated with fermentations. Proc Biochem 27:61–75
Huang W-C, Ramey DE, Yang S-T (2004) Continuous production of butanol by Clostridium acetobutylicum immobilized in a fibrous bed bioreactor. Appl Biochem Biotechnol 113–116:887–898
Jones DT, Woods DR (1986) Acetone-butanol fermentation revisited. Microb Rev 50:484–524
Maddox IS (1989) The acetone-butanol-ethanol fermentation: recent progress in technology. Biotechnol Genet Eng Rev 7:190–220
Maddox IS, Qureshi N, Roberts-Thomson K (1995) Production of acetone-butanol from concentrated substrates using Clostridium acetobutylicum in an integrated fermentation-product removal process. Proc Biochem 30:209–215
Mariano AP, Qureshi N, Filho RM, Ezeji TC (2011) Bioproduction of butanol in bioreactors: new insights from simultaneous in situ butanol recovery to eliminate product toxicity. Biotechnol Bioeng 108:1757–1765
Mawson AJ (1987) Ethanol production from whey in New Zealand. Aus J Biotechnol 1:64–73
Parekh SR, Parekh RS, Wayman M (1988) Ethanol and butanol production by fermentation of enzymatically saccharified SO2-prehydrolysed lignocellulosics. Enzym Microb Technol 10:660–668
Qureshi N, Maddox IS (2005) Reduction in butanol inhibition by perstraction: utilization of concentrated lactose/whey permeate by Clostridium acetobutylicum to enhance butanol fermentation economics. Food Bioprod Proc 83(C1):43–52
Qureshi N, Peterson AHJ, Maddox IS (1988) Model for continuous production of solvents from whey permeate in a packed bed reactor using cells of Clostridium acetobutylicum immobilized by adsorption onto bonechar. Appl Microbiol Biotechnol 29:323–328
Qureshi N, Maddox IS, Friedl A (1992) Application of continuous substrate feeding to the ABE fermentation: relief of product inhibition using extraction, perstraction, stripping and pervaporation. Biotechnol Prog 8:382–390
Qureshi N, Meagher MM, Hutkins RW (1999) Recovery of butanol from model solutions and fermentation broth using a silicalite/silicone membrane. J Membr Sci 158:115–125
Qureshi N, Lai LL, Blaschek HP (2004) Scale-up of a high productivity biofilm reactor to produce butanol by adsorbed cells of Clostridium beijerinckii. Trans I Chem E Food Bioprod Process 82:164–173
Qureshi N, Hughes S, Maddox IS, Cotta MA (2005) Energy efficient recovery of butanol from fermentation broth by adsorption. Bioprocess Biosyst Eng 27:215–222
Wongso DD (1993) Optimization of industrial whey ethanol fermentation process. Ph.D. dissertation, Biotechnology Department, Massey University, Palmerston North, New Zealand
Zverlov VV, Berezina O, Velikodvorskaya GA, Schwarz WH (2006) Bacterial acetone and butanol production by industrial fermentation in the Soviet Union: use of hydrolyzed agricultural waste for biorefinery. Appl Microbiol Biotechnol 71:587–597
Acknowledgments
NQ would like to thank Michael A. Cotta (United States Department of Agriculture, Agricultural Research Service, National Center for Agricultural Utilization Research, Peoria, IL, USA) for reading this paper and providing constructive comments that improved the quality of the article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mention of trade names or commercial products in this article is solely for the purpose of providing scientific information and does not imply recommendation or endorsement by the United States Department of Agriculture. USDA is an equal opportunity provider and employer.
Rights and permissions
About this article
Cite this article
Qureshi, N., Friedl, A. & Maddox, I.S. Butanol production from concentrated lactose/whey permeate: Use of pervaporation membrane to recover and concentrate product. Appl Microbiol Biotechnol 98, 9859–9867 (2014). https://doi.org/10.1007/s00253-014-6117-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6117-5