Abstract
Hydroxypropyl-β-cyclodextrin (HP-β-CD) enhances steroid 1-dehydrogenation biotransformation by Arthrobacter simplex. In this work, HP-β-CD-induced improvement of A. simplex CPCC 140451 cell envelope permeability which had positive effects on the steroid bioconversion was confirmed by a comparative investigation which showed a lower dehydrogenase activity and higher cell permeability of the cells after being incubated with HP-β-CD. Atomic force microscopy and transmission electron microscopy micrographs showed that HP-β-CD altered the size, sharpness, and surface structure of the cell envelope. The analysis of lipid composition revealed that the proportion of extractable lipids decreased and the fatty acids profile was considerably altered. The contents of unsaturated fatty acids and long-chain fatty acids were reduced by 11.77 and 14.98 %, respectively. The total leakage of protein level increased to 8 %. Proteins belonging to the ATP-binding cassette superfamily and major facilitator superfamily were observed outside the cell. These alterations can explain the change of permeability on the molecular level under HP-β-CD treatment. Results showed the material basis and mechanisms underlying the cellular changes, thus most likely contributing to the conversion rate in addition to cyclodextrins known effects on substrate solubility.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Steroid hormone production is a well-known example of the successful application of microbial biotransformation technology in large-scale industrial processes, as exemplified by the 1-dehydrogenation of steroids catalyzed by Arthrobacter simplex (Fernandes et al. 2003), which is obligatory aerobes, cocci in stationary phase, and Gram-positive. However, the main problem of steroid biotransformation remains to be its poor water solubility and low dissolution rate, which results in poor availability of substrate. Cyclodextrins (CDs) are a family of cyclic oligosaccharides with hydrophilic outer surface and lipophilic central cavity. These characteristics enable the formation of inclusion complexes with various molecules that fit inside the CD cavity. Given their complexing ability, CDs have been introduced to microbial transformations of steroids for the first time in 1985 by Udvardy, who also demonstrated that biotransformation can be intensified by adding CDs to the reaction mixture of the steroid substrate. Since their first use, CDs have been widely applied in microbial steroid transformation to enhance the solubility, stability, and bioavailability of steroids (Li et al. 2013; Manosroi et al. 2008).
Numerous studies have demonstrated that a variety of cellular functions are affected when cells are exposed to β-CD (Zidovetzki and Levitan 2007) for the reason that natural or chemically modified CDs can interact with cell components by virtue of either their complexing ability and/or their surface activity (Hapiot et al. 2011). When cells are exposed to high concentration of methyl-β-cyclodextrin (M-β-CD) (5–10 mmol L−1) for a prolong period of time (>2 h), 80–90 % of total cellular cholesterol can be removed (Kilsdonk et al. 1995; Levitan et al. 2000). Under the condition, cells typically lose their morphology, round up, and, in extreme cases, become nonviable. Puglisi et al. (1996) showed that the thermodynamic parameters of dipalmitoylphosphatidylcholine (DPPC) main phase transition were also affected by the appearance of CDs, indicating an interaction between CDs and DPPC. Indeed, M-β-CD was found to interact with proteins such as ubiquitin, chymotrypsin inhibitor 2 (CI2), S6, and insulin (Aachmann et al. 2008) for the hydrophobic character of its pocket.
Besides steroidal solubilization, CDs have multiple functions in microbial transformation of steroids. Thus, a general investigation of the interactions between CDs and transforming cells is necessary. Jadoun and Bar (1993a, b) studied the effect of CDs on Rhodococcus erythropolis and showed that CDs slightly inhibited microbial growth and limited the leakage of cellular proteins and cholesterol oxidase. Donova et al. (2007) investigated the effects of M-β-CD on sterol-transforming mycobacterial cell. M-β-CD increased the cell wall permeability to steroids and soluble nutrients. Such interactions disorganized the lipid bilayer and promoted the release of steroid-transforming enzymes weakly associated with the cell wall. Both R. erythropolis and Mycobacterium have an unusual cell envelope composition and architecture compared with other Gram-positive microorganisms. The low permeability of mycobacterial cell envelopes results from the unique composition and organization of the cell wall lipids, which form an asymmetric bilayer that hinders the entry of solutes into the cell (Nikaido and Jarlier 1991; Nikaido et al. 1993).
The cell envelope structures of A. simplex are closer to those of most Gram-positive microorganisms compared those of R. erythropolis and Mycobacterium. Shen et al. (2011) previously studied the effect of adding hydroxypropyl-β-cyclodextrin (HP-β-CD) to an A. simplex cell culture medium. Compared with M-β-CD, HP-β-CD has a better biocompatibility, an enhancement effect on steroid conversion, and a wide application range in the steroid biotransformation, although which has a relatively low solubility for steroidal compound (Ma et al. 2009; Manosroi et al. 2008; Shen et al. 2011). The presence of HP-β-CD improved cell wall permeability to lipophilic compounds but significantly inhibited cell growth and biocatalytic activity. Compared with studying the effect of CDs during the growth process, studying the CD-induced changes in cells during the bioconversion process can directly illustrate the effect of CDs on steroid microbial transformation and solubilization. Thus, the strategy that A. simplex cells were pre-incubated with HP-β-CD and washed three times with KH2PO4-NaOH buffer (PBS), which ensured that no HP-β-CD affected the solubilization of cortisone acetate (CA), was devised to imitate the interaction process between HP-β-CD and A. simplex cells during the bioconversion. Besides, the changes of dehydrogenase activity, cell permeability to steroids, and cell ultrastructure and the material basis of the further change in permeability, including the variations in lipid and protein contents, were investigated. The data obtained will improve our understanding of the mechanism underlying the effect of HP-β-CD on the bioconversion of hydrophobic compounds by altering cell envelope permeability.
Materials and methods
Materials
Substrate CA (99.4 % purity) was obtained from Tianjin Pharmaceutical Company. Standard C12–C28 fatty acids and phosphocholine (PC) were obtained from Sigma-Aldrich Co., USA. HP-β-CD (31.7 % degree of substitution, 1,523 average relative molecular mass) was obtained from Xi’an Deli Biology & Chemical Industry Co., Ltd. All chemical solvents and salts used were of analytical grade or higher.
Microorganism cultivation and treatment with HP-β-CD
A. simplex CPCC 140451 (ASP) was stored in our lab and cultivated in two stages as described previously (Ma et al. 2009). The cells were harvested by centrifugation at 6,000 × g for 5 min at 4 °C. Cells were washed three times with PBS (0.5 mol L−1, pH 7.2). The washed cells were then resuspended in PBS. Cell concentrations were maintained at 2.0 g dry weight L−1. HP-β-CD was added into the cell suspension at different concentrations after incubation at 32 °C for different times on a rotary shaker (160 rpm). Afterward, the pre-incubated ASP cells with HP-β-CD were washed with PBS three times and resuspended in the same buffer.
Steroid transformation and analysis
CA (5.0 mg mL−1) was added into 30 mL of cell suspension at 34 °C, 180 rpm. One hundred-microliter samples were withdrawn and extracted by ethyl acetate at various time intervals and dried in vacuum, and then, the solid extracts were redissolved in the high-performance liquid chromatography (HPLC) mobile phase (dichloromethane/ether/methanol, 86:12:2 (v)) and filtered through a 0.45-μm filter. HPLC (Agilent 1100, USA) analysis was performed on a Kromasil SIL, 5u, 250 × 4.6 mm column with UV absorbance detection at 240 nm as previously described (Ma et al. 2009). All experiments were done with two replicates at least thrice.
The activity of dehydrogenase
ASP cells were pre-incubated from 0.5 to 10 h by 50 mmol L−1 HP-β-CD. The relative activity of dehydrogenase compared with control (no HP-β-CD pre-incubating) was determined by triphenyl tetrazolium chloride (TTC) analysis. One milliliter PBS and 2 mL TTC solution (0.5 %) were added to the tube with 1-mL cell suspension, and after incubating at 37 °C for 30 min, the reaction was terminated by 0.5 mL formaldehyde, and then, the tubes were removed and centrifuged for 10 min. TF extraction was carried out using 80 % acetone (Xiong et al. 2013). The absorbance of the combined supernatants was measured at 485 nm. The dehydrogenase activity of different samples was expressed by the ratio of OD485 of treated sample with the control.
Preparation of liposome and transportation of CA
The liposome was prepared by the thin-film hydration method (Varona et al. 2011). Briefly, the phospholipid was dissolved in chloroform and dried under vacuum using a rotary evaporator. The film was then hydrated with 20 mL of Milli-Q water, and the resulting liposome dispersions were obtained by sonicating for 30 min. The transportation experiment was performed by adding liposome dispersions (V 1) to CA (1 g L−1) solution (V 2) with HP-β-CD at 0 to 100 mmol L−1. After the mixture was left to stand for 30 min at room temperature, centrifugation was performed at 15,000 × g for 20 min. The concentration of free CA (C 1) was determined by subjecting the supernatant to HPLC. The quantity (M) of CA transferred to liposomes was calculated using the following logistic function:
Preparation of AFM and TEM
The samples of treated and untreated cells were fixed overnight in 2.5 % glutaric aldehyde in PBS; after which, the cells were washed three times with PBS and resuspended in the same buffer. For atomic force microscopy (AFM), the procedure was performed as previously described, using a JSPM-5200 AFM (JEOL) in tapping mode (Shen et al. 2012). The sample solution was dropped on a freshly prepared mica surface and air-dried for more than 24 h. For transmission electron microscopy (TEM), the samples were dried, embedded in epoxy resin, sliced, stained, and observed under a HT7700 TEM instrument operated at an accelerating voltage of 120 kV (Hitachi, Japan).
Lipid analyses
The lipids were extracted from the samples of lyophilized cells (200 mg each) with 100 mL chloroform/methanol (2:1, v/v) by vigorous stirring for 48 h at 29 °C, as previously described (Donova et al. 2007). The residue was separated by filtration, and the filtrate was evaporated and weighed. The composition of fatty acids was determined in methanolysates of the obtained from lipid, as previously described (Fuchs et al. 2011). The chloroform phase was reduced in volume, and methanol/toluene/concentrated sulfuric acid (30:15:1,(v)) was added and incubated at 75 °C for 16 h. The formed fatty acid methyl esters were extracted twice with hexane. Fatty acid analysis was conducted using an Agilent 7890A gas chromatograph with a flame ionization detector on a CP-Sil 88 capillary column (50 m × 0.25 mm internal diameter, 0.2-μm film thickness) under a temperature gradient of 110 °C to 230 °C. The temperature of the injector was set at 250 °C, nitrogen flow rate was 20 mL min−1, and the detector was set at 260 °C. The ascending temperature program of the oven started at 110 °C for 4 min. Temperature was increased to 170 °C at 3 °C min−1, 200 °C at 2 °C min−1, and 230 °C at 3 °C min−1 for 6 min. The standard C12–C28 fatty acids were used for quantification.
Protein analysis
Sample preparation
Cells untreated and treated with 100 mmol L−1 HP-β-CD for 2 h were collected and washed three times with PBS. Pellets were resuspended in lysis buffer comprising 7.8 mol L−1 urea, 2 mmol L−1 thiourea, 4 % CHAPS, and 40 mmol L−1 dithiothreitol (DTT). The lysis buffer contained protease inhibitor phenylmethylsulfonyl fluoride. The suspensions were immersed in an ice bath and sonicated for 90 cycles (10 s per cycle at 10-s intervals). After sonication, the suspensions were withdrawn and centrifuged at 12,000 × g for 20 min. Protein contents in the supernatural fluids were estimated with a Bradford protein assay using bovine serum albumin as standard. The proteins were precipitated overnight with four volumes of acetone and carefully removed after centrifugation at 12,000 × g for 10 min at 4 °C.
Trypsin digestion
The pellet was redissolved in reducing solution containing 6 mol L−1 guanidine hydrochloride and 100 mmol L−1 ammonium bicarbonate at pH 8.3. The final concentration of the protein was 2 to 3 μg μL−1. A 200 μL aliquot of reducing solution was mixed with 2 μL of 1 mol L−1 DTT. The mixture was incubated at 56 °C for 1 h. A 10 μL aliquot of 1 mol L−1 iodoacetamide was added, and the mixture was incubated in the dark at room temperature for 40 min. The protein mixtures were spun, exchanged into 100 mmol L−1 ammonium bicarbonate buffer, and then incubated with trypsin (20:1 to 50:1) at 37 °C for 20 h.
LC-MS/MS analysis
Liquid chromatography-mass spectrometry/mass spectrometry (LC-MS/MS) experiments were performed using a Surveyor HPLC system coupled with an LTQ linear ion trap mass spectrometer (Thermo, San Jose, CA, USA). A reversed-phase C18 column (150 μm × 100 mm h; Column Technology Inc., USA) was used to separate the protein extract. The HPLC solvents used were 0.1 % formic acid (v/v) aqueous (A) and 0.1 % formic acid (v/v) acetonitrile (B). A 20-μL trypsin-digested peptide mixture was injected and washed out at a rate of 200 μL min−1. The gradient elution conditions were as follows: 5 % B for 30 min, from 5 % B to 32 % B for 60 min, to 90 % for 10 min, balance for 5 min, to 5 % in 0.1 min, and 5 % B for 35 min. The mass spectrometer used for this study is LTQ XL (Thermo Fisher). The temperature of the ion transfer capillary was set at 160 °C. A voltage of 3.0 kV was applied to the ESI needle, and the normalized collision energy was set at 35.0 %. System control and data collection were performed using Xcalibur software version 1.4 (Thermo).
Protein identification
The acquired MS/MS spectra were compared against the Arthrobacter database from NCBI using the TurboSEQUEST program in the Proteomics Discovery 1.2 software suite (Thermo). Peptide tolerance was set at ±1 Da, and MS/MS tolerance was set at ±0.5 Da. Peptides with a 1, 2, and 3 charge states were accepted when they were fully digested by trypsin and had a cross correlation (Xcorr) of at least 1.9, 2.2, and 3.75, respectively. The delta correlation score was set to 0.08, indicating a significant difference between the best-matched peptide reported and the next best-matched peptide. These criteria were established based on probability-based evaluation using a previously described sequence-reversed database search to provide >95 % overall confidence level for the entire set of unique peptide identifications (<5 % false positive rate) (Varona et al. 2011).
Results
Effect of ASP pre-incubation by HP-β-CD on the steroid transformation and activity of dehydrogenase
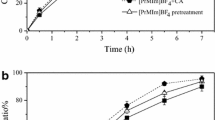
After the ASP cells being treated and washed, we choose 20 teams at random to determine the quantity of HP-β-CD remained on the cell surface. The result showed that the solubilization of CA was not affected by HP-β-CD, which was detected only in three teams at a concentration less than 0.01 mmol L−1 (data not shown). The dynamics of CA 1-dehydrogenation carried out by ASP cells of untreated or pre-incubated 0.5 h with 50 mmol L−1 HP-β-CD was studied. The results revealed that ASP pre-incubated by HP-β-CD obviously affected the transformation process. As shown in Fig. 1, CA conversion ratios were almost 20 % higher after 3-h conversion by the treated cells than those by control, and the time for reaching the final conversion ratio reduced by 1 h for the pre-incubated cells. Comparing the initial reaction rates, the pre-incubated cells was about two times of the initial (0.3080 and 0.1548 mg mL−1 h−1, respectively), suggesting that a pre-incubation of the cells with HP-β-CD might enhance their 1-dehydrogenation activity.
Effect of HP-β-CD pretreatment on the CA conversion process. The CA conversion ratios when ASP cell was pre-incubated 0.5 h with (triangle) and without (square) 50 mmol L−1 HP-β-CD. The conversion ratios of CA were determined by HPLC analysis. Standard deviations are indicated by error bars or are within each symbol
As shown in Fig. 2, the activity of dehydrogenase decreased after being treated by HP-β-CD. When the treated time was less than 1 h, HP-β-CD slightly affected the activity of dehydrogenase, as evidenced by the 8 % dehydrogenase activity inhibition of ASP cells treated 0.5 h with 50 mmol L−1 HP-β-CD compared with the untreated cells. With the prolonging of the treated time, the activity of dehydrogenase decreased significantly. The conversion rate of CA after HP-β-CD treatment significantly enhanced, whereas the activity of dehydrogenase reduced.
Effect of HP-β-CD on the activity of dehydrogenase. ASP cell pre-incubated from 0.5 to 10 h by 50 mmol L−1 HP-β-CD. The relative activity of dehydrogenase compared with control (no HP-β-CD pre-incubating) was determined by TTC analysis. Standard deviations are indicated by error bars or are within each symbol
Effect of pre-incubation with HP-β-CD on membrane permeability and substrate availability
Liposome had similar bilayer structure and fluid characteristics to real cell membrane (Bagatolli et al. 2010). To study the effect of HP-β-CD on cell permeability to the substrate, liposomes were used to substitute for ASP cells. The effect of HP-β-CD on the transportation of CA was studied. The results would help us to understand and predict the events that occurred during the transport of CA through the HP-β-CD-exposed cell membrane. The permeability of liposomes comprising PC is illustrated in Fig. 3. When the liposomes were mixed with HP-β-CD, the amount of CA that penetrated the liposomes increased with increasing HP-β-CD concentration. The CA amount in liposomes increased by 8.3 % with increasing HP-β-CD concentration from 20 to 100 mmol L−1. Thus, HP-β-CD changed the permeability of the liposomes to CA. And then, we speculated that HP-β-CD interacted with the cell envelope, and such interaction might cause the change in cell permeability to CA.
Alteration of cell ultrastructure
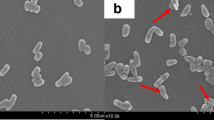
The AFM and TEM micrographs directly showed the cell morphology. As shown in the AFM images, untreated bacteria comprised typical rod-like cells, which showed rough and bright surfaces with regular creases. The exposure of bacteria to 100 mmol L−1 HP-β-CD caused remarkable cell surface changes, including wrinkles, gross creases, and large latitude (Fig. 4). The cell membrane surface changed. As shown in the TEM images, the cells pre-incubated without HP-β-CD (Fig. 5a) had a typical ASP ultrastructure. Cells remained intact, and the cell envelope was complete and clear. When the cells were exposed to 100 mmol L−1 HP-β-CD (Fig. 5b), the cell envelope became blurred and lost its integrity. Cell permeability is closely related to the integrity of cell membrane. Thus, these alterations may explain the increased permeability of ASP cells. The micrographs illustrated that HP-β-CD altered the size, sharpness, surface structure, and permeability of cells.
HP-β-CD affects cell lipid composition
The effect of HP-β-CD on the lipids of ASP cells was studied (Fig. 6). The preparations differed to a much greater extent in terms of lipid concentrations, which increased drastically with increasing HP-β-CD concentration. The percentage of loss lipid in cells pre-incubated with 100 mmol L−1 HP-β-CD was 33 %, which approximate sixfold of those in 10 mmol L−1 HP-β-CD.
Effect of HP-β-CD on ASP loss lipid concentration and protein leakage. The percentage of loss lipids (square) and leakage of proteins (triangle) with increasing HP-β-CD concentration from 10 to 100 mmol L−1. Lipids were extracted from samples of lyophilized cells (200 mg each) with 100 mL chloroform/methanol (2:1, v/v). The leakage of proteins were extracted from cell after ultrasonic decomposition. Protein contents in the supernatural fluids were estimated with a Bradford protein assay using bovine serum albumin as standard. Standard deviations are indicated by error bars or are within each symbol
The quantity of fatty acid components in the HP-β-CD-incubated cells was lower than that in the control. This finding agreed with the low overall content of noncovalently bound lipids in HP-β-CD-incubated cells (Fig. 6).
Gas chromatography analysis data showed that the C16 and C18 were the main fatty acid components of ASP (Table 1). C16 and C18 comprised more than 60 % of the total fatty acids in both preparations. HP-β-CD treatment only slightly affected the fatty acid composition. Only the relative percentage of fatty acids in the preparations varied significantly. The fatty acid profiles of the treated cells were not in agreement with those of the control cells, indicating that the selective change in fatty acids content was probably caused by HP-β-CD rather than by cell lysis. A similar effect was described for Mycobacterium vaccae cells exposed to polycations (Korycka-Machała et al. 2001) and for mycobacteria exposed to M-β-CD (Donova et al. 2007).
The contents of unsaturated fatty acids and long-chain fatty acids were reduced by 11.77 and 14.98 % with increasing HP-β-CD concentration from 0 to 100 mmol L−1. When HP-β-CD interacted with ASP cell, the long-chain fatty acids and unsaturated fatty acids were more obviously influenced by HP-β-CD than other fatty acids. The contents of long-chain fatty acid (C18:0–C20:0) residues in the treated cells were less than those detected in control lipids, indicating that these components were easily removed from the cell surface. Unsaturated fatty acids (C16:1, C17:1, and C18:1) were either absent (18:1 trans) or were present at lower levels in treated cell lipids than in the control cells. Other variations in the fatty acids patterns were not as evident as the abovementioned ones.
HP-β-CD affects cell protein composition
The protein contents of cells pre-incubated with or without the different HP-β-CD concentrations were investigated (Fig. 6). Elevated protein discharge was observed in the presence of 100 mmol L−1 HP-β-CD. The amount of eliminated proteins reached 8 % of the total protein content.
Proteomic profiling of ASP control cells and cells exposed to 100 mmol L−1 HP-β-CD was performed. HP-β-CD affected protein composition, and compared with the control, approximately 60 proteins were downregulated after pre-incubation with HP-β-CD. The downregulated proteins included intracellular and membrane proteins identified by LC-MS/MS (Table S1). The characteristics of the downregulated proteins are summarized in Figs. 7 and 8.
Different proteins were classified to delineate their functions (Fig. 7). Approximately 52 % of the identified proteins were located in the cytoplasm, indicating that the majority of proteins mainly functioned in cellular metabolism. Approximately one third of the identified proteins were situated in the plasma membrane, and such proteins were mainly used for material transportation of inorganic ion, carbohydrate, amino acids, and polypeptide. Many of these transport proteins belong to ATP-binding cassette (ABC) superfamily and major facilitator superfamily (MFS). A small percentage (2 %) of periplasmic space proteins was also detected in our dataset.
We characterized the identified proteins based on the grand average of hydropathy (GRAVY) and aromatic amino acid content (Fig. 8). The GRAVY and the aromatic amino acid content showed that the intracellular proteins were widely distributed, whereas the membrane proteins were mainly in the hydrophobic area and areas with a high concentration of aromatic amino acids.
Discussion
CDs have been shown previously to enhance steroid conversion (Donova et al. 2007; Druzhinina et al. 2008; Manosroi et al. 2008; Roglič et al. 2005; Shen et al. 2012). The enhancement effect was mainly attributed to steroidal solubilization by the formation of inclusion complexes with CDs (Kolomytseva et al. 2009; Ma et al. 2009; Mohamed et al. 2014). In this work, we investigated the interaction of HP-β-CD with ASP, particularly the effect of HP-β-CD on the permeability of steroids, such as CA, in ASP cells.
After ASP pre-incubating by 50 mmol L−1 HP-β-CD, the obvious promotion of steroid transformation process was achieved; no-residual HP-β-CD meant that the solubilization of CA was not affected by HP-β-CD. In other words, the solubilization was not the influence factor for transformation. The fact showed that when the inventories of CA were the same, the substrate availability was no difference between the two groups of experiment. It suggested that besides the solubilization, HP-β-CD could affect the bioconversion through other ways.
Korycka-Machała et al. (2001) had pointed out that apparent steroid-transforming activity was determined by the quantity and activity of steroid-transforming enzyme, the substrate availability, and the membrane permeability to steroids. The conversion rate of CA after HP-β-CD treatment was significantly enhanced, whereas the activity of dehydrogenase was reduced. Thus, we speculated that there was a significant increase in ASP cell permeability to CA upon exposure of the ASP cell to HP-β-CD, and it is another factor mediated by HP-β-CD that promotes the bioconversion besides solubilization. Because liposome had similar bilayer structure and fluid characteristics to real cell membrane and played an important role in mimetic biomembrane research (Bagatolli et al. 2010), the simulated cell membrane permeability experiment was performed using liposomes. Transmission efficiency and quantity of substrate (CA) increased when the liposomes were exposed to HP-β-CD. The liposomes revealed that the cell permeability increased under HP-β-CD treatment. CDs could form inclusion complexes with hydrophobic membrane compounds, which could affect the cell envelope composition and cause the change in cell permeability. The AFM and TEM micrographs further proved these changes, which illustrated that HP-β-CD altered the size, sharpness, surface structure, and permeability of cells.
The observed loss lipids in the presence of HP-β-CD agreed with the effect of M-β-CD on Mycobacterium sp. (Donova et al. 2007). Such effect resulted from the complexing ability and/or the surface activity of CDs; lipid content reduction disrupted the membrane and perturbed its fine structural arrangement. As shown in Fig. 4, wrinkles and invaginations were observed after cells were incubated with 100 mmol L−1 HP-β-CD for 2 h, but the bilayer structure of the membrane was maintained. Permeability was affected by changes in cell envelope structure and lipid content variation; a proper treatment could remarkably increase cell permeability for the steroids, CA (Piel et al. 2007).
The high accumulation of unsaturated and long-chain fatty acids outside the cells can be attributed to the enhanced release of lipids from the cell wall under HP-β-CD treatment; such enhanced release of lipids may contribute to the alteration in permeability (Donova et al. 2007; Wieslander et al. 1995). The thickness of the membrane was strongly affected by the length of the incorporated hydrocarbon chains. Acyl chain length in the fluid phase was approximately 50 % of the thickness of the hydrocarbon region of the bilayer (Thurmond et al. 1994). Thin and/or unsaturated membranes of Acholeplasma laidlawii are more permeable than thicker and more saturated membranes (Wieslander et al. 1995). Hence, the decrease in the number of long-chain fatty acids may lead to the formation of a thin membrane with increased permeability.
The interaction between CDs and proteins was previously described. Qiu et al. (2012) showed that HP-β-CD could form an inclusion complex with hydrophobic membrane protein of a parenzyme and superoxide dismutase (SOD), which was located intracellularly. Molecular docking studies and other relevant calculations of SOD with HP-β-CD suggested that HP-β-CD was located in the combined region of the two ligands; HP-β-CD interacted with the two ligands simultaneously (Qiu et al. 2012). Moreover, interacting with CDs and proteins, β-CD binds to glucoamylase 1 and its variants (Williamson et al. 1997), interacts with glycosyltransferase (Schmidt et al. 1998), and forms complexes of α-CD with pig pancreatic amylase (Larson et al. 1994).
The leakage of proteins was observed in ASP. These proteins included membrane and intracellular proteins. Given its chemical structure, molecular weight, and very low octanol/water partition coefficient, the CDs failed to readily permeate the biological membranes (Arun et al. 2008). So, the leakage of intracellular protein is probably occurred for the membrane was disordered by forming inclusion complexes between HP-β-CD and hydrophobic membrane proteins, and then, the cell permeability was increased, and this was consistent with the TEM observation. Thus, we preliminarily concluded that membrane protein directly interacted with HP-β-CD, but such direct interaction was not observed with the intracellular protein. HP-β-CD directly interacted with hydrophobic membrane proteins. An inclusion complex was formed between HP-β-CD and hydrophobic membrane proteins. HP-β-CD interacted with the membrane skeleton lipid to loosen the cytoskeleton and reduce the bonding force between the cell membrane and proteins. Thus, proteins were eliminated from the membrane, and cell permeability increased. The leakage of intracellular protein probably occurred along with increasing permeability. These findings were in agreement with the results of TEM and GRAVY.
Different proteins have special functions that are critical for maintaining normal bacteria growth and enzyme activity. Among the downregulated membrane proteins, two kinds of transport-related proteins were detected. The ABC superfamily and MFS account for nearly half of the solute transporters encoded in the genomes of microorganisms (Getsin et al. 2013). Transport can be regulated by manipulating the membrane protein interactions. The increased permeability resulted from interactions between CDs and cell membrane proteins.
As for the decrease of the activity of dehydrogenase, on the one hand, the downregulation of putative d-amino-acid dehydrogenase could directly illustrate it, and on the other hand, molecules of many peptides and proteins were too hydrophilic and bulky to be wholly included in the CD cavity, and the topological constraints of the peptide backbone might reduce the formation of inclusion complexes. Thus, their interaction with CDs could be only local; that was, accessible hydrophobic side chains might form inclusion complexes with CDs. Such interaction possibly affected the overall three-dimensional structure of peptides and proteins or inhibits their intermolecular association and thus changed their chemical and biological properties (Dahan et al. 2010).
In conclusion, the present study showed that HP-β-CD had multiple interactions with ASP cells. We found that HP-β-CD altered the lipid and protein profiles, leading to the change of membrane permeability, and then promoted the steroid-transforming activity. The mechanisms of HP-β-CD are very complex and have an effect not only on solubilization but also on permeability, which have important scientific significance and research value.
References
Aachmann FL, Otzen DE, Larsen KL, Wimmer R (2008) Structural background of cyclodextrin-protein interactions. Protein Eng 16:905–912
Arun RA, Kumar ACK, Sravanthi VVNSS (2008) Cyclodextrins as drug carrier molecule: a review. Sci Pharm 76:567–598
Bagatolli LA, Ipsen JH, Simonsen AC, Mouritsen OG (2010) An outlook on organization of lipids in membranes: searching for a realistic connection with the organization of biological membranes. Prog Lipid Res 49:378–389
Dahan A, Miller JM, Hoffman A, Amidon GE, Amidon GL (2010) The solubility-permeability interplay in using cyclodextrins as pharmaceutical solubilizers: mechanistic modeling and application to progesterone. J Pharm Sci 99:2739–2749
Donova MV, Nikolayeva VM, Dovbnya DV, Gulevskaya SA, Suzina NE (2007) Methyl-β-cyclodextrin alters growth, activity and cell envelope features of sterol-transforming mycobacteria. Microbiology 153:1981–1992
Druzhinina AV, Andryushina VA, Stytsenko TS, Voishvillo NE (2008) Conversion of 17α-methyltestosterone to methandrostenolone by the bacterium Pimelobacter simplex VKPM Ac-1632 with the presence of cyclodextrins. Appl Biochem Microbiol 4:580–584
Fernandes P, Cruz A, Angelova B, Pinheiro HM, Cabral JMS (2003) Microbial conversion of steroid compounds: recent developments. Enzyme Microb Technol 32:688–705
Fuchs B, Süß R, Teuber K, Eibisch M, Schiller J (2011) Lipid analysis by thin-layer chromatography—a review of the current state. J Chromatogr A 1218:2754–2774
Getsin I, Nalbandian GH, Yee DC, Vastermark A, Paparoditis PC, Reddy V, Saier MH (2013) Comparative genomics of transport proteins in developmental bacteria: Myxococcus xanthus and Streptomyces coelicolor. BMC Microbiol 13:279
Hapiot F, Ponchel A, Tilloy S, Monflier E (2011) Cyclodextrins and their applications in aqueous-phase metal-catalyzed reactions. CR Chim 14:149–166
Jadoun J, Bar R (1993a) Microbial transformations in a cyclodextrin medium. Part 3. Cholesterol oxidation by Rhodococcus erythropolis. Appl Microbiol Biot 40(2–3):230–240
Jadoun J, Bar R (1993b) Microbial transformations in a cyclodextrin medium. Part 4. Enzyme vs microbial oxidation of cholesterol. Appl Microbiol Biot 40(4):477–482
Kilsdonk EPC, Yancey PG, Stoudt GW, Bangerter FW, Johnson WJ, Phillips MC, Rothblat GH (1995) Cellular cholesterol efflux mediated by cyclodextrins. J Biol Chem 270:17250–17256
Kolomytseva MP, Randazzo D, Baskunov BP, Scozzafava A, Briganti F, Golovleva LA (2009) Role of surfactants in optimizing fluorene assimilation and intermediate formation by Rhodococcus rhodochrous VKM B-2469. Bioresource Technol 100:839–844
Korycka-Machała M, Ziółkowski A, Rumijowska-Galewicz A, Lisowska K, Sedlaczek L (2001) Polycations increase the permeability of Mycobacterium vaccae cell envelopes to hydrophobic compounds. Microbiology 147:2769–2781
Larson SB, Greenwood A, Cascio D, Day J, McPherson A (1994) Refined molecular structure of pig pancreatic α-amylase at 2.1 Å resolution. J Mol Biol 235:1560–1584
Levitan I, Christian AE, Tulenko TN, Rothblat GH (2000) Membrane cholesterol content modulates activation of volume-regulated anion current in bovine endothelial cells. J Gen Physiol 115:405–416
Li G, Li F, Deng LS, Fang X, Zou H, Xu K, Li T, Tan GS (2013) Increased yield of biotransformation of exemestane with β-cyclodextrin complexation technique. Steroids 78:1148–1151
Ma YH, Wang M, Fan Z, Shen YB, Zhang LT (2009) The Influence of host–guest inclusion complex formation on the biotransformation of cortisone acetate Δ1-dehydrogenation. J Steroid Biochem 117:146–151
Manosroi A, Saowakhon S, Manosroi J (2008) Enhancement of androstadienedione production from progesterone by biotransformation using the hydroxypropyl-β-cyclodextrin complexation technique. J Steroid Biochem 108:132–136
Mohamed SS, El-Refai AMH, Hashem AGM, Ali HA (2014) Approaches to improve the solubility and availability of progesterone biotransformation by Mucor racemosus. Biocatal Biotransfor 32:141–150
Nikaido H, Jarlier V (1991) Permeability of the mycobacterial cell wall. Res Microbiol 142:437–443
Nikaido H, Kim SH, Rosenberg EY (1993) Physical organization of lipids in the cell wall of Mycobacterium chelonae. Mol Microbiol 8:1025–1030
Piel G, Piette M, Barillaro V, Castagne D, Evrard B, Delattre L (2007) Study of the interaction between cyclodextrins and liposome membranes: effect on the permeability of liposomes. J Incl Phenom Macrocycl Chem 57:309–311
Puglisi G, Fresta M, Ventura CA (1996) Interaction of natural and modified β-cyclodextrins with a biological membrane model of dipalmitoylphosphatidylcholine. J Colloid Interf Sci 180:542–547
Qiu YZ, Huang ZH, Song FJ (2012) Enzymatic activity enhancement of non-covalent modified superoxide dismutase and molecular docking analysis. Molecules 17:3945–3956
Roglič U, Žnidaršič-Plazl P, Plazl I (2005) The influence of β-cyclodextrin on the kinetics of progesterone transformation by Rhizopus nigricans. Biocat Biotrans 23(5):299–305
Schmidt AK, Cottaz S, Driguez H, Schulz GE (1998) Structure of cyclodextrin glycosyltransferase complexed with a derivative of its main product β-cyclodextrin. Biochemistry-US 37:5909–5915
Shen YB, Wang M, Li HN, Wang YB, Luo JM (2012) Influence of hydroxypropyl-β-cyclodextrin on phytosterol biotransformation by different strains of Mycobacterium neoaurum. J Ind Microbiol Biot 39:1253–1259
Shen YB, Wang M, Zhang LT, Ma YH, Ma B, Zheng Y, Liu H, Luo JM (2011) Effects of hydroxypropyl-β-cyclodextrin on cell growth, activity, and integrity of steroid-transforming Arthrobacter simplex and Mycobacterium sp. Appl Microbiol Biot 90:1995–2003
Thurmond RL, Niemi AR, Lindblom G, Wieslander A, Rilfors L (1994) Membrane thickness and molecular ordering in Acholeplasma laidlawii strain A studied by 2H NMR spectroscopy. Biochemistry-US 33:13178–13188
Varona S, Martín Á, Cocero MJ (2011) Liposomal incorporation of lavandin essential oil by a thin-film hydration method and by particles from gas-saturated solutions. J Ind Eng Chem Res 50:2088–2097
Wieslander Å, Nordström S, Dahlqvist A, Rilfors L, Lindblom G (1995) Membrane lipid composition and cell size of Acholeplasma laidlawii strain A are strongly influenced by lipid acyl chain length. Eur J Biochem 227:734–744
Williamson MP, Le Gal-Coëffet MF, Sorimachi K, Furniss CS, Archer DB, Williamson G (1997) Function of conserved tryptophans in the Aspergillus niger glucoamylase 1 starch binding domain. Biochemistry-US 36:7535–7539
Xiong D, Gao Z, Fu B, Sun H, Tian S, Xiao Y, Qin Z (2013) Effect of pyrimorph on soil enzymatic activities and respiration. Eur J Soil Biol 56:44–48
Zidovetzki R, Levitan I (2007) Use of cyclodextrins to manipulate plasma membrane cholesterol content: evidence, misconceptions and control strategies. BBA-Biomembranes 1768:1311–1324
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21076158, 21276196), Natural Science Foundation of Tianjin (13JCQNJC04900), and Tianjin High School Science & Technology Fund Planning Project (20120629).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 107 kb)
Rights and permissions
About this article
Cite this article
Shen, Y., Liang, J., Li, H. et al. Hydroxypropyl-β-cyclodextrin-mediated alterations in cell permeability, lipid and protein profiles of steroid-transforming Arthrobacter simplex . Appl Microbiol Biotechnol 99, 387–397 (2015). https://doi.org/10.1007/s00253-014-6089-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6089-5