Abstract
We developed a sensitive quantitative assay for detecting Ralstonia solanacearum in soil by most probable number (MPN) analysis based on bio-PCR results. For development of the detection method, we optimized an elution buffer containing 5 g/L skim milk for extracting bacteria from soil and reducing contamination of polymerase inhibitors in soil extracts. Because R. solanacearum can grow in water without any added nutrients, we used a cultivation buffer in the culture step of the bio-PCR that contained only the buffer and antibiotics to suppress the growth of other soil microorganisms. To quantify the bacterial population in soil, the elution buffer was added to 10 g soil on a dry weight basis so that the combined weight of buffer, soil, and soil-water was 50 g; 5 mL of soil extract was assumed to originate from 1 g of soil. The soil extract was divided into triplicate aliquots each of 5 mL and 500, 50, and 5 μL. Each aliquot was diluted with the cultivation buffer and incubated at 35 °C for about 24 h. After incubation, 5 μL of culture was directly used for nested PCR. The number of aliquots showing positive results was collectively checked against the MPN table. The method could quantify bacterial populations in soil down to 3 cfu/10 g dried soil and was successfully applied to several types of soil. We applied the method for the quantitative detection of R. solanacearum in horticultural soils, which could quantitatively detect small populations (9.3 cfu/g), but the semiselective media were not able to detect the bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ralstonia solanacearum is the causal agent of bacterial wilt in more than 100 species of plants, including economically important crops such as tomato, potato, pepper, and banana (Hayward 1991). R. solanacearum can survive for long periods in water or soil under natural conditions, although the number of viable cells decreases rapidly under dry or low-temperature conditions. The life history of this organism remains poorly understood. In disease management, it is important to understand the ecology of a pathogenic organism in the natural environment, and in studying a pathogen, its detection is one of the most important factor. To this end, various techniques have been developed for detecting R. solanacearum.
Semiselective media have been developed for detecting viable cells of R. solanacearum in soil and in plant tissues (Chen and Echandi 1982; Elphinstone et al. 1996; Granada and Sequeira 1983; Hara and Ono 1983; Karganilla and Buddenhagen 1972; Nesmith and Jenkins 1979; Okabe 1969). These media are able to detect 102–103 colony-forming units (cfu) per gram of dry soil, and colonies are countable within 48–72 h. There are, however, some saprophytic bacteria with colony morphology similar to that of R. solanacearum that make detection difficult.
Serological techniques are used for the primary screening of samples because these techniques are generally quick and reliable. They can detect as few as 104 cells per gram or per milliliter of sample (Elphinstone et al. 1996; Janse 1988; Pradhanang et al. 2000; Robinson-Smith et al. 1995). However, serological techniques can lack specificity because of cross-reactions of polyclonal antibodies with other bacteria and can have limited sensitivity. Serological techniques also have the disadvantage in that they do not discriminate between live and dead cells. Serological kits for detecting R. solanacearum are commercially available and are being used (Denny 2006; Ji et al. 2007).
Polymerase chain reaction (PCR) assays have also been developed for detection of R. solanacearum. PCR-based techniques are usually sensitive, and specific primers for R. solanacearum have been developed (Boudazin et al. 1999; Chen et al. 2010; Elphinstone et al. 1996; Huang et al. 2009; Kang et al. 2007; Kutin et al. 2009; Lee and Wang 2000; Opina et al. 1997; Pastrik and Maiss 2000; Pastrik et al. 2002; Schönfeld et al. 2003; Seal et al. 1993; Thammakijjawat et al. 2006; Weller et al. 2000). Nested PCR, co-operational PCR, and pre-PCR processing can enhance detection sensitivity (Caruso et al. 2003; Elphinstone et al. 1996; Grover et al. 2009; Pradhanang et al. 2000). Real-time PCR methods enable quantitative detection in soil and in plant tissues (Chen et al. 2010; Ha et al. 2012; Huang et al. 2009; Weller et al. 2000).
By combining these methods, it is possible to detect populations of R. solanacearum in a soil sample below 102 cells/g. However, PCR is often inhibited by compounds introduced into the reaction along with the sample. Therefore, methods for removing or reducing PCR inhibitors are applied. The method most often used to solve this problem is extraction of DNA from the sample. This method increases analysis time and cost while reducing detection sensitivity. Other methods involve washing the bacteria by centrifugation or immunocapture to reduce the concentration of inhibitors (Dittapongpitch and Surat 2003; Ha et al. 2012; Poussier et al. 2003), although target cells are also lost to some degree. Another method involves enriching the target population of R. solanacearum by culturing samples on semiselective medium prior to the PCR step (Elphinstone et al. 1996; Ito et al. 1998; Ozakman and Schaad 2003; Pradhanang et al. 2000). This method, known as bio-PCR, can reduce not only the concentration of PCR inhibitors but also the detection threshold. Furthermore, this method avoids the problem of detecting dead cells that is associated with general PCR techniques, because bio-PCR detects only cultured viable cells. One drawback, however, is that populations of saprophytic bacteria often have higher growth rates than R. solanacearum, making detection difficult, and in liquid culture, PCR inhibitors cannot be removed completely.
There is information available that suggests ways to improve the PCR detection method as applied to R. solanacearum. First, R. solanacearum can be grown to a density of 106 cells/mL in distilled water (Goto 1992). The use of distilled water can control the growth of saprophytic bacteria because there are no added nutrients, and a cell density of 106 cells/mL is sufficient for detection by PCR or serological techniques. Second, a solution of skim milk is known to improve the efficiency of DNA extraction from soil (Hoshino and Matsumoto 2004; Volossiouk et al. 1995), and it is possible that the presence of skim milk reduces the effects of PCR-inhibiting substances. Third, a combination of the most probable number (MPN) method with a PCR procedure (MPN-PCR) has recently been used for quantitative detection of microorganisms in soil, food, and water (Caruso et al. 2005; Fredslund et al. 2001; Kikuchi et al. 2001; Luan et al. 2008; Miwa et al. 2003, 2006; Thaithongnum et al. 2006). MPN-PCR has not yet been used for the quantitative detection of R. solanacearum in soil.
The goal of this study was to develop a sensitive method for the quantitative detection of R. solanacearum in soil. We tested several methods for eluting bacterial cells from soil and for cultivating them efficiently, and we investigated the conditions for detection by PCR combined with the MPN method.
Materials and methods
Bacterial strains and culture
We used Ralstonia solanacearum strains 8107R (a rifampicin-resistant mutant derived from strain 8107; Ministry of Agriculture, Forestry and Fisheries, Japan [MAFF] no. 107632, race 1, biovar 4, phylotype I, isolated from tomato), TG1-1 (MAFF no. 107639, race 4, biovar 3, phylotype I, isolated from ginger), and PS82-1 (MAFF no. 301559, race 3, biovar N2, phylotype IV, isolated from potato). These strains were stored at −70 °C and cultured on triphenyl tetrazolium chloride medium (Kelman 1954) or potato semisynthetic medium (PSA) (Wakimoto 1960) at 28 °C for 48 h. For the method development steps outlined below, we used bacteria suspended in a buffer solution of 1 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; pH 7.0). Bacteria were suspended in the sterile HEPES buffer solution and adjusted to an optical density of 0.30 at a wavelength of 600 nm. The viable bacterial population in this suspension was estimated at 1 × 108 cfu/mL after dilution plating on PSA or Hara-Ono medium (Hara and Ono 1983) at 28 °C for 72 h. During method development, this suspension was added to soil samples and then different elution buffers were tested for recovery efficiency. R. solanacearum was isolated from the soil extracts using Hara-Ono medium with or without 20 mg/L of rifampicin, at 28 °C for 72 h.
Soil extracts
The water content of the soil samples was determined by weight loss after drying at 150–180 °C for 3 h. Elution buffer (1 mM HEPES with varying concentrations of skim milk) was added to 10 g soil on a dry weight basis so that the combined weight of buffer, soil, and soil-water was 50 g. The mixture was shaken at 280 rpm for at least 30 min on an orbital shaker (SA31; Yamato Scientific, Tokyo, Japan) and then allowed to sit until the supernatant had fully separated. The supernatant was collected as the soil extract. We assumed that 5 mL of soil extract originated from 1 g of soil. In our preliminary experiments aimed at developing the detection method and MPN-PCR, we used an Andosol soil sample collected in Tsukuba, Ibaraki, Japan, where bacterial wilt has not yet appeared and R. solanacearum was not detected by conventional methods. After perfecting our method, it was tested on agricultural soil from locations in Ibaraki, Hokkaido, Yamaguchi, and Niigata prefectures. These soil extracts were also tested for the presence of R. solanacearum using Hara-Ono medium with or without 20 mg/L of rifampicin at 28 °C for 72 h, for comparison with our newly developed method.
PCR amplification
We performed two-stage nested PCR. The region around the phcA gene sequence of R. solanacearum strain GMI1000 (available from GenBank; accession number AL646053) was used to derive primer sets for the first stage (phcA2981f [5′-TGGATATCGGGCTGGCAA-3′] and phcA4741r [5′-CGCTTTTGCGCAAAGGGA-3′]) and the second stage (phcA3538f [5′-GTGCCACAGCATGTTCAGG-3′] and phcA4209r [5′-CCTAAAGCGCTTGAGCTCG-3′]). For the first stage, 5 μL of bacterial suspension or a cultured soil extract was heated for 2 min at 98 °C. This solution was added to a 15 μL reaction mixture containing 0.5 U KAPA2G Robust HotStart DNA Polymerase (KAPA Biosystems, Woburn, MA, USA), PCR reaction buffer for high GC content (KAPA Biosystems), 4 nmol of each dNTP, and 3.2 pmol of each first-stage primer. The PCR was performed using the following conditions: 2 min at 95 °C; 30 cycles of 30 s at 95 °C, 30 s at 57.5 °C, and 60 s at 72 °C; and a final extension for 5 min at 72 °C.
For the second stage, the same 15 μL reaction mixture was used, but with the second-stage primer set and 5 μL of the mixture from the first-stage reaction. The PCR reaction conditions were the same as in the first stage except the extension time was changed to 30 s. For comparison, we also performed a conventional PCR with Rsol_fliC primers, as described by Schönfeld et al. (2003).
MPN-PCR conditions
Soil extracts were separated into three aliquots each of 5 mL and 500, 50, and 5 μL. These correspond to 1, 0.1, 0.01, and 0.001 g of the original soil sample, respectively. Each aliquot was diluted 10-fold (5 mL and 500 and 50 μL aliquots) or 20-fold (5 μL aliquot) with the cultivation buffer and incubated at 35 °C for at least 20 h. The cultivation buffer consisted of 1 mM HEPES, with the antibiotics penicillin G potassium (0.5 or 5 mg/L), bacitracin (25 mg/L), chloramphenicol (0.5 or 5 mg/L), and polymyxin B sulfate (8 or 80 mg/mL) and the pigment Crystal Violet (0.5 or 5 mg/L), alone or in combination. After incubation, 5 μL of culture was used for PCR amplification. Duplicate two-stage nested PCR was performed, and when specific amplification was detected by either or both assays, the aliquot was considered to contain viable R. solanacearum cells. The number of aliquots at each dilution showing positive results was collectively checked against the MPN table (Supplementary Table S1). MPN is evaluated using the number of positive results from three sequential soil extract sample volumes. If the results from the 5 mL to 50 μL aliquots are used, the number of bacteria per 10 g can be predicted; if the results from the 500 to 5 μL aliquots are used, the number of bacteria per 1 g can be predicted. Therefore, using our series of soil extract aliquots, the lowest usable results can predict the existence of three viable cells per 10 g soil, and the highest can predict the existence of at least 2,400 cells/1 g. For example, when positive detections numbered 3, 3, 1, and 0 from the respective 5 mL to 5 μL aliquots, the values 3, 3, and 1 (from 5 mL to 50 μL) applied to the MPN table yield an expected cell density of 460 cells/10 g (46 cells/g) soil, and the values 3, 1, and 0 (from 500 to 5 μL) yield an expected cell density of 43 cells/g soil. The number adopted is the larger of the two predictions. The steps in the MPN-PCR method are shown in Fig. 1. The limit of detection by semiselective media is around 102–103 cfu/g of soil. We therefore designed our MPN-PCR method so that the maximum level of detection overlapped the minimum limit of detection by the semiselective medium.
Results
Preliminary development of the detection method
For comparative purposes, we first assessed the limit of detection using conventional PCR. R. solanacearum strain 8107R was serially diluted 10-fold to contain from 108 to 101 cfu/mL. Conventional PCR with Rsol_fliC primers detected densities as low as 104 cfu/mL (Supplementary Fig. S1). In contrast, the nested PCR with new primer sets detected densities down to 103 cfu/mL (Supplementary Fig. S1). However, the nested PCR with new primer sets did not amplify the DNA fragment from biovar N2 strains of R. solanacearum (Supplementary Fig. S2). In this study, we used the nested PCR technique with the higher detection sensitivity.
We next tested different media for cultivating R. solanacearum. For this test, we followed the growth of R. solanacearum strain 8107R cultured in Hara-Ono medium with 10 μg/mL of rifampicin. When about 10–20 cells of 8107R were added to 10 mL of sterilized HEPES buffer solution (pH 7.0) and incubated at 35 °C for 24 h, the number of cells increased about 200-fold (Table 1). The same number of cells increased by a factor of 105–6 when added to the HEPES buffer solution containing 10 % by volume of a filter-sterilized (0.22 μm) soil extract from Andosol. In contrast, in nonsterilized soil extract diluted by a factor of 10 with the buffer, there was extensive growth of a saprophyte, and cell numbers of strain 8107R increased by a factor of about 103. When the nonsterilized soil extract was diluted by a factor of 10 with the buffer solution containing 0.5 mg/L of penicillin G potassium, 25 mg/L of bacitracin, and 0.5 mg/L of chloramphenicol, 8107R cell numbers increased by a factor of about 104. For this study, we used this latter buffer solution (without soil extract) containing the three antibiotics as the cultivation buffer. The antibiotic polymyxin B sulfate and the pigment Crystal Violet inhibited growth of R. solanacearum in water (data not shown).
In a third experiment, we varied the solution used to elute R. solanacearum cells from the soil. We tested solutions with skim milk concentrations of 0, 2.5, 5, 10, and 20 g/L in the HEPES buffer. We used soil artificially inoculated with R. solanacearum and estimated the number of eluted bacteria by counting colonies on Hara-Ono medium with rifampicin (data not shown). There was no difference in the extraction efficiency of 8107R between the concentrations of skim milk. In a separate check of extraction efficiency, the soil extracts were diluted by a factor of 10 with the cultivation buffer and incubated at 35 °C for 24 h. After incubation, 5 μL of the solution was used for nested PCR. Although R. solanacearum was always detected in soil extracts using the extraction buffer with 2.5, 5, or 10 g/L skim milk, it was often not detected using the buffer containing 0 or 20 g/L skim milk (Fig. 2). In this study, we used the buffer solution with 5 g/L skim milk as the elution buffer.
PCR product banding patterns amplified by nested PCR from a suspension containing soil extract incubated at 35 °C for 24 h. Ralstonia solanacearum cells were eluted from artificially inoculated soil using buffer solutions (1 mM HEPES-HCl, pH 7.0) with skim milk concentrations of 0 g/L (lanes 1 and 2), 2.5 g/L (lanes 3 and 4), 5 g/L (lanes 5 and 6), 10 g/L (lanes 7 and 8), and 20 g/L (lanes 9 and 10). Lanes 11 and 12 show PCR product banding patterns amplified from a suspension containing only R. solanacearum cells. Lane M contains the 1-kb Plus DNA Ladder marker (Invitrogen, Carlsbad, CA, USA)
Finally, we checked the sensitivity of the detection procedure. We produced a soil extract using the elution buffer. Ten microliters of the 8107R suspension at the concentration of ca. 100 cfu/mL was plated onto Hara-Ono medium. In addition, 10 μL of the same suspension was added to 5 mL soil extract, diluted by a factor of 10 with the cultivation buffer, and incubated at 35 °C for at least 20 h. After incubation, 5 μL of the solution was used for PCR. In four repeat trials, the PCR method detected bacteria in 5–8 of 10 replicates (Fig. 3). In contrast, out of four trials of direct plating onto Hara-Ono medium, using 10 plates in each trial, bacterial colonies grew on three to seven plates, with one to five bacterial colonies per plate (Fig. 3). These results suggest that our new detection procedure can detect one viable cell in 50 mL of diluted soil extract; it was converted that 5 mL of soil extract originated in 1 g of soil.
The number of plates showing colony growth after plating with a soil extract suspension containing about one cell of Ralstonia solanacearum strain 8107R and the PCR product banding patterns from a suspension containing the extract from 1 g soil and about one added cell, incubated at 35 °C for 24–48 h and amplified by nested PCR
MPN-PCR
Two tubes containing 10 g (dry weight) of nonsterile soil were inoculated with 100 μL of suspensions of 8107R previously prepared at 5 × 104 and 5 × 102 cfu/mL, respectively. Elution was carried out within 5 min of inoculation. Soil extracts were separated into three aliquots each of 5 mL and 500, 50, and 5 μL, added to the cultivation buffer, and incubated at 35 °C for 20 h. In addition, 10 μL of the 8107R suspension at 5 × 102 cfu/mL was spread on Hara-Ono medium with 10 replicates to get an exact measure of the bacteria population in the suspension. Both the MPN-PCR test and the plate counts were repeated three times. The averages of the number of colonies on a plate were 4.0, 3.6, and 5.6, respectively. The number of colonies per plate ranged from one to eight. In the MPN-PCR analysis of the soil inoculated with about 5 × 103 cfu of 8107R, the profiles of detection were (3, 3, 3, 1), (3, 3, 3, 2), and (3, 3, 3, 2), respectively. The average of the three resulting MPN values for R. solanacearum is 8.9 × 102 cfu/g. In the extract from soil inoculated with about 50 cfu of 8107R, the profiles of detection were (2, 1, 1, 0), (3, 0, 0, 0), and (1, 0, 1, 0), respectively. The average of the three resulting MPN values for R. solanacearum is 1.67 cfu/g. There is close agreement between the number of bacteria inoculated into the soil and the number detected by MPN-PCR. There was no significant difference between the number of bacteria inoculated into the soil and the number detected by MPN-PCR (t test, P > 0.05). For comparison, 50 μL of soil extract that was prepared from 10 g of soil inoculated with about 50 cfu of 8107R was spread on each of 20 plates containing Hara-Ono medium. No R. solanacearum colonies formed.
Detection of R. solanacearum in horticultural soil samples
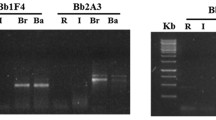
The horticultural soils used to test the new method were collected in Ibaraki, Hokkaido, Yamaguchi, and Niigata prefectures from fields in which tomatoes were cultivated and bacterial wilt was observed. The soil collected from Tsukuba in Ibaraki Prefecture was an Andosol. There were positive results in 3, 2, 0, and 0 aliquots from the dilutions representing 1, 0.1, 0.01, and 0.001 g of soil, respectively (Fig. 4a). These values applied to the MPN table give an expected count of 9.3 cells/g soil. In addition, 50 μL of soil extract was spread on each of 10 plates containing Hara-Ono medium. From this total of 500 μL of the soil extract, no R. solanacearum colonies formed.
PCR product banding patterns amplified by nested PCR from a suspension incubated at 35 °C overnight, containing the extract from 1 g (lanes 1–3), 0.1 g (lanes 4–6), 0.01 g (lanes 7–9), or 0.001 g (lanes 10–12) of horticultural soil. The soil was collected from Ibaraki (a), Niigata (b), Hokkaido (c), and Yamaguchi (d) prefectures
The soil collected in Niigata, Niigata Prefecture, was a brown lowland soil. R. solanacearum was detected in 3, 3, 0, and 0 replicate aliquots from dilutions representing 1, 0.1, 0.01, and 0.001 g soil, respectively (Fig. 4b). These values applied to the MPN table give an expected value of 24 cells/g. A total of 500 μL of soil extract was spread on Hara-Ono medium as described in the previous paragraph. A total of two colonies of R. solanacearum formed, indicating an estimated 0.2 × 102 cfu/g.
The soil collected in Sunagawa, Hokkaido, was a brown lowland soil. R. solanacearum was detected in 3, 3, 3, and 3 replicate aliquots from dilutions representing 1, 0.1, 0.01, and 0.001 g soil, respectively (Fig. 4c). These values applied to the MPN table give an expected count of at least 2,400 cells/g. In addition, 5 μL of soil extract was spread on each of 20 plates containing Hara-Ono medium for a total of 100 μL of soil extract. A total of 48 colonies of R. solanacearum formed, indicating an estimated 2.4 × 103 cfu/g.
The soil collected in Yamaguchi, Yamaguchi Prefecture, was a sandy soil. R. solanacearum was detected in 3, 3, 3, and 3 replicate aliquots representing 1, 0.1, 0.01, and 0.001 g soil, respectively (Fig. 4d). These values applied to the MPN table give an expected value of at least 2,400 cells/g. In addition, 5 μL of soil extract was spread on each of 10 plates containing Hara-Ono medium, and a total of 144 colonies of R. solanacearum formed, indicating an estimated 14.4 × 103 cfu/g.
Discussion
We developed a new quantitative detection method for R. solanacearum in soil. This method has unique features in that it does not use a culture medium with added nutrients for cultivation, and PCR is performed without extracting DNA from the culture. The bio-PCR method can detect the presence of bacteria at densities as low as 1 cfu/g of soil. Furthermore, the number of bacteria in 10 g of soil can be predicted by the MPN-PCR method. The new method was successfully applied to various kinds of soil.
The buffer containing skim milk was effective for eluting R. solanacearum from the soil. Several concentrations of skim milk were first tested. The bacterium was often not detected using the buffer containing 20 g/L skim milk, even though it was present, and the extract often had a foul smell after incubation. This might be because any excess skim milk remaining in the supernatant could inhibit PCR and enhance the growth of saprophytic microorganisms. Hoshino and Matsumoto (2004) showed that the amount of DNA extracted with 100 mg of skim milk per 0.5 g of soil was sometimes less than with 40 mg of skim milk; the excess skim milk inhibited the extraction of DNA from soils. In our method, about 200 mg of skim milk (included in 40 mL of a 5 g/L solution) is added to 10 g of soil, similar to the method of Volossiouk et al. (1995).
When developing detection technology, it is important to encourage the growth of only the target microorganism. Conventional methods involve cultivation in enriched medium and increase bacterial densities to about 107 cfu/mL, if only R. solanacearum cells are present (Pradhanang et al. 2000). However, the growth rate of saprophytic bacteria often exceeds that of R. solanacearum, and if these organisms are present, the R. solanacearum population remains low or decreases in the enrichment medium (Pradhanang et al. 2000). This makes detection of R. solanacearum difficult by these methods. On the other hand, PCR methods that use bacterial suspensions can detect R. solanacearum at 103–104 cfu/mL (Dittapongpitch and Surat 2003; Ha et al. 2012; Weller et al. 2000; this study); therefore, large populations are not necessary. Goto (1992) reported that R. solanacearum can be grown to a density of 106 cells/mL in distilled water. We confirmed that the cell numbers of strain 8107R increased about 200-fold in our buffer solution (Table 1) and by a factor of about 104 in the soil extract, after dilution by a factor of 10 with the cultivation buffer and incubation at 35 °C for 24 h. Thus, although the bacterial population does not greatly increase in a solution that does not contain nutrients, the growth is sufficient for detection by PCR. In this study, we used the HEPES buffer to stabilize the pH of the suspension. In preliminary tests, we confirmed that there was no difference in the increase in bacterial numbers between the HEPES buffer and water (data not shown).
The MPN-PCR method is a powerful tool for detecting low numbers of bacterial cells and has been used for quantitative detection of microorganisms in soil, food, and water (Caruso et al. 2005; Fredslund et al. 2001; Kikuchi et al. 2001; Luan et al. 2008; Miwa et al. 2003, 2006; Thaithongnum et al. 2006). The MPN method requires highly reliable, sensitive, and selective detection of the target bacteria. However, there is no direct method for sensitive and selective detection of R. solanacearum in soil. Therefore, the MPN method has been used for the quantitative detection of R. solanacearum in river water (Caruso et al. 2005) but not in soil. Quantitative detection is possible by using semiselective media if the bacteria exist at densities above 102–103 cfu/g (Denny 2006); however, detection is difficult at low bacterial numbers. Therefore, in this study, we designed the method to be able to detect bacterial numbers from 0.3 cfu/g of soil up to 2,400 cfu/g of soil; the maximum limit of our method overlaps the minimum detection limit by the semiselective medium method.
The new primer sets developed as part of this study can be used to specifically detect R. solanacearum from horticultural soils. However, these primer sets did not amplify a specific DNA fragment from the biovar N2 (phylotype IV) strain of R. solanacearum. Group-specific PCR primers have been reported (Fegan and Prior 2005). It is possible that our new primers can also be used for grouping. The phcA 4741r primer was designed for a specific region of the genome of R. solanacearum strain GMI1000. To detect a phylotype IV strain, it will be necessary to redesign the primer. The conventional nested PCR technique described by Pradhanang et al. (2000) was unable to amplify their target DNA fragment from the soil extract containing 8107R (data not shown). We did not, however, use the same DNA polymerase or thermal cycler that was used in their study. If PCR conditions are optimized, it might be possible to successfully amplify the target DNA. Conventional PCR with Rsol_fliC primers was able to detect the amplified fragments but sometimes failed (data not shown). In soil extract containing saprophytic bacteria, increases in R. solanacearum densities may be limited to a maximum of 103 to 104 cells/mL. To detect R. solanacearum with certainty, improvements in the conventional PCR method are required, such as in the design of nested primers.
We applied methods for the quantitative detection of R. solanacearum to the soil of four tomato fields in which bacterial wilt disease was observed. In samples from two of these fields, the results from our new method were in agreement with those from the method using semiselective medium. This shows that the semiselective medium could be useful for the quantitative detection of R. solanacearum from soil containing more than 103 cfu/g soil. However, the semiselective medium method did not work in soil containing bacterial populations at less than 102 cfu/g soil because of contamination by saprophytic bacteria on the plates (Elphinstone et al. 1996; Pradhanang et al. 2000) and the need for many plates of medium (at least 10 plates are necessary to detect approximately 20 cfu of the pathogen in 1 g of soil). In contrast, our MPN-PCR method could easily quantitatively detect bacterial populations at fewer than 102 cfu/g soil (in fact, as low as 3 cfu/10 g dry soil), a detection limit more than 100-fold below that by the usual selective medium methods. The MPN-PCR method is not much different in terms of time and effort than conventional bio-PCR and can be completed in 2 days, whereas the detection by a selective medium takes 3 days. Our new method will be useful for detection of R. solanacearum in horticultural soils. We are currently investigating the relationship between the extent of bacterial wilt outbreaks and pathogen density in the soil.
References
Boudazin G, Le Roux AC, Josi K, Labarre P, Jouan B (1999) Design of division specific primers of Ralstonia solanacearum and application to the identification of European isolates. Eur J Plant Pathol 105:373–380
Caruso P, Bertolini E, Cambra M, Lopez MM (2003) A new and sensitive co-operational polymerase chain reaction for rapid detection of Ralstonia solanacearum in water. J Microbiol Meth 55:257–272
Caruso P, Palomo JL, Bertolini E, Alvarez B, Lopez MM, Biosca EG (2005) Seasonal variation of Ralstonia solanacearum biovar 2 populations in a Spanish river, recovery of stressed cells at low temperatures. Appl Environ Microbiol 71:140–148
Chen W-Y, Echandi E (1982) Bacteriocin production and semiselective medium for detection, isolation and quantification of Pseudomonas solanacearum in soil. Phytopathology 72:310–313
Chen Y, Zhang W-Z, Liu X, Ma Z-H, Li B, Allen C, Guo J-H (2010) A real-time PCR assay for the quantitative detection of Ralstonia solanacearum in horticultural soil and plant tissues. J Microbiol Biotechnol 20:193–201
Denny TP (2006) Plant pathogenic Ralstonia species. In: Gnanamanickam SS (ed) Plant-associated bacteria. Springer, Dordrecht, The Netherlands, pp 573–644
Dittapongpitch V, Surat S (2003) Detection of Ralstonia solanacearum in soil and weeds from commercial tomato fields using immunocapture and the polymerase chain reaction. J Phytopathol 151:239–246
Elphinstone JG, Henessy J, Wilson JK, Stead D (1996) Sensitivity of different methods for the detection of Ralstonia solanacearum in potato tuber extracts. Bull OEPP/EPPO Bull 26:663–678
Fegan M, Prior P (2005) How complex is the Ralstonia solanacearum species complex. In: Allen C, Prior P, Hayward AC (eds) Bacterial wilt disease and the Ralstonia solanacearum species complex. APS Press, St. Paul, MN, pp 449–461
Fredslund L, Ekelund F, Jacobsen CS, Kaare Johnsen K (2001) Development and application of a most-probable-number-PCR assay to quantify flagellate populations in soil samples. Appl Environ Microbiol 67:1613–1618
Goto M (1992) Lethal dilution effect. Pages 61–63 in. Fundamentals of bacterial plant pathology. Academic Press, New York
Granada GA, Sequeira L (1983) A new selective medium for Pseudomonas solanacearum. Plant Dis 67:1084–1088
Grover A, Azmi W, Khurana SMP, Chakrabarti SK (2009) Multiple displacement amplification as a pre-polymerase chain reaction (pre-PCR) to detect ultra low population of Ralstonia solanacearum (Smith 1896) Yabuchi et al. (1996). Lett Appl Microbiol 49:539–543
Ha Y, Kim J-S, Denny TP, Schell MA (2012) A rapid, sensitive assay for Ralstonia solanacearum race 3 biovar 2 in plant and soil samples using magnetic beads and real-time PCR. Plant Dis 96:258–264
Hara H, Ono K (1983) Ecological studies on the bacterial wilt of tobacco, caused by Pseudomonas solanacearum E. F. Smith. I. A selective medium for isolation and detection of P. solanacearum. Bull Okayama Tob Exp Stn 42:127–138
Hayward AC (1991) Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu Rev Phytopathol 29:65–87
Hoshino YT, Matsumoto N (2004) An improved DNA extraction method using skim milk from soils that strongly adsorb DNA. Microbes Environ 19:13–19
Huang J, Wu J, Li C, Xiao C, Wang G (2009) Specific and sensitive detection of Ralstonia solanacearum in soil with quantitative, real-time PCR assays. J Appl Microbiol 107:1729–1739
Ito S, Ushijima Y, Fujii T, Tanaka S, Kameya-Iwaki M, Yoshiwara S, Kishi F (1998) Detection of viable cells of Ralstonia solanacearum in soil using a semiselective medium and a PCR technique. J Phytopathol 146:379–384
Janse JD (1988) A detection method for Pseudomonas solanacearum in symptomless potato tubers and some data on its sensitivity and specificity. Bull OEPP/EPPO Bull 18:343–351
Ji P, Allen C, Sanchez-Perez A, Yao J, Elphinstone JG, Jones JB, Momol MT (2007) New diversity of Ralstonia solanacearum strains associated with vegetable and ornamental crops in Florida. Plant Dis 91:195–203
Kang MJ, Lee MH, Shim JK, Seo ST, Shrestha R, Cho MS, Hahn JH, Park DS (2007) PCR-based specific detection of Ralstonia solanacearum by amplification of cytochrome c1 signal peptide sequences. J Microbiol Biotechnol 17:1765–1771
Karganilla AD, Buddenhagen IW (1972) Development of a selective medium for Pseudomonas solanacearum. Phytopathology 62:1373–1376
Kelman A (1954) The relationship of pathogenicity in Pseudomonas solanacearum to colony appearance on a tetrazolium medium. Phytopathology 44:693–695
Kikuchi T, Iwasaki K, Nishihara H, Takamura Y, Yagi O (2001) Quantitative and specific detection of a trichloroethylene-degrading methanotroph, Methylocystis sp. strain M, by a most probable number-polymerase chain reaction method. Biosci Biotechnol Biochem 65:2673–2681
Kutin RK, Alvarez A, Jenkins DM (2009) Detection of Ralstonia solanacearum in natural substrates using phage amplification integrated with real-time PCR assay. J Microbiol Meth 76:241–246
Lee YA, Wang CC (2000) The design of specific primers for the detection of Ralstonia solanacearum in soil samples by polymerase chain reaction. Bot Bull of Acad Sin 41:121–128
Luan X, Chen J, Liu Y, Li Y, Jia J, Liu R, Zhang X-H (2008) Rapid quantitative detection of Vibrio parahaemolyticus in seafood by MPN-PCR. Curr Microbiol 57:218–221
Miwa N, Nishio T, Arita Y, Kawamori F, Masuda T, Akiyama M (2003) Evaluation of MPN method combined with PCR procedure for detection and enumeration of Vibrio parahaemolyticus in seafood. J Food Hyg Soc Japan 44:289–293
Miwa N, Kashiwagi M, Kawamori F, Masuda T, Sano Y, Hiroi M, Kurashige H (2006) Levels of Vibrio parahaemolyticus and thermostable direct hemolysin gene-positive organisms in retail seafood determined by the most probable number-polymerase chain reaction (MPN-PCR) method. J Food Hyg Soc Japan 47:41–45
Nesmith WC, Jenkins SF Jr (1979) A selective medium for the isolation and quantification of Pseudomonas solanacearum from soil. Phytopathology 69:182–185
Okabe N (1969) Population changes of Pseudomonas solanacearum and soil microorganisms in artificially infested natural field soil. Bull Fac Agr Shizuoka Univ 19:1–29
Opina N, Tavner F, Hollway G, Wang J-F, Li T-H, Maghirang R, Fegan M, Hayward AC, Krishnapillai V, Hong WF, Holloway BW, Timmis JN (1997) A novel method for development of species and strain specific DNA probes and PCR primers for identifying Burkholderia solanacearum (formerly Pseudomonas solanacearum). Asia Pac J Mol Biol Biotechnol 5:19–30
Ozakman M, Schaad NW (2003) A real-time BIO-PCR assay for detection of Ralstonia solanacearum race 3, biovar 2, in asymptomatic potato tubers. Can J Plant Pathol 25:232–239
Pastrik KH, Maiss E (2000) Detection of Ralstonia solanacearum in potato tubers by polymerase chain reaction. J Phytopathol 148:619–626
Pastrik KH, Elphinstone JG, Pukall R (2002) Sequence analysis and detection of Ralstonia solanacearum by multiplex PCR amplification of 16S-23S ribosomal intergenic spacer region with internal positive control. Eur J Plant Pathol 108:831–842
Poussier S, Cheron JJ, Couteau A, Luisetti J (2003) Evaluation of procedures for reliable PCR detection of Ralstonia solanacearum in common natural substrates. J Microbiol Meth 51:349–359
Pradhanang PM, Elphinstone JG, Fox RTV (2000) Sensitive detection of Ralstonia solanacearum in soil: a comparison of different detection techniques. Plant Pathol 49:414–422
Robinson-Smith A, Jones P, Elphinstone JG, Forde SMD (1995) Production of antibodies to Pseudomonas solanacearum, the causative agent of bacterial wilt. Food Agric Immunol 7:67–79
Schönfeld J, Heuer H, van Elsas JD, Smalla K (2003) Specific and sensitive detection of Ralstonia solanacearum in soil on the basis of PCR amplification of fliC fragments. Appl Environ Microbiol 69:7248–7256
Seal SE, Jackson LA, Young JPW, Daniels MJ (1993) Differentiation of Pseudomonas solanacearum, Pseudomonas syzygii, Pseudomonas pickettii and the blood disease bacterium by partial 16S rRNA sequencing: construction of oligonucleotide primers for sensitive detection by polymerase chain reaction. J Gen Microbiol 139:1587–1594
Thaithongnum S, Ratanama P, Weeradechapol K, Sukhoom A, Vuddhakul V (2006) Detection of V. harveyi in shrimp postlarvae and hatchery tank water by the most probable number technique with PCR. Aquaculture 261:1–9
Thammakijjawat P, Thaveechai N, Kositratana W, Chunwongse J, Frederick RD, Schaad NW (2006) Detection of Ralstonia solanacearum in ginger rhizomes by real-time PCR. Can J Plant Pathol 28:391–400
Volossiouk T, Robb E, Nazar R (1995) Direct DNA extraction for PCR-mediated assays of soil organisms. Appl Environ Microbiol 61:3972–3976
Wakimoto S (1960) Classification of strains of Xanthomonas oryzae on the basis of their susceptibility against bacteriophages. Ann Phytopathol Soc Jpn 25:193–198
Weller SA, Elphinstone JG, Smith NC, Boonham N, Stead DE (2000) Detection of Ralstonia solanacearum strains with a quantitative, multiplex, real-time, fluorogenic PCR (TaqMan) assay. Appl Environ Microbiol 66:2853–2858
Acknowledgments
We thank Ms. A Notsu (Ornamental Plants and Vegetables Research Center, Hokkaido Research Organization), Dr. M Maeda (Niigata Agricultural Research Institute), and Dr. H Kajihara (Yamaguchi Prefectural Technology Center for Agricultural and Forestry) for kindly providing the horticultural soil samples. This work was supported by a Grant-in-Aid for Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries and Food Industry from the Ministry of Agriculture, Forestry and Fisheries, Japan (25062C).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 159 kb)
Rights and permissions
About this article
Cite this article
Inoue, Y., Nakaho, K. Sensitive quantitative detection of Ralstonia solanacearum in soil by the most probable number-polymerase chain reaction (MPN-PCR) method. Appl Microbiol Biotechnol 98, 4169–4177 (2014). https://doi.org/10.1007/s00253-014-5604-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5604-z