Abstract
Fibroblast growth factor receptor 3 (FGFR3) is a noted proto-oncogene involved in the pathogenesis of many tumors, so more and more studies focus on the potential use of receptor kinase inhibitor and therapeutic antibodies against FGFR3. In this study, we designed a novel fusion protein containing the single-chain Fv (ScFv) against FGFR3 and 9-arginine, denoted as ScFv-9R. To achieve the high-level production and soluble expression, ScFv and ScFv-9R were fused with small ubiquitin-related modifier (Sumo) by polymerase chain reaction and expressed in Escherichia coli BL21 (DE3). The recombinant bacteria was induced by 0.5 mM isopropyl-β-d-thiogalactopyranoside for 20 h at 20 °C; supernatants of Sumo-ScFv was harvested and purified by DEAE Sepharose FF and Ni-NTA orderly, and supernatants of Sumo-ScFv-9R was harvested and purified by Ni-NTA. After cleaved by the Sumo protease, the recombinant ScFv or ScFv-9R was released from the fusion protein, respectively. The purity of ScFv or ScFV-9R was shown to be higher than 90 %, and their yield reached 3–5 mg per liter of bacterial culture. In vitro data showed that ScFV-9R can attenuate the phosphorylation of FGFR3 and ERK in the absence or presence of FGF9. Gel retardation assay showed that 1 μg of ScFv-9R could efficiently bind to about 4 pmol siRNA. Fluorescent microscope analysis showed that ScFv-9R can efficiently bind and deliver siRNA into RT112 cells. In conclusion, we use Sumo fusion system to acquire high-level production, soluble expression, and bifunctional activity of ScFv-9R in E. coli. Our results also revealed that ScFv-9R, as a novel carrier, may have potential applications in antitumor studies and pharmaceutical development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroblast growth factors (FGFs) are composed of over 20 members that bind to fibroblast growth factor receptors (FGFRs). As multifunctional signaling moleculars, FGFs exert important functions in regulating cell proliferation, differentiation, and survival during embryo development and adult stage (Wesche et al. 2011). Many reports indicated deregulation of FGFRs involved in a large number of diseases, especially cancer. Of all the FGFRs, overexpression or activating mutations of FGFR3 was highlighted and defined in many types of cancer, such as multiple myeloma (MM) (Plowright et al. 2000), bladder cancer (di Martino et al. 2012), breast cancer (Glénisson et al. 2012), and colorectal cancer (Sonvilla et al. 2010). Enhancing FGFR3 signaling promotes tumor growth, metastasis, and resistance to drug. Thus, FGFR3 has been considered as a novel therapy target for several tumors, e.g., multiple myeloma (Qing et al. 2009) and bladder cancer (Qing et al. 2009;Du et al. 2012).
There is no doubt that antibody-based therapeutics have been a prospective direction for clinical therapy and pharmaceutical development of human malignancies. Monoclonal antibody or single-chain Fv (ScFv) against FGFR3 has been screened and applied to study antitumor activity in vitro and in vivo. PRO-001, a monoclonal antibody against FGFR3 inhibits phosphorylation of FGFR3 and downstream signaling ERK activation in MM cells (Trudel et al. 2006). ScFv directed against FGFR3 was shown to inhibit proliferation of bladder cancer cell line RT112 in vitro (Martínez-Torrecuadrada et al. 2005). Immunotoxin, 3C/rGel, which consists of FGFR3-specific Fv fragments and NH2 terminus of rGel significantly exhibited antitumor activity in RT112 tumor xenografts by inducing cell apoptosis (Martínez-Torrecuadrada et al. 2008).
In recent years, RNA interference (RNAi) has been considered as a therapeutic method against cancer. However, how to deliver siRNA into target cells becomes a key question for its application. A new type of fusion protein containing ScFv and basic polypeptide (e.g., protamine, 9-arginine) was created by combination of ScFv technique and RNAi theory. It can deliver siRNA into ScFv-targeting cancer cells as a novel siRNA carrier. Up to now, there exist many fusion protein targeting different types of cancer cells, such as ErbB2+ breast cancer cells (Li et al. 2001), HIV env+ cells (Song et al. 2005), HBsAg+ cells (Wen et al. 2007), and Her2+ breast cancer cells (Yao et al. 2012).
In our studies, we designed a new fusion molecular, ScFv-9R-containing ScFv against FGFR3 (Martínez-Torrecuadrada et al. 2005) and 9-arginine, which specifically binds to siRNA and delivers siRNA into FGFR3+ cancer cells via silencing specific gene expression to suppress tumor growth. In order to get better bioactivity of ScFv, recombinant ScFv was often expressed in eukaryocyte, such as SF21 cells (Li et al. 2001), SF9 cells (Yao et al. 2012), and HEK293 T cells (Jäger et al. 2013), but low protein production and high cost impede its application. Compared with eukaryocyte expression system, the Escherichia coli expression system is very popular to get recombinant protein in vitro, but it is also easier to form inclusion body which needs to be denatured, refolded, and purified through many chromatography columns. Previous studies report that recombinant ScFv often forms inclusion body using traditional E. coli expression method (Ye et al. 2012; Wang et al. 2013). To overcome these shortcomings, we chose a novel expression system, small ubiquitin-related modifier (Sumo) molecular partner, which had been testified by our group and many other labs. Various proteins, such as MMP13 (Marblestone et al. 2006), FGF21 (Wang et al. 2010), and FGF23 (Liu et al. 2012), have been expressed successfully by being fused with Sumo. Our results showed that Sumo is very helpful to promote soluble expression of recombinant ScFV-9R, which is a promising drug carrier to efficiently deliver siRNA into FGFR3-positive cancer cells.
Materials and methods
Reagents
Prime STAR®GXL DNA Polymerase was purchased from TaKaRa Company (Japan). Restriction enzymes NdeI and XhoI were purchased from NEB (England). Gel extraction kit and plasmid miniprep kit were obtained from the CW Bio Company (China). DEAE Sepharose FF and Ni-NTA agarose were purchased from GE Healthcare (Sweden). Lipofectamine® 2000 (Lipo-2000) was purchased from Invitrogen Co. Anti-His antibody was provided from Proteintech (Chicago). p-FGFR3 (Tyr724) antibody was purchased from Santa Cruz (USA). Anti-FGFR3 antibody was obtained from Abcam (USA). Anti-ERK (1/2), anti-phospho-ERK (1/2), and anti-beta-actin antibody were purchased from Cell Signaling (USA). All siRNA duplexes were synthesized and labeled with FAM by GenePharma Co. Ltd. (China). Pierce® BCA Protein Assay Kit was purchased from Thermo Company (USA). The human bladder cancer cell line RT112 was supported by JENNIO Company (China). pET-20b-Sumo plasmid was kept in Biochemistry Lab, College of Basic Medical Science, Jilin University. Dr. Qi Xiang from Jinan University generously supplied us with the Sumo protease.
Design of fusion genes and constructions of pET-Sumo-ScFv and pET-Sumo-ScFv-9R
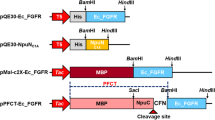
The gene sequence of anti-FGFR3 ScFv was synthesized by Generay Biotech Co. (China), and its format is VH-linker-VL according to the prior report (Martínez-Torrecuadrada et al. 2005). The fusion protein comprised of anti-FGFR3 ScFv and 9-arginine was denoted as ScFv-9R. According to the sequence of ScFv, ScFv-9R, and Sumo fragment, five primers were designed and synthesized as shown in Table 1. Psumo-linker contained 3′ terminal sequence of Sumo and 5′ terminal sequence of ScFv. As shown in Fig. 1, Sumo-linker was created by polymerase chain reaction (PCR) using SF1 and Psumo-linker as forward and reverse primers, using pET-20b-Sumo as a template. PCR parameters consisted of 5 min of Prime STAR®GXL DNA Polymerase activation at 98 °C, followed by 35 cycles of denaturation at 98 °C for 10 s, annealing at 58 °C for 15 s, extension at 68 °C for 60 s, and then a final single extension at 68 °C for 5 min.
Schematic illustration of ScFv or ScFv-9R synthesis. According to the sequence of ScFv, ScFv-9R, and Sumo fragment, five primers were designed and synthesized (as shown in Table 1). Sumo-linker was created by PCR using SF1 and Psumo-linker as primers; Sumo-ScFv or Sumo-ScFv-9R was then synthesized by PCR using Sumo-linker, ScFv or ScFv-9R as templates, SF1, and P2 or PR2 as primers
Using Sumo-linker and ScFv or ScFv-9R as templates, SF1 and P2 or PR2 as the forward and reverse primers, the Sumo-ScFv or Sumo-ScFv-9R was generated by PCR. Further, the fusion gene was digested with NdeI and XhoI, and then inserted into the E. coli expression vector pET-20b to create a new expression vector. The inserted fragment in the recombinant plasmid was confirmed by the restriction enzyme digestion and DNA sequencing. The sequence of fusion gene has been submitted to GenBank (accession number, KF732845).
Inducible expression and soluble detection of Sumo-ScFv and Sumo-ScFv-9R
The recombinant bacteria containing pET-ScFv or pET-ScFv-9R was inoculated in the LB medium (1 % peptone, 1 % yeast extract, and 0.5 % sodium chloride, pH 7.0) and incubated in a shaker at 37 °C until OD600 was 0.6 to 0.8. Isopropyl-β-d-thiogalactopyranoside (IPTG) was added to a final concentration of 0.5 mM for 20 h induction at 20 °C. Recombinant bacteria was collected by centrifugation and lysed by sonication into the buffer. The supernatants were harvested by centrifugation at 14,000 rpm for 30 min at 4 °C, and the remaining pellets (insoluble fraction) containing inclusion bodies were resuspended into an equal volume of lysis buffer. Both soluble and insoluble fractions were analyzed by 12 % sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The amount of fusion protein yield was measured by densitometer scanning.
Purification of Sumo-ScFv and Sumo-ScFv-9R
The cell lysate of Sumo-ScFv was purified by DEAE Sepharose FF column chromatography. Sumo-ScFv was eluted with buffer containing different concentrations of NaCl (0.1–1 mol/L, pH 8.0), followed by further purification with Ni-NTA resin. The Ni-NTA resin was washed with bufferI (50 mmol/L Tris-HCl containing 20 mM imidazole and 300 mM NaCl, pH 8.0) until OD280 of effluent reached the base line. Contaminating proteins were eluted from the column with wash bufferII (50 mmol/L Tris-HCl containing 40 mM imidazole and 300 mM NaCl, pH 8.0). Finally, His6-tagged Sumo-ScFv protein was collected from the column with elution buffer (50 mmol/L Tris-HCl containing 300 mM imidazole and 300 mM NaCl, pH 8.0). The fusion protein of Sumo-ScFv-9R was purified by Ni-NTA resin according to the prior descriptions. Samples taken at the elution peak were pooled. The purity of fusion protein was assessed using SDS-PAGE, and its concentration was evaluated with BCA Protein Assay Kit.
Cleavage of Sumo from Sumo-ScFv and Sumo-ScFv-9R and further purification
The Sumo-ScFv or Sumo-ScFv-9R protein was diluted to a concentration of 0.5 mg/mL. Ten units of Sumo protease were added to the dilution in high-salt buffer (50 mM Tris-HCl, pH 7.0, 0.1 M NaCl, 1 mM DTT), and the mixture was incubated for 10 h at 4 °C, then the mixture was loaded into an Ni-NTA resin column to obtain ScFv or ScFv-9R, which was further desalted with dialysis bag and concentrated by ultrafiltration at 4 °C.
Authenticity assay of fusion protein
The immunogenic activity of purified Sumo-ScFv and Sumo-ScFv-9R was assayed by Western blot. Total cellular protein was boiled in an equal volume of sample-loading buffer, a Tris-HCl buffer (pH 6.8) containing 20 % glycerol, 2.5 % SDS, 10 % β-mercaptoethanol, and 0.005 % bromophenol blue. Protein samples were electrophoresed on 12 % polyacrylamide gels containing SDS. Proteins were electrophoretically transferred into the PVDF membrane and overnight block nonspecific binding with 5 % nonfat milk powder. The membrane was incubated with a polyclonal anti-histidine antibody (1:3,000), then washed and incubated with a 1:2,000 dilution of secondary HRP-conjugated antibody. Immunoreactive bands were visualized using an ECL kit.
Authenticity assay of the recombinant ScFv-9R
The recombinant ScFv-9R was identified for amino acid compositions by liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis (technical services provided by Beijing Protein Institute Co. Ltd).
RT112 cells were cultured in DMEM medium containing 10 % FBS. Before stimulation, cells were firstly starved for 4 h, and then treated with 10 ng/mL FGF9 and 40 μg/mL heparin for 30 min in the absence or presence of ScFv-9R. Then total protein from RT112 cellular extracts was analyzed by SDS-PAGE, followed by immunoblotting with specific antibody against p-FGFR3, FGFR3, p-ERK, and ERK. β-actin was detected as a loading control.
Gel retardation assay
Ten picomoles of noncoding siRNA were mixed with increasing amounts of ScFv-9R in the phosphate-buffered saline (PBS) buffer and incubated at 4 °C for 30 min. A 2.5 μg ScFv was applied as a negative control. The mixture was then performed by electrophoresis on 2 % (w/v) agarose gels and detected by ethidium bromide (EB) staining.
ScFv-9R delivered siRNA into RT112 cells
Forty picomoles of noncoding siRNA labeled with FAM were mixed with 10 μg of ScFv-9R in the PBS buffer totalling 50 μL and incubated at 4 °C for 60 min. ScFv was used as a negative control, and lipo-2000 was used as a positive control. The mixture was added to the medium of RT112 cells and cultured at 37 °C for 4 h in the cell incubator. The cells were then harvested and washed two times with PBS and observed under a fluorescent microscope. Further, the cells preincubated with ScFv-9R/FAM-siRNA were fixed by 4 % paraformaldehyde and stained with 5 μg/mL 4′,6-diamidino-2-phenylindole (DAPI) and observed under a fluorescent microscope (Olympus, Japan).
Results
Construction of pET-Sumo-ScFv and pET-Sumo-ScFv-9R
The gene sequences of ScFv and ScFv-9R were synthesized by Generay Biotech Co. (China). The strategies for Sumo-ScFv and Sumo-ScFv-9R constructs were described in “Materials and methods” (Fig. 1). As shown in Fig. 2a, the PCR product of ScFv and Sumo-ScFv is 708 and 1,026 bp, respectively. Additionally, the molecular weight of ScFv-9R and Sumo-ScFv-9R is 780 and 1,098 bp, respectively (Fig. 2b). The final PCR products (Sumo-ScFv and Sumo-ScFv-9R) were digested and cloned into pET-20b plasmid, respectively. The sequence of recombinant plasmid containing Sumo-ScFv or Sumo-ScFv-9R was confirmed by automated DNA sequencing, and results showed that their sequences are in conformity with the expected sequence.
Synthesis of Sumo-ScFv and Sumo-ScFv-9R by PCR. The sequence of ScFv and ScFv-9R were synthesized by Generay Biotech Co. (China). The strategies for Sumo-ScFv and Sumo-ScFv-9R synthesis were described in “Materials and methods.” As shown in panel a, the molecular weight of ScFv and Sumo-ScFv is 708 and 1,026 bp, respectively. Additionally, the molecular weight of ScFv-9R and Sumo-ScFv-9R is 780 and 1,098 bp (panel b), respectively
Expression, purification, and identification of Sumo-ScFv and Sumo-ScFv-9R
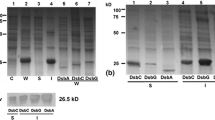
Recombinant bacterial cells containing Sumo-ScFv or Sumo-ScFv-9R were induced by 0.5 mM IPTG for 20 h at 20 °C, then the recombinant bacterial cells were harvested by centrifugation, and protein was extracted and separated by sonication and centrifugation. The supernatants and pellets were collected and performed to 12 % SDS-PAGE analysis. As shown in the left panel of Fig. 3, the results showed that Sumo-ScFv was expressed highly in the total bacteria and supernatant but less in precipitation. The molecular weight of recombinant protein is 38 kDa, which is consistent with the predicted size of Sumo-ScFv. The target protein is more than 50 % of the total bacterial protein, and the soluble fraction (supernatant) reaches 95 % of the total target protein. The right panel of Fig. 3 showed the expression of Sumo-ScFv-9R in total bacteria, supernatant, precipitation of recombinant bacterial cells. The results showed that the molecular weight of recombinant protein is 39 kDa,which is consistent with the predicted size of Sumo-ScFv. The target protein is more than 50 % of total bacterial protein, and the soluble fraction (supernatant) reaches 80 % of total target protein.
SDS-PAGE analysis of recombinant Sumo-ScFv and Sumo-ScFv-9R. Bacterial cells containing Sumo-ScFv or Sumo-ScFv-9R were induced by 0.5 mM IPTG for 20 h at 20 °C, then the recombinant bacterial cells were harvested by centrifugation, and protein was extracted and separated by sonication and centrifugation. In bacterial cells containing blank plasmid, pET-20b was used as negative control. M represents the molecular weight marker of protein. Total bacteria, supernatant, and precipitation of bacterial cells containing Sumo-ScFv was shown in the left panel. The right panel showed total bacteria, supernatant, and precipitation of bacterial cells containing Sumo-ScFv-9R
According to the isoelectric point of fusion protein, DEAE Sepharose FF was chosen for the first-step purification of Sumo-ScFv. SDS-PAGE analysis showed that most of the host proteins were removed and still residual proteins exist in the target proteins. Further, Ni-NTA was applied to the second-step purification because His6 was located in the N-terminal of Sumo. Sumo-ScFv was eluted from Ni-NTA column using Tris-HCl containing 200 mM imidazole. SDS-PAGE analysis showed that the purity of Sumo-ScFv is more than 96 % (Fig. 4a). Sumo-ScFv-9R was successfully purified by Ni-NTA column, and its purity reached 95 % (Fig. 4b).
SDS-PAGE analysis of Sumo-ScFv and Sumo-ScFv-9R purification and their identification by Western blot. The recombinant protein of Sumo-ScFv supernatant shown above was loaded on a DEAE Sepharose FF and Ni-NTA orderly (a). b Showed the purification of Sumo-ScFv-9R by Ni-NTA. Immunoactivity of purified Sumo-ScFv (lane 1 and lane 3) and Sumo-ScFv-9R (lane 2 and lane 4) was performed with anti-histidine antibody by Western blot
To assay the authenticity of fusion protein, immunoactivity of the purified Sumo-ScFv and Sumo-ScFv-9R was performed with anti-histidine antibody by Western blot. The results revealed that both Sumo-ScFv (Fig. 4c, lane 1 and lane 3) and Sumo-ScFv-9R (lane 2 and lane 4) could specifically react with anti-histidine antibody.
Further purification and determination of ScFv and ScFv-9R after cleavage of Sumo
To cleave the Sumo from the fusion protein, the purified Sumo-ScFv or Sumo-ScFv-9R was digested by Sumo protease for 10 h at 4 °C, and then loaded on the Ni-NTA. The Sumo and Sumo protease containing His6 tags were affiliated by Ni-NTA resin, and only ScFv or ScFv-9R was eluted from the column when washed off by the resin with elution buffer. As shown in Fig. 5, SDS-PAGE analysis showed that the Sumo fragment from Sumo-ScFv or Sumo-ScFv-9R could be efficiently cut by Sumo protease and separated with ScFv or ScFv-9R by Ni-NTA; AKTA analysis showed a major peak of ScFv or ScFv-9R, and their purity exceed 90 and 95 %, respectively (Fig. S1). To confirm the authenticity of the fusion protein, the ScFv-9R was successfully identified by mass spectrometric method. Details of sequenced ScFv-9R were attached in Fig. S2.
SDS-PAGE analysis of purification and cleavage of Sumo-ScFv and Sumo-ScFv-9R. The purified Sumo-ScFv or Sumo-ScFv-9R was digested by Sumo protease for 10 h at 4 °C, and then loaded into the Ni-NTA column. SDS-PAGE analysis showed the results of before and after digestion as well as that of after purification
ScFv-9R can specifically target FGFR3 and efficiently deliver siRNA into RT112 cells
To investigate whether ScFv-9R can specifically inhibit FGFR3 signaling pathway by competing FGFR3 with FGFs, RT112, a FGFR3-positive bladder cancer cell line, was stimulated by FGF9 and heparin in the absence or presence of ScFv-9R. The phosphorylation of FGFR3 and ERK which represent receptor kinase activity and its downstream major signaling molecular were detected by Western blot with a specific antibody. The results showed that ScFv-9R can efficiently attenuate the phosphorylation of FGFR3 and ERK in the absence or presence of FGF9 (Fig. 6).
Inhibition of FGFR3 signaling pathway by ScFv-9R. RT112 cells were stimulated with 10 ng/mL FGF9 and 40 μg/mL heparin in the absence or presence of ScFv-9R for 30 min. Then total protein from RT112 cell lysis solution was analyzed by SDS-PAGE and performed to immunoblotting with p-FGFR3, FGFR3, p-ERK, and ERK. β-actin was detected as a loading control
To determine the ability of ScFv-9R to bind siRNA, 10 pmol noncoding siRNA were mixed with increasing amounts of ScFv-9R and incubated at 4 °C for 30 min. The mixture was then analyzed on 2 % agarose gel. As shown in Fig. 7, ScFV-9R was able to shift siRNA in a concentration-dependent manner, whereas ScFv itself displayed no siRNA-binding ability. Clearly, to bind 10 pmol, siRNA needs no less than 2.4 μg ScFv-9R. In other words, 1 μg ScFv-9R can efficiently bind about 4 pmol siRNA.
Gel retardation assay of siRNA binding to ScFv-9R. Ten picomoles of noncoding siRNA were mixed with increasing amounts of ScFv-9R in the PBS buffer and incubated at 4 °C for 30 min. A 2.5 μg ScFv was applied as a negative control. The mixture was then performed by electrophoresis on 2 % (w/v) agarose gels and detected by EB staining
To confirm whether ScFv-9R can efficiently deliver siRNA into RT112 cells, 40 pmol noncoding siRNA labeled with FAM were mixed with 10 μg of ScFv-9R and incubated at 4 °C for 60 min. Then the mixture was added to the medium of RT112 cells and cultured at 37 °C for 4 h in the cell incubator. The cells were harvested and observed under a fluorescent microscope. Fluorescent microscope analysis showed that the FAM-positive cells only exist in ScFv-9R/siRNA and lipo-2000/siRNA-treated cells (Fig. 8), and no FAM fluorescent was found in control groups (ScFv-9R and ScFv/siRNA, data not shown). These results demonstrate that ScFv-9R can efficiently deliver siRNA into RT112 cells and also suggest that the ability of lipo-2000 in siRNA delivery is better than that of ScFv-9R.
ScFv-9R efficiently delivered siRNA into RT112 cells. Forty picomoles of noncoding siRNA labeled with FAM were mixed with 10 μg of ScFv-9R in the PBS buffer totalling 30 μl and incubated at 4 °C for 60 min. lipo-2000 was used as positive control. Then the mixture was added to the medium of RT112 cells and cultured at 37 °C for 4 h in the cell incubator. The cells were harvested and fixed with 4 % paraformaldehyde and stained with DAPI, then observed under a fluorescent microcopy. Upper panel lipo-2000 plus FAM-siRNA, lower panel ScFv-9R plus FAM-siRNA, bar = 10 μm
Discussion
Targeted gene therapy needs efficient carrier system. Liposome is an extremely efficient tool to deliver DNA, but it is not selective. Virus is also highly efficient in gene transfer, but safety and immunogenicity are impossible to avoid (Li et al. 2001). The creation of fusion protein that consisted of ScFv and basic peptides opens the way to resolve these difficulties. It could target the defined cell population and deliver siRNA to silence the specific gene expression. As we know, tumor development involved numerous genes and multiple signaling pathways. Only inhibiting one gene or one pathway is easier to induce cancer cell resistance to drug or generate new mechanisms of escape by altering other genes’ characterizations or signaling transduction. For example, non-small cell lung cancer (NSCLC) cell lines generate acquired resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in that FGFR-mediated ERK signaling was activated (Ware et al. 2010), so simultaneous inhibition of EGFR and FGFR would be required for the treatment of NSCLC patients. In view of clinical drawbacks of single drug, ScFv fusion protein can deliver siRNA cocktail which can disturb several signaling pathway and downregulate many gene expressions because it contains numerous siRNAs against different proto-oncogene. Facts proved that siRNA cocktail is more effective than one siRNA alone in tumor suppression (Yao et al. 2012).
In this study, we construct fusion protein ScFv-9R containing 9-arginine and ScFv against FGFR3 which specifically binds to both soluble and cell-surface expressed FGFR3 on cancer cells (Martínez-Torrecuadrada et al. 2005). To get high production and better bioactivity of ScFv-9R, we used Sumo molecular partner as a fusion expression system, by which we acquired high-level, soluble, and pretty purity of recombinant ScFv-9R. So far, the reported recombinant protein from E. coli expression system almost contained all various types of tag or start codon. Thus, the other advantage of Sumo system is that the recombinant protein has a natural amino acid sequence without any tags after cleavage of Sumo, and it is very important for safety in vivo and pharmaceutical development. To select the optimal inducible conditions, we try to use different temperature and culture time after induction for the expression of recombinant ScFv-9R. The results showed that high temperature (37 °C) can get the high yield of ScFv-9R at the short induction time(4 h); however, most of recombinant proteins do not exist in soluble fraction, but in the inclusion body (data not shown). In contrast, low temperature (20 °C) and long induction time (20 h) can significantly promote ScFv-9R expression in soluble fraction, which contained more than 90 % recombinant protein (Fig. 3). Using this expression and purification system, we can finally get 5 mg ScFv or 3 mg ScFv-9R protein in per liter of bacterial culture. As far as we know, this yield is high enough to meet the demand of animal experiments in the future.
To confirm the specificity and target ability of ScFv-9R, we applied FGF9 to activate FGFR3 signaling pathway in the absence or presence of ScFv-9R, and Western blot results showed that ScFv-9R can inhibit FGF9 or basal condition-stimulated FGFR3 phosphorylation and its downstream signaling molecular ERK phosphorylation. These results also suggest that the target activity of ScFv-9R is similar as the previous report (Martínez-Torrecuadrada et al. 2005) and also confirmed the recent finding which is bladder cancer cells can secrete FGF (Chung and Koh 2013). Further, we also performed ScFv-9R/siRNA to treat THP-1 cells, an FGFR3-negative cell line (Fig. S3), and the results showed that ScFv-9R/siRNA cannot deliver siRNA into THP-1 cells (data not shown). In contrast, ScFv-9R can efficiently deliver siRNA into FGFR3-positive RT112 cells (Fig. 8), implying better specificity of ScFv-9R. In our experiments, we used DAPI to stain nuclear so that we can clearly distinguish whether ScFv-9R can deliver siRNA into cells. The results showed FAM fluorescent distributes around the nuclear in the ScFv-9R/siRNA-treated RT112 cells (Fig. 8), not in the cell surface. These results all demonstrate that ScFv-9R is efficient and a bifunctional carrier that binds to and delivers siRNA and selectively targets FGFR3-positive cells.
In summary, we acquired successfully high yield and better purity of ScFv-9R by Sumo fusion expression in E. coli. The results showed that recombinant ScFv-9R have bifunctional characteristics that not only bind to siRNA but also target and deliver siRNA into FGFR3-positive cancer cells. Future studies need to investigate whether delivered siRNA can silence specific gene expression, leading to suppression of tumor growth in vitro and in vivo.
References
Chung SS, Koh CJ (2013) Bladder cancer cell in co-culture induces human stem cell differentiation to urothelial cells through paracrine FGF10 signaling. Vitro Cell Dev Biol Anim 49(10):746–751
Di Martino E, Tomlinson DC, Knowles MA (2012) A decade of FGF receptor research in bladder cancer: past, present, and future challenges. AdvUrol 2012:429213
Du X, Wang QR, Chan E, Merchant M, Liu J, French D, Ashkenazi A, Qing J (2012) FGFR3 stimulates stearoyl CoA desaturase 1 activity to promote bladder tumor growth. Cancer Res 72(22):5843–5855
Glénisson M, Vacher S, Callens C, Susini A, Cizeron-Clairac G, Le Scodan R, Meseure D, Lerebours F, Spyratos F, Lidereau R, Bièche I (2012) Identification of new candidate therapeutic target genes in triple-negative breast cancer. Genes Cancer 3(1):63–70
Jäger V, Büssow K, Wagner A, Weber S, Hust M, Frenzel A, Schirrmann T (2013) High level transient production of recombinant antibodies and antibody fusion proteins in HEK293 cells. BMC Biotechnol 13(1):52
Li X, Stuckert P, Bosch I, Marks JD, Marasco WA (2001) Single-chain antibody-mediated gene delivery into ErbB2-positive human breast cancer cells. Cancer Gene Ther 8(8):555–565
Liu X, Chen Y, Wu X, Li H, Jiang C, Tian H, Tang L, Wang D, Yu T, Li X (2012) SUMO fusion system facilitates soluble expression and high production of bioactive human fibroblast growth factor 23 (FGF23). Appl Microbiol Biotechnol 96(1):103–111
Marblestone JG, Edavettal SC, Lim Y, Lim P, Zuo X, Butt TR (2006) Comparison of SUMO fusion technology with traditional gene fusion systems: enhanced expression and solubility with SUMO. Protein Sci 15(1):182–189
Martínez-Torrecuadrada J, Cifuentes G, López-Serra P, Saenz P, Martínez A, Casal JI (2005) Targeting the extracellular domain of fibroblast growth factor receptor 3 with human single-chain Fv antibodies inhibits bladder carcinoma cell line proliferation. Clin Cancer Res 11(17):6280–6290
Martínez-Torrecuadrada JL, Cheung LH, López-Serra P, Barderas R, Cañamero M, Ferreiro S, Rosenblum MG, Casal JI (2008) Antitumor activity of fibroblast growth factor receptor 3-specific immunotoxins in a xenograft mouse model of bladder carcinoma is mediated by apoptosis. Mol Cancer Ther 7(4):862–873
Plowright EE, Li Z, Bergsagel PL, Chesi M, Barber DL, Branch DR, Hawley RG, Stewart AK (2000) Ectopic expression of fibroblast growth factor receptor 3 promotes myeloma cell proliferation and prevents apoptosis. Blood 95(3):992–998
Qing J, Du X, Chen Y, Chan P, Li H, Wu P, Marsters S, Stawicki S, Tien J, Totpal K, Ross S, Stinson S, Dornan D, French D, Wang QR, Stephan JP, Wu Y, Wiesmann C, Ashkenazi A (2009) Antibody-based targeting of FGFR3 in bladder carcinoma and t(4;14)-positive multiple myeloma in mice. J Clin Invest 119(5):1216–1229
Song E, Zhu P, Lee SK, Chowdhury D, Kussman S, Dykxhoorn DM, Feng Y, Palliser D, Weiner DB, Shankar P, Marasco WA, Lieberman J (2005) Antibody mediated in vivo delivery of small interfering RNAs via cell-surface receptors. Nat Biotechnol 23(6):709–717
Sonvilla G, Allerstorfer S, Heinzle C, Stättner S, Karner J, Klimpfinger M, Wrba F, Fischer H, Gauglhofer C, Spiegl-Kreinecker S, Grasl-Kraupp B, Holzmann K, Grusch M, Berger W, Marian B (2010) Fibroblast growth factor receptor 3-IIIc mediates colorectal cancer growth and migration. Br J Cancer 102(7):1145–1156
Trudel S, Stewart AK, Rom E, Wei E, Li ZH, Kotzer S, Chumakov I, Singer Y, Chang H, Liang SB, Yayon A (2006) The inhibitory anti-FGFR3 antibody, PRO-001, is cytotoxic to t(4;14) multiple myeloma cells. Blood 107(10):4039–4046
Wang H, Xiao Y, Fu L, Zhao H, Zhang Y, Wan X, Qin Y, Huang Y, Gao H, Li X (2010) High-level expression and purification of soluble recombinant FGF21 protein by SUMO fusion in Escherichia coli. BMC Biotechnol 10:14
Wang W, Luo J, Xu L, Zeng J, Cao L, Dong J, Cai S (2013) Expression of scFv-Mel-Gal4 triple fusion protein as a targeted DNA-carrier in Escherichia coli. Cell Biochem Funct. doi:10.1002/cbf.2958
Ware KE, Marshall ME, Heasley LR, Marek L, Hinz TK, Hercule P, Helfrich BA, Doebele RC, Heasley LE (2010) Rapidly acquired resistance to EGFR tyrosine kinase inhibitors in NSCLC cell lines through de-repression of FGFR2 and FGFR3 expression. PLoS One 5(11):e14117
Wen WH, Liu JY, Qin WJ, Zhao J, Wang T, Jia LT, Meng YL, Gao H, Xue CF, Jin BQ, Yao LB, Chen SY, Yang AG (2007) Targeted inhibition of HBV gene expression by single-chain antibody mediated small interfering RNA delivery. Hepatology 46(1):84–94
Wesche J, Haglund K, Haugsten EM (2011) Fibroblast growth factors and their receptors in cancer. Biochem J 437(2):199–213
Yao YD, Sun TM, Huang SY, Dou S, Lin L, Chen JN, Ruan JB, Mao CQ, Yu FY, Zeng MS, Zang JY, Liu Q, Su FX, Zhang P, Lieberman J, Wang J, Song E (2012) Targeted delivery of PLK1-siRNA by ScFv suppresses Her2+ breast cancer growth and metastasis. SciTransl Med 4(130):130ra48
Ye Q, Hu H, Wang Z, Lu T, Hu Z, Zeng X, Zhang S, Liu J, Lei P, Wang CY, Ye Z, Shen G (2012) Generation and functional characterization of the anti-transferrin receptor single-chain antibody-GAL4 (TfRscFv-GAL4) fusion protein. BMC Biotechnol 12:91
Acknowledgments
This work was supported by National Natural Science Foundation of China (No.81370640), the key Science & technology funding of Jilin province (20130206003YY) and biopharmaceutical grant from Jilin provincial Science & technology department (20130727041YY).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 232 kb)
Rights and permissions
About this article
Cite this article
Zhang, X., Xie, J., Sun, Y. et al. High-level expression, purification, and characterization of bifunctional ScFv-9R fusion protein. Appl Microbiol Biotechnol 98, 5499–5506 (2014). https://doi.org/10.1007/s00253-014-5541-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5541-x