Abstract
To clarify the effect of type of foods on the intestinal environment, Far East Asian- (FEA; rich in rice starch, soy protein and soy oil) and Far East Asian marine- (FEAM; rich in rice starch, fish meal, fish oil and brown alga) modelled diets and sucrose, casein and beef tallow-rich (SCB) diet were prepared. After the 2-week administration of diets in rats, caecal organic acids and putrefactive compounds (ammonia, indole, phenol and H2S, which are regarded as putative risk factors for tumours) were determined. The caecal microbiota was also analyzed using denaturing gradient gel electrophoresis and pyrosequencing with bar-coded primers targeting the bacterial 16S rRNA gene. Levels of n-butyrate, acetate, indole and phenol were high in rats fed FEA. On the other hand, H2S was clearly suppressed by both FEA and FEAM comparing with SCB. These results suggest that FEAM is preferable to FEA for optimal intestinal environment and host health. Both microbial analyses showed that the diversity of microbiota in the FEAM group was lower than in the other diet groups. Ratio of Firmicutes, Bacteroidetes and Proteobacteria in the SCB group was about 5:4:1. Firmicutes, particularly Lachnospiraceae, was promoted by FEA and FEAM.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The adult human intestine contains 1013 to 1014 bacteria, involving at least 500 different species or strains, which make up the gut microbiota (Dethlefsen et al. 2008). While up to ten different bacterial phyla typically comprise the microbiota, the three phyla Firmicutes, Bacteroidetes and Actinobacteria predominate (Simrén et al. 2013). The intestinal microbiota plays an important role in host health that can be seen as twofold, with both beneficial and harmful effects on the host. Beneficial effects include prevention of pathogen colonization and stimulation of immune responses (Chung et al. 2012; Feng et al. 2011), assistance in digestion, absorption and synthesis of nutrients (Willing and Van Kessel 2010), and aging (Biagi et al. 2011). Harmful effects include the production of intestinal putrefactive compounds, such as ammonia, H2S, amines, phenols and indoles; these putrefactive compounds are regarded as putative carcinogens and toxins (Davila et al. 2013; Kuda et al. 2005). The intestinal microbiota depends on various factors, such as aging, stress, climate, infectants, disease, drugs and diet (Biagi et al. 2011). Moreover, diet composition is dependent on geographic location and culture. Such differences can also affect the intestinal microbiota (De-Filippo et al. 2010).
Food materials, food consumption and cuisine vary not only by climate but also by culture, region and society. Diets of Far East Asian areas, such as Korea, Japan and East coasts of China, are characterized by the consumption of rice, vegetables and marine products, including various fishes and algae (Kuda and Ikemori 2009; Ogawa et al. 2002). Additionally, various processed soy products, such as miso, shoyu, natto and tofu, are utilized in the cuisines of these countries. There are reports of food-associated beneficial effects, such as hypolipidemic, antihypertensive and/or antioxidant effects of fish oil and canned fish products (Aguilera et al. 2004; Biagi et al. 2011; Kuda and Yano 2009), as well as antioxidant, antibacterial, immune promoting and anti-inflammatory activities of edible algae and soybean products (Kim et al. 2008; Kuda et al. 2007; Kuda et al. 2012a; Kuda et al. 2012b).
In our previous studies, soluble polysaccharides (dietary fibres) of brown algae, laminaran and alginic acid, showed inhibitory effects on intestinal putrefactive compounds with organic acid fermentation (An et al. 2013a; Kuda et al. 2005). On the other hand, although n-3 polyunsaturated fatty acids improved hypocholesterolemia in rats, an increase in intestinal oxidation, evaluated as thiobarbituric acid reactive substances (TBARS), was observed (Kuda et al. 2000). Furthermore, compared with milk-casein, the intake of soy protein and fish meal increased intestinal putrefactive compound levels (An et al. 2014). Therefore, an analysis of the combined effects of various food materials on fermentation by the intestinal microbiota and the generation of putrefactive compounds is anticipated. It was reported that Lactobacillus, Bifidobacterium, Eubacterium, Clostridium and Enterococcus (Gram positive) in the rat caecum were impaired by a European-modelled diet compared with a Japanese-modelled diet (Morishita 1990). However, this determination was made using only culture-dependent methods.
It is well known that most of the intestinal microbiota is non-cultureable using general medium. Therefore, since the 1990s, the microbiota has been analyzed using a variety of molecular techniques, such as denaturing gradient gel electrophoresis (DGGE) and terminal restriction fragment length polymorphism (T-RFLP), because these methods are not culture-based (Kibe et al. 2005). However, these methods can detect only tens of mainly microbes, though there are so many species. Recently, pyrosequencing of 16S rRNA genes has been used for the analysis of complex microbiota in various environments, including the animal intestine (An et al. 2013a; An et al. 2014; Van den Bogert et al. 2011). This method can evaluate thousands to tens of thousands of reads of genes from a sample in one run.
In this study, to clarify the effects of the types of foods on the intestinal environment and host health, caecal organic acids and putrefactive compounds in rats fed the sucrose, casein and beef tallow-rich diet, Far East Asian-modelled diet (rich in rice starch, soybean protein and soy oil) and Far East Asian marine modelled diet (rich in rice starch, fish protein and fish oil) were analysed. Furthermore, the caecal microbiota was analyzed using DGGE and amplicon pyrosequencing with bar-coded primers targeting the bacterial 16S rRNA gene.
Materials and methods
Animal and diets
The animal experiment was performed in compliance with the guidelines for the proper conduct of animal experiments and related activities in academic research institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan, and approved by the animal experiment committee of Tokyo University of Marine Science and Technology (approval no. 2012-10).
As shown in Table 1, the three diets were prepared as sucrose, wheat starch, milk-casein and beef tallow-rich (SCB) diet, Far East Asian-modelled (FEA; rich in rice starch, soybean protein and soy oil) diet and Far East Asian marine-modelled (FEAM; rich in rice starch, fish protein and fish oil) diet; diets in this study were based on the report of Murata et al. (2002); however, diets had some changes comparing with diets of the report. In the report, the SCB diet was regarded as European-modelled diet. Rice starch, soy protein, soy oil and high dietary fibre were found in FEA. FEAM contained fish meal, fish oil and wakame (Undaria pinnatifida).
Four-week-old male Wistar rats were housed separately in metal wire cages and allowed free access to water and food. After acclimation with AIN-76 based diet for 7 days, the animals were divided into three groups (n = 6) and given the SCB, FEA or FEAM for 14 days. Faecal frequency was a number of faeces and faeces of rats were counted in every morning. Then, rats were anesthetized with diethyl ether and exsanguinated from the abdominal aorta. The caecum were excised and weighed. A portion of the caecal content was used for direct total cell counts using the Gram stain method (Miriam and Buenviaje 1989). The remaining caecal content was stored at −80 °C until used in subsequent experiments.
Blood lipid and glucose levels
Blood samples were placed in tubes with heparin and centrifuged at 2,190 × g for 10 min to collect plasma. Levels of plasma total-cholesterol, triglyceride, phospholipid, non-esterified fatty acid and glucose were determined using commercial test kits (T-Cho E Test Wako, TG E Test Wako, PL C Test Wako, NEFA C Test Wako and Glucose CII Test Wako; Wako Pure Chemical Industries, Ltd., Osaka, Japan) and a grating microplate reader (SH-1000 Lab; Corona Electric, Ibaraki, Japan).
Caecal chemical compounds
Caecal contents were diluted with four volumes of distilled water, and levels of organic acids and putrefactive compounds were determined. Organic acids (lactic acid, acetic acid, propionic acid and n-butyric acid) were determined using high-pressure liquid chromatography (HPLC) according to a previous report (Kuda et al. 2010). Briefly, 0.2 ml of the caecal suspension was acidified with 0.05 ml of 1 mol/l sulphuric acid and centrifuged at 15,000×g for 3 min at 4 °C. After centrifugation, the supernatant was passed through a 0.45-μm-pore-size filter and injected into the HPLC instrument under the following conditions: column, ICSep ICE-ORH-801 (Tokyo Chemical Industry Co., Ltd., Tokyo, Japan); operating temperature, 35 °C; elution, 0.005 mol/l of H2SO4; flow rate, 0.8 ml/min. Eluted compounds were detected by a refractive index detector.
Levels of ammonia, phenol and sulphide compounds were determined using reagent sets for water analysis (nos. 7, 17 and 53, respectively; Kyoritsu Co., Tokyo, Japan) with the grating microplate reader, after dilution with four volumes of distilled water (Kuda et al. 2005). Indole level was measured using Kovac's reagent (Lombard and Dowell 1983).
DNA extraction
Bacterial DNA from each caecal sample was extracted using a NuCleoSpin Tissue (Takara Bio, Shiga, Japan). With DNA extraction, caecal content impurities were removed before being placed on the columns by centrifugation. Purified DNA was dissolved in Tris-EDTA buffer (pH 8) and used as the DNA template in DGGE and pyrosequencing analyses.
DGGE analysis
DGGE analysis was performed as described previously (An et al. 2010; An et al. 2013b). The primer pair was chosen for the amplification of the V3 region (approximately 220 bp) of the 16S rRNA gene: forward primer with GC clamp GC-339f (50-CGC CCG CCG CGC CCC GCG CCC GTC CCG CCG CCC CCG CCC GCT CCTACG GGA GGC AGC AG-30) and reverse primer V3–53r (50-GTATTA CCG CGG CTG CTG G-30). Polyacrylamide gels (8 %, w/v acrylamide-bis-acrylamide (37.5:1)) in 1x Tris–acetate-EDTA buffer (pH 8), with a denaturing gradient ranging from 30 to 60 % denaturant (100 % denaturation corresponds to 7 mol/l urea and 40 %, v/v formamide), were electrophoresed at a constant voltage of 200 V at 60 °C for 3 h using the DCode System apparatus (Bio-Rad, Hercules, CA, USA).
Bacterial analysis using pyrosequencing
DNA sequences of caecal contents were amplified individually with primer pairs for the 16S rDNA gene following a previous report (An et al. 2014). The region of the 16S rRNA gene was amplified by PCR using bar-coded primers targeting 27 to 338 bp. The primers contained five-base sample-specific bar code sequences denoted as “X” and common linker (AC) sequences in the 5’ end (Turnbaugh et al. 2009). The forward primer was 5’-ctatgcgccttgccagcccgctcag NNNNNNNN AGAGTTTGATCCTGGCTCAG - 3’, where the sequence of the A adapter is shown in small letters and N represents an 8-bp bar code that is unique for each sample. The reverse primer was 5’-ctatgcgccttgccagcccgctcag TGCTGCCTCCCG TAGGAGT-3’, where the sequence of the A adapter is shown in lowercase letters. PCR amplification was performed in 0.1 ml reaction mixtures composed of 10 mmol/l Tris-HCl (pH 8.3), 50 mmol/l KCl, 1.5 mmol/l MgCl2, 50 pmol of each primer, 0.2 mol/l of each deoxynucleoside triphosphate (dNTP), 2.5 U of TaKaRa Ex Taq DNA polymerase (TaKaRa Bio) and 50 ng of template DNA.
PCR was performed using the Genome Sequencer Junior System (Roche, CT, USA). The pooled DNA samples were adapter ligated with beads and amplified by emulsion PCR using a GS Junior Titanium emPCR kit (Lib-A; Roche). Beads were enumerated after emulsion PCR, and the same amount of beads was put in a PicoTiterPlate and sequencing was carried out using Genome Sequencer Junior System. Sequences obtained from the pyrosequencing were analyzed with GS Run Browser in the Genome Sequencer Junior System Software 2.5 (Roche).
Taxonomy-based analyses at the phylum, family and genus levels were performed by assigning taxonomic status to each sequence using Classifier of Ribosomal Database Project (http://rdp.cme.msu.edu/classifier/classifier.jsp). In this study, pyrosequencing data was analyzed at the species level by BLAST analysis through DDBJ (http://blast.ddbj.nig.ac.jp/blast/blastn?lang = ja). Diversity of the microbiota, indicated by operational taxonomy units (OTUs) and the Shannon–Wiener index (SWi), was calculated using FastGroupII (http://fastgroup.sdsu.edu/fg_tools.htm). Data was accepted under the conditions that sequences should be at least 300 bp in length and the Percent Sequence Identity (PSI) was >97 %.
Nucleotide sequence accession number
The sequence obtained from DGGE analysis was in DDBJ under accession numbers AB873092-6. Furthermore, the sequence data of pyrosequencing was in DDBJ under submission accession numbers DRA001220 (Study; DRP001285, Sample; DRS012837-48, Experiment; DRX013029-40, Run; DRR014509-20).
Statistical analysis
Data for body, organ and faecal weights, and caecal chemical compounds were expressed as means ± SEM (standard error of the mean). Statistical analysis for the animal experiment was performed using EXCEL Statistic 5.0 (Esumi Co., Tokyo, Japan). One-way ANOVA was used to assess the effects of treatments. Significant differences were accepted at p < 0.05. When significant variation was found, the Tukey test was used to determine the exact nature of the difference.
Results
Faecal frequency and caecal weights
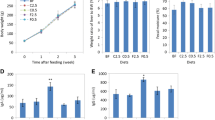
The initial weight of rats was about 125 g, and the rats gained 82 to 92 g of body weight in the three groups. There were no significant differences in body weight gained between the SCB, FEA and FEAM groups. As shown in Fig. 1a, the faecal frequency of rats fed FEA was 1.8 times higher than rats fed SCB, while that of the FEAM group was 1.6 times higher than the FEA group. Caecal weight (including content) of rats fed FEAM was 1.3 to 1.4 times higher than that of rats fed the other diets.
Blood lipid and glucose levels
Levels of plasma TC, TG, PL, NEFA and Glu in rats fed the test diets are summarized in Table 2. In rats fed SCB, the plasma TC and PL levels were 1.4 and 1.5 times higher, respectively, than the other diet groups. Although there were no significant differences in TG, NEFA and Glu levels among the three groups, TG and NEFA levels showed an increased tendency in rats fed FEAM.
Caecal organic acids and putrefactive compounds
Organic acid contents are shown in Fig. 2a. Lactic and n-butyric acid levels (La and Ba in Fig. 2a) and total organic acid were higher in rats fed FEA than in the other groups. Acetic acid (Aa) was high in the FEA and FEAM groups.
Caecal organic acid content (a) and putrefactive compounds (b) in rats fed a model SCB, FEA or FEAM diet. Values are means ± SEM (n = 6). a Ba, n-butyric acid; Pa, propionic acid; Aa, acetic acid; La, lactic acid. *p < 0.05, **p < 0.01. b Different letters indicate significant differences (p < 0.05) between groups
As shown in Fig. 2b, caecal indole and phenol contents in the FEA group were higher than in the other groups. Caecal ammonia content was tended to be high in rats fed FEA. On the other hand, caecal H2S of SCB fed rats was obviously (4 to 16 times) higher than that of rats fed FEA or FEAM.
Caecal microbiota evaluated by DGGE
Direct bacterial counts (using Gram staining) of the caecal content of rats fed SCB, FEA and FEAM were about 10.2 log cells/g, respectively. No differences were observed in the cell counts between the three diet groups.
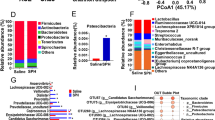
Diversity in the caecal microbiota was analyzed by DGGE and is shown in Fig. 3. Over 20 bands were visually detected on the DGGE gel for each group. On the lanes representing SCB, many bands were observed in a small region (A in Fig. 3). In the case of FEA, many bands were located in the middle region of the gel (B). Only five of the bands were excised from the gel, since several bands were closely situated.
Representative band patterns from DGGE analysis of the intestinal microbiota of rats fed SCB, FEA or FEAM diet. The main bands were identified as follows: 1, Not identified; 2, Fusobacterium / Lachnospiraceae bacterium / Eubacterium xylanophilum / Moryella indoligenes 98 % similarity; 3, Oscillospira guilliermondii 100 % similarity; 4, Barnesiella intestinihominis 88 % similarity; 5, Clostridium difficile / Cl. bifermentans 100 % similarity
Although band 1 was clear in rats fed SCB, it could not be identified. Band 4 was clearly detected on the lanes for SCB and was identified as Barnesiella intestinihominis; however, the sequence similarity was low (88 %). From the lanes for FEA, bands 2, 3 and 5 were identified. However, the sequence of band 2 showed similarity to the four genera, Fusobacterium naviforme, Lachnospiraceae bacterium, Eubacterium xylanophilum and Moryella indoligenes. Band 3 was identified as Oscillospira guilliermondii and band 5 was identified as Clostridium difficile, Clostridium lituseburense or Clostridium bifermentans.
Pyrosequencing analysis of caecal microbiota
In the pyrosequencing analysis, total reads of each sample were 4,462, 4,398 and 4,521 in rats fed SCB, FEA or FEAM, respectively (Table 3). Most of the reads passed the Fast Group II filter: >300 bp in length and 97 % similarity. On the other hand, the number of OTUs and the SWi showed a decreased tendency in rats fed FEAM (OTU 1206; SWi 5.34) compared to rats fed SCB (OTU 1402; SWi 5.73) and JPD (OTU 1462, SWi 5.76).
The microbiotas at the phylum and family levels are shown in Fig. 4. The predominant phyla in the three diet groups were Firmicutes, Bacteroidetes and Proteobacteria (outer circles in Fig. 4). The proportion of Firmicutes was the lowest in rats fed SCB, accounting for about 50 % of the genes, and the proportions in the FEA and FEAM groups were 66.8 and 66.1 %, respectively. On the other hand, the proportion of Bacteroidetes was the highest in the SCB group (about 41 %). Proteobacteria was high in rats fed FEA (8.1 %).
Characterization of the caecal microbiota of rats fed SCB, FEA or FEAM, using pyrosequencing of bacterial 16S rDNA genes, at the phylum and family levels. The outer circle indicates the phylum percentages. FE is Firmicutes, BA is Bacteroidetes, and PR is Proteobacteria. Additionally, the circle is separated at the family level; (a) Unclassified Bacteroidetes, (b) Unclassified Bacteroidales, (c) Rikenellaceae, (d) Prevotellaceae, (e) Bacteroidaceae, (f) Porphyromonadaceae, (g) Unclassified Proteobacteria, (h) Helicobacteraceae, (i) Sutterellaceae, (j) Unclassified Firmicutes, (k) Unclassified Clostridiales, (l) Lachnospiraceae, (m) Ruminococcaceae, (n) Peptostreptococcaceae, (o) Lactobacillaceae, (p) Unclassified Selenomonadales
At the family level, Lachnospiraceae (l in Fig. 4) was predominant in the three diet groups, though the proportion in each group differed, from 17 % of genes for FEA to 34 % for FEAM. Ruminococcaceae (m) was the second most predominant family in the diet groups, accounting for between 15 and 18 % of genes. Of the Firmicutes, Peptostreptococcaceae (n) was detected in all groups at 1 to 2 %. Lactobacillaceae (o) was not detected in the FEA rats. Bacteroidaceae (e) was the highest in rats fed SCB (9 %). All Proteobacteria genes were identified as Helicobacteraceae (h) or Sutterellaceae (i).
In this study, a large number of the detected genes could not be classified to the genus level (Fig. 5). At this level, unclassified Lachnospiraceae (l) was dominant, though the proportion differed from 14 % for the SCB group to 34 % for the FEAM group. The second most dominant group was unclassified Bacteroidetes (a) and unclassified Ruminococcaceae (n), with proportions ranging from 10 to 16 %. In the case of FEA rats, unclassified Bacteroidales (b) was low (6 %) and unclassified Firmicutes (j) was high (13 %). Bacteroides (e), Parasutterella (i) and Helicobacter (h) were high in rats fed SCB.
Characterization of the caecal microbiota of rats fed SCB, FEA or FEAM, using pyrosequencing of bacterial 16S rDNA genes, at the genus level. (a) Unclassified Bacteroidetes, (b) Unclassified Bacteroidales, (c) Alistipes, (d) Unclassified Prevotellaceae, (e) Bacteroides, (f) Unclassified Porphyromonadaceae, (g) Parabacteroides, (h) Helicobacter, (i) Parasutterella, (j) Unclassified Firmicutes, (k) Unclassified Clostridiales, (l) Unclassified Lachnospiraceae, (m) Clostridium XlVb, (n) Unclassified Ruminococcaceae, (o) Ruminococcus, (p) Oscillibacter, (q) Clostridium XI, (r) Lactobacillus, (s) Unclassified Selenomonadales
Discussion
In traditional Japanese cuisine, the main food materials are rice, vegetables, soybean, fishes and algae. However, the intake of animal products, such as pork, beef, chicken and dairy products, as well as sugar and wheat flour has increased recently in Japan (Murata et al. 2002). It is well known that the overconsumption of animal products and sugar is associated with life style-related illnesses, such as hyperlipidemia, hypertension, diabetes, obesity and arteriosclerosis (Poothullil 1993). In this study, levels of plasma TC and PL in the SCB group were the highest among the three diets (Table 2), which agreed with previous studies (Murata et al. 2002). Furthermore, the plasma NEFA level in the FEAM group was lower than the other diet groups. FEAM contained fish oil and brown alga (wakame). Fish oil is rich in eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Many studies have indicated that the cholesterol lowering effect of fish oil is related to EPA and DHA (Kuda et al. 2000). Furthermore, it was reported that fish oil and wakame showed an ameliorating effect on blood and liver lipid levels (Murata et al. 2002).
Among caecal organic acids (Fig. 2a), n-butyric and lactic acids were the highest in the FEA group. FEA was rich in soy protein and fish meal. In our previously study, the AIN-76-based diet containing soy protein or fish meal instead of milk-casein increased caecal n-butyric or butyric acids, respectively (An et al. 2014). This suggests that these proteins were degraded and the amino acids fermented, thereby constituting an increased contribution to organic acid levels. However, lactic acid levels were not higher in rats fed FEAM compared to other groups, even though the diet was rich in fish meal. It is thought that the other food materials in the diet, such as fish oil, which was high in FEAM, affected organic acid generation (Kuda et al. 2000).
In the previous study, 20 % w/w soy protein or fish meal increased caecal indole, phenol and H2S levels (An et al. 2014). In this study, rats fed FEA also showed the highest caecal indole and phenol levels. However, these putrefactive compounds were not high in rats fed FEAM. The inhibitory effect of brown algal dietary fibres, alginate and laminaran, on the generation of caecal putrefactive compounds was reported (An et al. 2013a; Kuda et al. 2005). By extension, it is thought that the production of putrefactive compounds from dietary proteins was suppressed by the dietary fibres contained in FEAM. Additionally, caecal H2S was suppressed in rats fed FEA or FEAM. Because caecal putrefactive compounds are regarded in the risk factors of cancer and tumours (Davila et al. 2013; Kuda et al. 2005), it is considered FEAM is better in optimal intestinal environment and host health than SEM and FEM.
The results of DGGE analysis revealed that the caecal microbiota diversity of the FEAM group showed a decreased tendency compared to the other diet groups (Fig. 3). This is in agreement with the number of OTUs and the SWi of the pyrosequencing data (Table 3). In the DGGE analysis, the species closely related to Barnesiella intestinihominis was detected as one of the dominant components in the SCB group. On the other hand, this species was not detected by pyrosequencing. Barnesiella belongs to Bacteroidales and was identified from a human faecal sample using 16S rRNA gene pyrosequencing (Kulagina et al. 2012; Wylie et al. 2012). It is possible that Barnesiella was included as unclassified Bacteroidetes in the pyrosequencing result in this study. The DGGE band of Oscillospira guilliermondii was clearly visible on the lanes for the FEA group. Though this genus also could not be identified by pyrosequencing, Oscillospira belongs to Ruminococcaceae. In previous reports, Bifidobacterium spp., such as Bifidobacterium pseudolongum and Bifidobacterium animalis, were detected as main bands on the DGGE gel of caecal contents of rats and hamsters fed AIN-76 or AIN-93 M diets these are rich in sucrose and casein (An et al. 2013b; Martínez et al. 2009). However, a clear DGGE band of Bifidobacterium was not detected in this study, even in rats fed SCB. Comparing the AIN-76 and AIN-93 M diets, the main polysaccharide and lipid in SCB were wheat starch and beef tallow instead of corn starch and corn or soybean oil.
According to the pyrosequencing results, the proportion of Firmicutes (Gram positives) to Bacteroidetes and Proteobacteria (Gram negatives) was high in rats fed FEA or FEAM. This agrees with the effect of dietary fibres, such as brown algal polysaccharides (An et al. 2013a) and beet pulp (Middelbos et al. 2010) on intestinal microbiota. On the other hand, there are several reports that indicated that a high ratio of Firmicutes was associated with a high energy intake and obesity (De-Filippo et al. 2010; Ley et al. 2006). Therefore, researchers have proposed that the ratio of Firmicutes/Bacteroidetes can be used as an index of obesity. However, Firmicutes is comprised of many families with various characteristics. Furthermore, although, lactic acid bacteria was detected as predominance in ceacal of rats fed 20 % w/w fish meal in previous reports (An et al. 2014), it was detected only 3 % in ceacal of rats fed diets containing fish meal. It was indicated that food materials affected each other in the combination diet, not individually.
Among the Firmicutes, Lachnospiraceae was high in rats fed FEA and FEAM. Lachnospiraceae belongs to the order Clostridiales and includes various genera, such as Eubacterium, Roseburia, Oribacterium, butyric acid-producing bacteria, such as Butyrivibrio, Pseudobutyrivibrio, as well as Oscillibacter (Lee et al. 2013). On the other hand, acetic acid levels were high in FEA and FEAM groups. It is well known that a number of species inhabiting the intestine can produce acetate from dietary fibres. For example, several intestinal acetate-producing bacteria, such as Ruminococcus flavefacien, Bacteroides ovatus and Clostridium ramosum, can degrade cellulose, alginate and/or laminaran (Kuda et al. 1992; Salyers et al. 1977; Varel et al. 1984).
As mentioned above, the FEA group showed high content of caecal indole and phenol with high organic acids, though reduced caecal H2S was clearly observed. It is thought that the dietary fibres in FEAM promoted excretion of putrefactive compounds from the intestine. Results of this study suggest that FEAM is preferable to FEA. However, the effect of alterations in the intestinal environment and microbiota on host health requires clarification. Further studies on the effects of these diets on inflammation bowl disease and food borne pathogen infection are now in progress.
References
Aguilera AA, Díaz GH, Barceleta ML, Guerrero OA, Roes RMO (2004) Effects of fish oil on hypertension, plasma lipids, and tumour necrosis factor-α in rats with sucrose-induced metabolic syndrome. J Nutr Biochem 15:350–357. doi:10.1016/j.jnutbio.2003.12.008
An C, Takahashi H, Kimura B, Kuda T (2010) Comparison of PCR-DGGE and PCR-SSCP analysis for bacterial flora of Japanese traditional fermented fish products, aji-narezushi and iwashi-nukazuke. J Sci Food Agric 90:1796–1801. doi:10.1002/jsfa.4015
An C, Kuda T, Yazaki T, Takahashi H, Kimura B (2013a) FLX pyrosequencing analysis of the effects of the brown-algal fermentable polysaccharides alginate and laminaran on rat caecal microbiotas. Appl Environ Microbiol 79:860–866. doi:10.1128/AEM.02354-12
An C, Yazaki T, Takahashi H, Kuda T, Kimura B (2013b) Diet-induced changes in alginate- and laminaran-fermenting bacteria levels in the caecal contents of rats. J Funct Foods 5:389–394. doi:10.1016/j.jff.2012.11.011
An C, Kuda T, Yazaki T, Takahashi H, Kimura B (2014) Caecal fermentation, putrefaction and microbiotas in rats fed milk casein, soy protein or fish meal. Appl Microbiol Biotechnol. doi:10.1007/s00253-013-5271-5
Biagi E, Candela M, Franceschi C, Brigidi P (2011) The aging gut microbiota: new perspectives. Ageing Res Rev 10:428–429. doi:10.1016/j.arr.2011.03.004
Chung H, Pamp SJ, Hill JA, Surana NK, Edelman SM, Troy EB, Reading NC, Villablanca EJ, Wang S, Mora JR, Umesaki Y, Mathis D, Benoist C, Relman DA, Kasper DL (2012) Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149:1578–1593. doi:10.1016/j.cell.2012.04.037
Davila AM, Blachier F, Gotteland M, Andriamihaja M, Benetti PH, Sanz Y (2013) Intestinal luminal nitrogen metabolism: role of the gut microbiota and consequences for the host. Pharmacol Res 68:95–107. doi:10.1016/j.phrs.2012.11.005
De-Filippo CD, Cavalieri D, Paola MD, Ramazzoth M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107:14691–14696. doi:10.1073/pnas.1005963107
Dethlefsen L, Huse S, Sogin ML, Relman DA (2008) The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol 6:e280. doi:10.1371/journal. pbio.0060280
Feng T, Elson CO, Cong Y (2011) Treg cell–IgA axis in maintenance of host immune homeostasis with microbiota. Int Immunopharmacol 11:589–592. doi:10.1016/j.intimp.2010.11.016
Kibe R, Sakamoto M, Yokota H, Ishikawa H, Aiba Y, Koga Y, Benno Y (2005) Movement and fixation of intestinal microbiota after administration of human feces to germfree mice. Appl Environ Microbiol 71:3171–3178. doi:10.1128/AEM.71.6.3171-3178.2005
Kim NY, Song EJ, Kwon DY, Kim HP, Heo MY (2008) Antioxidant and antigenotoxic activities of Korean fermented soybean. Food Chem Toxicol 46:1184–1189. doi:10.1016/j.fct.2007.12.003
Kuda T, Ikemori T (2009) Minerals, polysaccharides and antioxidant properties of aqueous solutions obtained from macroalgal beach-casts in the Noto Peninsula, Ishikawa, Japan. Food Chem 112:575–581. doi:10.1016/j.foodchem.2008.06.008
Kuda T, Yano T (2009) Changes of radical-scavenging capacity and ferrous reducing power in chub mackerel Scomber japonicus and Pacific saury Cololabis saira during 4 °C storage and retorting. LWT Food Sci Technol 42:1070–1075. doi:10.1016/j.lwt.2009.02.005
Kuda T, Fujii T, Hasegawa A, Okuzumi M (1992) Effects of degraded products of laminaran by Clostridium ramosum on the growth of intestinal bacteria. Nippon Suisan Gakkaishi 58:1307–1311
Kuda T, Enomoto T, Yano T, Fujii T (2000) Caecal environment and TBARS level in mice fed corn oil, beef tallow and menhaden fish oil. J Nutr Vitaminol 46:65–70
Kuda T, Yano T, Matsuda N, Nishizawa M (2005) Inhibitory effects of laminaran and low molecular alginate against the putrefactive compounds produced by intestinal microflora in vitro and in rats. Food Chem 91:745–749. doi:10.1016/j.foodchem.2004.06.047
Kuda T, Kunii T, Goto H, Suzuki T, Yano T (2007) Varieties of antioxidant and antibacterial properties of Ecklonia stolonifera and Ecklonia kurome products harvested and processed in the Noto peninsula, Japan. Food Chem 103:900–905. doi:10.1016/j.foodchem.2006.09.042
Kuda T, Kaneko N, Yano T, Mori M (2010) Induction of superoxide anion radical scavenging capacity in Japanese white radish juice and milk by Lactobacillus plantarum isolated from aji-narezushi and kaburazushi. Food Chem 120:517–522. doi:10.1016/j.foodchem.2009.10.046
Kuda T, Nakamura S, An C, Takahashi H, Kimura B (2012a) Effect of soy and milk protein-related compounds on Listeria monocytogenes infection in human enterocyte Caco-2 cells and A/J mice. Food Chem 134:1719–1723. doi:10.1016/j.foodchem.2012.03.031
Kuda T, Nakamura S, An C, Takahashi H, Kimura B, Nishizawa M (2012b) Effects of holdfast of Laminaria japonica on Listeria invasion on enterocyte-like Caco-2 cells and NO production of macrophage RAW 264.7 cells. Appl Biochem Biotechnol 168:928–935. doi:10.1007/s12010-012-9831-4
Kulagina EV, Efimov BA, Maximov PY, Kafarskaia LI (2012) Species composition of Bacteroidales order bacteria in the feces of healthy people of various ages. Biosci Biotechnol Biochem 76:169–171. doi:10.1271/bbb.110434
Lee GH, Rhee MS, Chang DH, Lee J, Kim S, Yoon MH, Kim BC (2013) Oscillibacter ruminantium sp. nov., isolated from the rumen of Korean native cattle. Int J Syst Evol Microbiol 63:1942–1946. doi:10.1099/ijs.0.041749-0
Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Human gut microbes associated with obesity. Nature 444:1022–1023. doi:10.1126/science.1155725
Lombard GL, Dowell VR (1983) Comparison of three reagents for detecting indole production by anaerobic bacteria in microtest systems. J Clin Microbiol 18:609–613
Martínez I, Wallace G, Zhang C, Legge R, Benson AK, Carr TP, Moriyama EN, Walter J (2009) Diet-induced metabolic improvements in a hamster model of hypercholesterolemia are strongly linked to alterations of the gut microbiota. Appl Environ Microbiol 75:4175–4184. doi:10.1128/AEM.00380-09
Middelbos IS, Boler BMV, Qu A, White BA, Swanson KS, Fahey GC Jr (2010) Phylogenetic characterization of fecal microbial communities of dogs fed diets with or without supplemental dietary fiber using 454 pyrosequencing. PloS One 5:e9768. doi:10.1371/journal.pone.0009768
Miriam B, Buenviaje MD (1989) Quantitative sputum culture and gram stain: pulmonary infection vs. colonization. Philipp J Microbiol Infect Dis 18:350–356
Morishita Y (1990) Structure and function of the intestinal flora. Asakurasyoten, Tokyo, pp 116–126, in Japanese
Murata M, Sano Y, Ishihara K, Uchida M (2002) Dietary fish oil and Undaria pinnatifida (wakame) synergistically decrease rat serum and liver triacylglycerol. J Nutr 132:742–747
Ogawa K, Tsubono Y, Nishino Y, Watanabe Y, Ohkubo T, Watanabe T, Nakatsuka H, Takahashi N, Kawamura M, Tsuji I, Hisamichi S (2002) Dietary sources of nutrient consumption in a rural Japanese population. J Epidemiol 12:1–8
Poothullil JM (1993) Obesity, hyperlipidemia and non-insulin-dependent diabetes: a unified theory. Neurosci Biobehav Rev 17:85–89
Salyers AA, Vercellotti JR, West SE, Wilkins TD (1977) Fermentation of mucin and plant polysaccharides by strains of Bacteroides from the human colon. Appl Environ Microbiol 33:319–322
Simrén M, Barbara G, Flint HJ, Spiegel BMR, Spiller RC, Vanner S, Verdu EF, Whorwell PJ, Zoetendal EG, Rome Foundation Committee (2013) Intestinal microbiota in functional bowel disorders: a Rome foundation report. Gut 62:159–176. doi:10.1136/gutjnl-2012-302167
Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI (2009) A core intestinal microbiome in obese and lean twins. Nature 457:480–484. doi:10.1038/nature07540
Van den Bogert B, de Vos WM, Zoetendal EG, Kleerebezem M (2011) Microarray analysis and barcoded pyrosequencing provide consistent microbial profiles depending on the source of human intestinal samples. Appl Environ Microbiol 77:2071–2080. doi:10.1128/AEM.02477-10
Varel VH, Fryda SJ, Robinson IR (1984) Cellulolytic bacteria from pig large intestine. Appl Environ Microbiol 47:219–221
Willing BP, Van Kessel AG (2010) Host pathways for recognition: establishing gastrointestinal microbiota as relevant in animal health and nutrition. Livest Sci 133:82–91. doi:10.1016/j.livsci.2010.06.031
Wylie KM, Truly RM, Sharpton TJ, Mihindukulasuriya KA (2012) Novel bacterial taxa in the human microbiome. PLoS One 7:e35294. doi:10.1371/journal.pone.0035294
Acknowledgments
The present study was supported in part by the Ministry of Education, Science, Sports and Culture, Japan, Grant-in-Aid for Scientific Research (C), #25450300, 2013–2015 and was also supported by a 2013 Grant from Fuji Foundation for Protein Research (Osaka, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
An, C., Kuda, T., Yazaki, T. et al. Caecal environment of rats fed far East Asian-modelled diets. Appl Microbiol Biotechnol 98, 4701–4709 (2014). https://doi.org/10.1007/s00253-014-5535-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5535-8