Abstract
The human cell line rF2N78 produces an antibody with a high galactosylation ratio which resembles human IgG. However, it has been observed that the aglycosylated antibody starts to appear when glucose is depleted. To determine whether glucose depletion is a main cause for aglycosylation of the antibody, fed-batch cultures of rF2N78 cells were performed using different feeding cocktails (glucose only, nutrient feeding cocktail without glucose, and nutrient feeding cocktail with glucose). In the fed-batch culture with nutrient feeding cocktail without glucose, aglycosylated antibody was produced in a later phase of culture, when glucose was depleted. Approximately 44 % of antibodies produced were aglycosylated at the end of culture. In contrast, aglycosylated antibody was not produced in cultures with glucose feeding. The expression levels of oligosaccharyl transferases determined by Western blot analysis were similar among the cultures, suggesting that aglycosylation of the antibody was not due to altered expression of oligosaccharyl transferases under glucose-deficient conditions. Thus, it is likely that glucose deficiency led to insufficiency of the precursor for glycosylation and induced aglycosylation of the antibody. Taken together, glucose feeding in fed-batch cultures successfully prevented occurrence of aglycosylated antibody during the cultures, confirming that glucose depletion is a main cause for aglycosylation of antibody.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recently, a human cell line, F2N78, was established by somatic fusion of human embryonic kidney 293 and Namalwa cells (Lee et al. 2013). F2N78 was demonstrated to be an appropriate host cell line for production of therapeutic antibodies with the features of safety expected of a production cell line for human use (Lee et al. 2013). More importantly, the antibody produced from F2N78 cells has more galactose capping than that from Chinese hamster ovary (CHO) cells, resembling human plasma IgG (Seo et al. 2013).
Therapeutic antibodies produced in mammalian cells contain two N-glycans at asparagine 297 (Asn-297) in the CH2 domain of the Fc region. This glycosylation is one of the important factors affecting the functional efficiency of the antibody. Most dominant functions of the glycosylated antibody are antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) (Jefferis 2009a, b). The complete removal of the oligosaccharide at Asn-297 in the CH2 domain of the Fc region of the antibody reduces its binding to various Fc receptors expressed by B cells, natural killer (NK) cells, granulocytes, and monocytes, as well as to the complement protein C1q, thus significantly diminishing ADCC and CDC (Borrok et al. 2012; Nesspor et al. 2012; Tao and Morrison 1989; Walker et al. 1989). In addition, aglycosylated antibodies have potential for immunogenicity, instability during storage, and aggregation of antibody (Adair 1992; Kayser et al. 2011). For these reasons, the biopharmaceutical industry has focused on the development of processes for the production of therapeutic antibodies with a consistent human glycoform profile (Jefferis 2009a, b).
Therefore, it is necessary to reduce aglycosylation and maintain constant glycosylation for the antibody produced from rF2N78 cells. Previously, it was observed that the aglycosylated antibody started to appear when glucose was limited in cultures of recombinant F2N78 (rF2N78) cells producing a monoclonal antibody against rabies virus (Seo et al. 2013).
In this study, we performed fed-batch cultures of rF2N78 cells with glucose feeding in a bioreactor in order to control aglycosylation of the antibody. Furthermore, to determine whether glucose depletion is a main cause for aglycosylation of the antibody, fed-batch cultures were performed using different feeding cocktails (glucose only, nutrient feeding cocktail without glucose, and nutrient feeding cocktail with glucose). After the antibody was secreted during fed-batch cultures with different feeding cocktails and purified with fast protein liquid chromatography (FPLC), its glycosylation profile and aggregation were evaluated by electrospray ionization (ESI)-TOF spectrometry and size exclusion chromatography (SEC), respectively.
Material and method
Cell line and culture medium
The rF2N78 cell line producing a monoclonal antibody against rabies virus (F2N78-S19) was established as described previously (Seo et al. 2013). The F2N78-S19 maintains its antibody production capability over 30 passages.
The commercial serum-free medium EX-CELL293TM (SAFC Biosciences, Lenexa, KS, USA) supplemented with 5 mM glutamine (Thermo Fisher Scientific, Waltham, MA, USA) was used for suspension cultures of rF2N78 cells. Cells were maintained as suspension cultures in 125-mL Erlenmeyer flasks (Corning, Corning, NY, USA) on a shaker (Adolf Kühner AG, Birsfelden, Switzerland) at 110 rpm.
Fed-batch culture
Cultures were performed in a 3-L bioreactor with a marine blade impeller (Applikon, Netherlands). Exponentially growing cells were inoculated at 0.5 × 106 cells/mL into a bioreactor with a working volume of 2 L. The agitation speed was initially 50 rpm and then increased to 60 rpm when the cell concentration reached 3 × 106 cells/mL. The dissolved oxygen (DO) concentration was controlled at 50 % of air saturation. The culture temperature was controlled at 37.0 °C within ±0.5 °C. The culture pH was controlled at pH 7.0 within ±0.05 by addition of 1 M sodium carbonate or CO2 gas.
Fed-batch cultures with four different feeding protocols (F1–F4) were performed: (F1) glucose concentration was adjusted to 2 g/L (11.1 mM) daily from day 4; (F2) glucose concentration was adjusted to 4 g/L (22.2 mM) daily from day 3; (F3) chemically defined nutrient feeding cocktail (IS CHO Feed-CD without glucose, Irvine Scientific, Santa Ana, CA, USA) was fed at 50 mL/day every other day, starting from day 5; and (F4) glucose concentration was adjusted to 4 g/L daily from day 3 and IS CHO Feed-CD without glucose was fed at 50 mL/day every other day, starting from day 5. Approximately 7 mL of culture medium was taken daily from the bioreactor. Cell concentration and viability were determined using a Vi-Cell XR automatic cell counter (Beckman Coulter, Brea, CA, USA). Culture supernatants, after centrifugation at 2,000×g for 5 min, were aliquoted and kept frozen at −80 °C for later analyses.
Antibody, glucose, lactate, glutamine, and ammonia assays
The secreted antibody concentration was quantified by an enzyme-linked immunosorbent assay (ELISA) as described previously (Hwang et al. 2008). Antibody assay was repeated three times for each sample to ensure the reproducibility of assay results. Glucose, lactate, glutamine, and ammonia concentrations were measured using a YSI 7100 multiparameter bioanalytical system (Yellow Spring Instrument, Yellow Springs, OH, USA).
Glycosylation analysis
For glycosylation analysis, approximately 50 mL of culture medium was taken from the bioreactor. The secreted antibody was purified using MabSelect SuRe resin and AKTA explorer 100 (GE Healthcare Biosciences, Piscataway, NJ, USA) according to the manufacturer’s instructions. Antibody was reduced using 10 mM dithiothreitol (DTT) prior to analysis. Glycosylation profiles were analyzed using ESI-TOF mass spectrometry following the separation of antibody with Zorbax 300SB C18 column (Agilent, New Castle, DE, USA), as described previously (Seo et al. 2013).
Western blot analysis
Western blot analyses were performed as described previously (Hwang and Lee 2008). The antibodies used for the analysis were anti-ribophorin I antibody (Novus Biologicals, Littleton, CO, USA), anti-ribophorin II, anti-DAD1 (Abcam, Cambridge, UK), and anti-β-actin (Clone AC-74; Sigma-Aldrich, St. Louis, MO, USA).
Result
To investigate the effect of glucose feeding on the aglycosylation of antibodies produced from rF2N78 cells, fed-batch cultures with four different feeding protocols (F1–F4) were performed in a bioreactor with pH control. Batch culture (B) was also performed as a control. Cultures were performed twice at separate times. Cultures were terminated when cell viability was lower than 50 %. In all cases except for F4, the osmolality of the medium, which was initially 285–295 mOsm/kg, did not exceed 360 mOsm/kg at the end of the cultures. The osmolality of F4 at the end of the cultures was 572 mOsm/kg.
Cell growth and antibody production
Figure 1 shows the profiles of cell growth, cell viability, and antibody production during the cultures. Cumulative volumes of feed during the cultures of F1, F2, F3, and F4 were approximately 182, 165, 300, and 600 mL, respectively. Viable cell concentration, viability, and antibody concentrations were estimated and plotted daily before feeding nutrients, to avoid the complexity of the profiles. In batch culture (B), a maximum viable cell concentration of (7.4 ± 0.1) × 106 cells/mL was achieved on day 5. The culture longevity, determined on the basis of the 50 % cell viability, was approximately 11 days.
Cell growth (a), viability (b), and antibody production (c) profiles during batch and fed-batch cultures with various feeding strategies (filled circle, F1: glucose feeding adjusted to 2 g/L; empty circle, F2: glucose feeding adjusted to 4 g/L; filled triangle, F3: nutrient feeding cocktail without glucose; empty triangle, F4: glucose feeding adjusted to 4 g/L + nutrient feeding cocktail; filled square, B: batch culture without feeding)
Feeding of nutrient cocktail without glucose (F3) neither increased the maximum viable cell concentration nor extended the culture longevity. In contrast, glucose feeding together with nutrient cocktail (F4) increased the maximum viability and extended the culture longevity. The maximum viable cell concentration and culture longevity were (8.2 ± 0.4) × 106 cells/mL on day 5 and approximately 13 days, respectively. Glucose feeding only (F2) extended the culture longevity as well. The culture longevity in F2 was approximately 12 days.
Due to the extended culture longevity by glucose feeding, the maximum antibody concentration in F1 (141.6 ± 11.0 mg/L) and F2 (135.1 ± 8.8 mg/L) was higher than that (109.3 ± 3.6 mg/L) in batch culture (B). The maximum antibody concentration in F4 (116.2 ± 17.2 mg/L) was comparable to that in batch culture (B), though approximately 600 mL of feed was added to a culture of initially 2 L.
Metabolism
Figure 2 shows the profiles of glucose, lactate, glutamine, and ammonia concentrations during the cultures. Glucose concentrations estimated daily before and after feeding nutrients were plotted, while other concentrations were estimated and plotted daily only before feeding nutrients, to avoid the complexity of the profiles.
Glucose, lactate (a), glutamine, and ammonia (b) profiles during batch and fed-batch cultures with various feeding strategies (filled circle, F1; empty circle, F2; filled triangle, F3; empty triangle, F4; filled square, B). Glucose (solid line), lactate (dotted line), glutamine (solid line), and ammonia (dotted line)
In batch (B) and fed-batch culture without glucose feeding (F3), glucose was almost depleted on day 5. Glucose utilization was accompanied by a corresponding accumulation of lactate up to approximately 26 mM. After glucose was depleted, cells started to consume lactate. Lactate concentrations at the end of B and F3 were 6.4 ± 1.0 and 8.5 ± 0.1 mM, respectively. In fed-batch cultures with glucose feeding (F1, F2, and F4), glucose concentration was adjusted to 11.1 or 22.2 mM to avoid glucose deficiency. Therefore, glucose was not limited during the cultures. Unlike B and F3, lactate concentration did not decrease in a later phase of the cultures. The highest lactate concentration of 56.4 ± 1.9 mM was observed in F4.
In all cultures including B, most of glutamine was consumed in the first 5 days of cultivation. In fed-batch cultures with glucose feeding only (F1 and F2), glutamine concentration, thereafter, increased slightly. Glutamine utilization was accompanied by a corresponding accumulation of ammonia for the first 4 days of cultivation. Unlike the glutamine profiles, the profiles of ammonia after 4 days of cultivation were significantly different among the cultures. In F3, F4, and F5, ammonia concentration continued to increase until the end of the cultures but to a different degree. The highest maximum ammonia concentration of 5.5 ± 0.1 mM was obtained in F3. In contrast, ammonia concentration, in F1 and F2 with glucose feeding only, decreased in a later phase of the culture. Therefore, ammonia concentration was maintained at a low level throughout the cultures.
Glycosylation
Since the glycosylation of the antibody is a critical factor in terms of efficacy and safety, it is important to understand the glycosylation patterns of the antibody produced in batch and fed-cultures with different feeding protocols shown in Fig. 1. The samples for glycosylation assay were harvested at two times, on day 4 and at the end of the cultures. N-linked oligosaccharide profiles of the antibody were analyzed under both reduced and nonreduced forms to identify individual oligosaccharide species of the antibody.
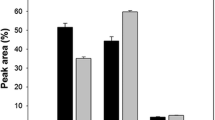
Figure 3 shows the variation of N-linked oligosaccharide profiles of the reduced antibody during the cultures. On day 4, the patterns of glycosylation were similar regardless of culture methods. Most of the N-glycans contained a core fucose and varying terminal galactose contents (G0F, G1F, and G2F). G1F (approximately 52 %) was dominant. G2F (approximately 32 %) occupied a larger portion than G0F (approximately 11 %). A small portion of nonfucosylated, nongalactosylated N-glycan (G0, less than 1.0 %) and mannose-5 (Man5, less than 1.4 %) was also evident.
N-linked oligosaccharide profiles of the reduced antibody produced during batch and fed-batch cultures with various feeding strategies. Antibodies harvested on day 4 (a) and at the end of culture (b) were analyzed. The five bars in each glycoform indicate five different cultures: F1: first bar, F2: second bar, F3: third bar, F4: fourth bar, B: fifth bar. The error bars represent standard deviations calculated from the data obtained in two independent experiments
However, the patterns of glycosylation at the end of the cultures were different among the cultures. In F3, where no significant antibody production was observed in the later phase of the culture, the pattern of glycosylation did not vary significantly during the culture. Even the largest variation of F3 was less than 0.6 %. In contrast, the ratio of G0F increased, whereas that of G1F and G2F decreased at the end of cultures in the cultures, except for F3. In F4, where the largest variation in the patterns of glycosylation was observed, the ratio of G1F decreased by 10.5 % and the ratio of G0F increased by 9.7 % during the culture.
The variation of N-linked oligosaccharide profiles of the nonreduced antibody was also analyzed through the whole IgG intact mass. The trend of paired glycosylation was similar to that of reduced glycosylation. In F3, the pattern of glycosylation did not vary significantly during the culture. In the other cultures except for F3, a low galactosylated form such as G0F-G0F and G1F-G0F increased, whereas a high galactosylated form such as G1F-G1F, G0F-G2F, and G1F-G2F decreased at the end of cultures (Fig. S1).
Aglycosylation
Aglycosylated antibody, without N-linked oligosaccharide at N297 of the Fc domain, was measured simultaneously through the whole IgG intact mass.
Figure 4 shows the variations of an aglycosylated antibody from day 4 to the end of cultures. On day 4, when glucose was not depleted, no aglycosylated antibody was detected regardless of culture methods. In cultures with glucose feeding (F1, F2, and F4), aglycosylated antibody was not detected, either, at the end of culture. However, it was detected at the end of cultures without glucose feeding (F3 and B). The percentages of aglycosylated antibodies at the end of the cultures of F3 and B were 44.2 ± 1.1 and 11.9 ± 0.7 %, respectively. Thus, it appears that aglycosylated antibody was produced only under glucose-depleted conditions.
Aglycosylated antibody profiles during batch and fed-batch cultures with various feeding strategies. Antibodies harvested on day 4 (first bar) and at the end of culture (second bar) were analyzed. Asterisk indicates that aglycosylated antibody was not detected. The error bars represent standard deviations calculated from the data obtained in two independent experiments
Aggregation
Since antibody aggregates may cause immune responses, the level of aggregates was measured using SEC-HPLC. Using SEC-HPLC, two abnormal structures outranged from a main molecular weight peak were detected and were designated as high molecular weight (HMW, antibody aggregate) and low molecular weight (LMW, cleaved antibody) structures, respectively. Regardless of culture methods, antibody aggregation was insignificant (Fig. S2).
Western blotting analysis
In N-linked glycosylation, oligosaccharyl transferases catalyze the biosynthesis of N-linked glycoproteins, in which they transfer a tetradecasaccharide from a dolichol pyrophosphate donor to asparagine side chains within nascent polypeptide during dolichol pathway. Accordingly, oligosaccharyl transferases are believed to be key enzymes affecting aglycosylation (Burda and Aebi 1999; Dempski and Imperiali 2002).
Figure 5 shows the expression level of representative oligosaccharyl transferases (ribophorin I and II, and DAD1) determined by Western blotting. β-actin was used as a loading control. As in F2 culture, cells in F1 culture did not experience glucose limitation. Therefore, they were excluded from Western blot analysis. Cells in cultures of F2, F3, F4, and B were sampled for Western blot analysis on days 3, 6, and 9.
Changes in the expression level of oligosaccharyl transferases during the culture were similar among the cultures. Regardless of culture methods, ribophorin I and II did not show a significant variation during the cultures. In contrast, DAD1 increased from day 3 to day 9. The increased expression level of DAD1 toward the end of culture was expected because DAD1 is a well-known apoptotic maker of human cell lines (Kelleher and Gilmore 1997).
Discussion
For therapeutic antibodies, a consistent human-type glycosylation should be maintained throughout development, irrespective of the system in which they are produced. Previously, it was observed that a human cell line, rF2N78 cells, started to produce aglycosylated antibody at the later part of batch culture when glucose was depleted (Seo et al. 2013). Aglycosylation under a glucose-deficient condition was also observed with rCHO cells (Hayter et al. 1992; Hayter et al. 1993; Seo et al. 2013) and human hybridoma cells (Tachibana et al. 1994).
An aglycosylated antibody lacks N-linked oligosaccharide at the N297 site of the Fc domain and does not show antibody-dependent cell-mediated cytotoxicity (ADCC) activity (Jefferis 2009a, b; Jung et al. 2010). To determine whether the aglycosylation of the antibody can be minimized through the manipulation of glucose concentration in a fed-batch culture, fed-batch cultures with four different protocols were carried out in a bioreactor with pH control.
In cultures without glucose feeding (F3 and B), glucose was almost depleted on day 5. No aglycosylated antibody was detected on day 4, whereas a significant amount of aglycosylated antibody was detected at the end of cultures. Thus, aglycosylated antibody was produced only in a later phase of culture when glucose was depleted. Nutrient feeding cocktails without glucose in culture F3 could not reduce the aglycosylation of antibody. In contrast, aglycosylated antibody was not detected throughout the cultures with glucose feeding (F1, F2, and F4). Glucose concentration of 2 g/L was high enough to avoid aglycosylation of antibody.
When specific antibody productivity (q Ab) was calculated based on the data collected from day 5 to the end of culture (Renard et al. 1988), it was similar among the cultures. Thus, it is unlikely that the q Ab affected aglycosylation of antibody. In addition, Western blot analysis of oligosaccharyl transferases in Fig. 5 indicates that the expression levels of oligosaccharyl transferases were similar among the cultures, suggesting that aglycosylation of the antibody was not due to altered expression of oligosaccharyl transferases under glucose-deficient conditions.
The percentage of aglycosylated antibody at the end of culture F3 was much higher than that at the end of culture B. In culture F3, it was approximately 44 %, suggesting that most of the antibodies produced under the glucose-depleted condition from day 4 to the end of culture were aglycosylated. Previously, it was reported that an elevated ammonia level increased the intracellular pH and inhibited the activities of enzymes related to the branching mechanism of glycosylation (Chen and Harcum 2006; Gawlitzek et al. 2000). Under glucose-deficient conditions, various amino acids were used as a carbon source, from which the ammonia molecule, NH4 +, might be released. The highest ammonia concentration (5.5 mM) in culture F3 fed with amino acid cocktails might also have contributed to aglycosylation of the antibody, though the relationship between aglycosylation and ammonia concentration has not been clearly understood yet.
To estimate the correlation between aglycosylation and aggregation of the antibody during the cultures, antibody samples on day 4 and at the end of cultures were analyzed using SEC-HPLC. We obtained three peaks, which were one main peak for normal antibody structure and two peaks distinct from the main peak: HMW structure for aggregated antibodies and LMW structure for cleaved antibodies. During all cultures, the percentages of HMW and LMW structures did not vary significantly and were below 2 and 0.1 %, respectively. Previously, it was reported that the aglycosylated form aggregated faster than the glycosylated form (Kayser et al. 2011). However, the aglycosylated form did not aggregate faster than the glycosylated form in this study. In fact, the aggregate form in cultures of F3, which produced a significant amount of aglycosylated antibody, did not increase significantly from day 4 to the end of culture. On the other hand, the aggregate form in cultures of F1, F2, and F4, which produced no aglycosylated antibody, increased slightly from day 4 to the end of culture. Therefore, it seems that the antibody produced in rF2N78 is stable in the culture conditions tested and that aglycosylation of the antibody does not influence its aggregation.
As an initial step of glycosylation, a dolichol-linked oligosaccharide requires three different nucleotide-activated sugar donors, which include UDP-N-acetylglucosamine, GDP-mannose, and UDP-glucose. Glucose capping on oligosaccharide is a key element in the oligosaccharide recognition by the oligosaccharyl transferase, which eventually allows the transfer of the oligosaccharide to asparagine residues in the endoplasmic reticulum (Burda and Aebi 1999). Thus, it is likely that glucose deficiency led to insufficiency of the precursor for glycosylation and induced aglycosylation of the antibody.
In cultures with glucose feeding, no aglycosylated antibody was observed and antibody aggregation was insignificant at the end of culture. Furthermore, as observed with other human cell lines, the antibody produced in rF2N78 had more galactose capping than that in rCHO cells (Seo et al. 2013). G1F was dominant and G2F occupied a larger portion than G0F. These results demonstrate the potential of F2N78 as a new antibody-producing cell line that has a glycosylation pattern similar to a human serum IgG.
As observed with rF2N78 cells, it is likely that occurrence of aglycosylated antibody during recombinant CHO cell cultures can be prevented by glucose feeding.
In conclusion, rF2N78 cells produced aglycosylated Ab at the later part of batch culture when glucose was depleted. Glucose feeding in fed-batch cultures successfully prevented occurrence of aglycosylated antibody during the cultures, confirming that glucose depletion is a main cause for aglycosylation of antibody.
References
Adair JR (1992) Engineering antibodies for therapy. Immunol Rev 130:5–40
Borrok MJ, Jung ST, Kang TH, Monzingo AF, Georgiou G (2012) Revisiting the role of glycosylation in the structure of human IgG Fc. ACS Chem Biol 7(9):1596–1602
Burda P, Aebi M (1999) The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta 1426(2):239–257
Chen P, Harcum SW (2006) Effects of elevated ammonium on glycosylation gene expression in CHO cells. Metab Eng 8(2):123–132
Dempski RE, Imperiali B (2002) Oligosaccharyl transferase: gatekeeper to the secretory pathway. Curr Opin Chem Biol 6(6):844–850
Gawlitzek M, Ryll T, Lofgren J, Sliwkowski MB (2000) Ammonium alters N-glycan structures of recombinant TNFR-IgG: degradative versus biosynthetic mechanisms. Biotechnol Bioeng 68(6):637–646
Hayter PM, Curling EM, Baines AJ, Jenkins N, Salmon I, Strange PG, Tong JM, Bull AT (1992) Glucose-limited chemostat culture of Chinese hamster ovary cells producing recombinant human interferon-gamma. Biotechnol Bioeng 39(3):327–335
Hayter PM, Curling EM, Gould ML, Baines AJ, Jenkins N, Salmon I, Strange PG, Bull AT (1993) The effect of the dilution rate on CHO cell physiology and recombinant interferon-gamma production in glucose-limited chemostat culture. Biotechnol Bioeng 42(9):1077–1085
Hwang SO, Lee GM (2008) Autophagy and apoptosis in Chinese hamster ovary cell culture. Autophagy 4(1):70–72
Hwang SJ, Kim SH, Kim HZ, Steinmetz MO, Koh GY, Lee GM (2008) High-level expression and purification of a designed angiopoietin-1 chimeric protein, COMP-Ang1, produced in Chinese hamster ovary cells. Protein J 27(5):319–326
Jefferis R (2009a) Glycosylation as a strategy to improve antibody-based therapeutics. Nat Rev Drug Discov 8(3):226–234
Jefferis R (2009b) Recombinant antibody therapeutics: the impact of glycosylation on mechanisms of action. Trends Pharmacol Sci 30(7):356–362
Jung ST, Reddy ST, Kang TH, Borrok MJ, Sandlie I, Tucker PW, Georgiou G (2010) Aglycosylated IgG variants expressed in bacteria that selectively bind FcgammaRI potentiate tumor cell killing by monocyte-dendritic cells. Proc Natl Acad Sci U S A 107(2):604–609
Kayser V, Chennamsetty N, Voynov V, Forrer K, Helk B, Trout BL (2011) Glycosylation influences on the aggregation propensity of therapeutic monoclonal antibodies. Biotechnol J 6(1):38–44
Kelleher DJ, Gilmore R (1997) DAD1, the defender against apoptotic cell death, is a subunit of the mammalian oligosaccharyltransferase. Proc Natl Acad Sci 94(10):4994–4999
Lee H-J, Chang M, Kim J-M, Hong H, Maeng K, Koo J, Chang S, Cho M-S (2013) Application of a new human cell line, F2N78, in the transient and stable production of recombinant therapeutics. Biotechnol Prog 29(2):432–440
Nesspor TC, Raju TS, Chin CN, Vafa O, Brezski RJ (2012) Avidity confers FcgammaR binding and immune effector function to aglycosylated immunoglobulin G1. J Mol Recognit 25(3):147–154
Renard JM, Spagnoli R, Mazier C, Salles MF, Mandine E (1988) Evidence that monoclonal antibody production kinetics is related to the integral of the viable cells curve in batch systems. Biotechnol Lett 10(2):91–96
Seo JS, Kim YJ, Cho JM, Baek E, Lee GM (2013) Effect of culture pH on recombinant antibody production by a new human cell line, F2N78, grown in suspension at 33.0 °C and 37.0 °C. Appl Microbiol Biotechnol 97(12):5283–5291
Tachibana H, Taniguchi K, Ushio Y, Teruya K, Osada K, Murakami H (1994) Changes of monosaccharide availability of human hybridoma lead to alteration of biological properties of human monoclonal antibody. Cytotechnology 16(3):151–157
Tao MH, Morrison SL (1989) Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol 143(8):2595–2601
Walker MR, Lund J, Thompson KM, Jefferis R (1989) Aglycosylation of human IgG1 and IgG3 monoclonal antibodies can eliminate recognition by human cells expressing Fc gamma RI and/or Fc gamma RII receptors. Biochem J 259(2):347–353
Acknowledgments
This research was supported in part by a grant from the Fundamental R&D Program for Technology of World Premier Materials funded by the Ministry of Knowledge Economy and a grant from the Intelligent Synthetic Biology Center of Global Frontier Project funded by the MEST (2011-0031962), Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 310 kb)
Rights and permissions
About this article
Cite this article
Seo, J.S., Min, B.S., Kim, Y.J. et al. Effect of glucose feeding on the glycosylation quality of antibody produced by a human cell line, F2N78, in fed-batch culture. Appl Microbiol Biotechnol 98, 3509–3515 (2014). https://doi.org/10.1007/s00253-013-5462-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5462-0