Abstract
Although many efforts had been performed to engineer Escherichia coli for succinate production, succinate efflux system had not been investigated as an engineering target for improving succinate production. In this work, four Dcu transporters, which had been reported to be responsible for C4-dicarboxylates transportation of E. coli, were investigated for their succinate efflux capabilities. These four dcu genes were deleted individually in a previously constructed succinate-producing strain to study their effects on succinate production. Deleting dcuA and dcuD genes had nearly no influence, while deleting dcuB and dcuC genes led to 15 and 11 % decrease of succinate titer, respectively. Deleting both dcuB and dcuC genes resulted in 90 % decrease of succinate titer, suggesting that DcuB and DcuC were the main transporters for succinate efflux and they functioned as independent and mutually redundant succinate efflux transporters. Furthermore, RBS library having strengths varied from 0.17 to 8.6 times of induced E. coli lacZ promoter was used to modulate dcuB and dcuC genes for improving succinate production. Modulating these two genes in combination led to 34 % increase of succinate titer. To the best of knowledge, this was the first report about improving succinate production through engineering succinate efflux system.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Succinate is a four-carbon dicarboxylate that has many applications in food, agricultural, pharmaceutical, and biodegradable plastic industries (McKinlay et al. 2007). Although succinate is currently produced from petroleum-derived maleic anhydride, considerable interest in microbial production from renewable biomass has emerged during the past two decades (Lee et al. 2005; Lin et al. 2005; Wang et al. 2006; McKinlay et al. 2007; Zhang et al. 2009a). Many efforts had been performed to improve succinate production in Escherichia coli. Competitive fermentation pathways, such as formate pyruvate-lyase, lactate dehydrogenase, and alcohol dehydrogenase, were usually inactivated to eliminate production of byproducts and increase succinate yield (Stols and Donnelly 1997; Lee et al. 2005; Wang et al. 2006; Jantama et al. 2008a, b; Singh et al. 2011). In addition, precursor and energy supplies were increased for improved cell growth and succinate production under anaerobic conditions (Chatterjee et al. 2001; Zhang et al. 2009a, b; Singh et al. 2011).
On the other hand, transportations, such as substrate uptake and product efflux, are very important for efficient production of target compounds. The phosphoenolpyruvate (PEP): carbohydrate phosphotransferase system (PTS) of E. coli was usually inactivated to increase PEP supply for succinate production, and alternative glucose utilization pathways were recruited to improve cell growth and glucose consumption rates (Wang et al. 2006; Lu et al. 2012; Tang et al. 2013). However, there has been no report about engineering export system to improve succinate production.
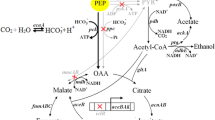
E. coli contains four Dcu carries (encoded by dcuA, dcuB, dcuC, and dcuD genes) for C4-dicarboxylates (succinate, fumarate, and malate) transportation. The uptake, antiport, and efflux of C4-dicarboxylates are mediated by the Dcu transporters. DcuA and DcuB are homologous proteins that appear to function as independent and mutually redundant C4-dicarboxylate transporters during anaerobic conditions (Golby et al. 1998). DcuB is the major C4-dicarboxylate carrier for fumarate respiration with high fumarate-succinate exchange activity, while the precise physiological purpose of DcuA remains unclear (Golby et al. 1998). Although DcuC is able to compensate for DcuA and DcuB in fumarate-succinate exchange and fumarate uptake, the preferential function of DcuC is suggested to be involved in succinate efflux during glucose fermentation (Zientz et al. 1999). DcuD, which is homologous to DcuC, apparently is not expressed under most conditions, and its role is unknown (Janausch and Unden 1999).
In this work, the four dcu genes were individually deleted in a succinate-producing strain Suc-T110 (Tan et al. 2013) to investigate their roles in succinate production under anaerobic glucose fermentation conditions. Deleting dcuA and dcuD genes had nearly no influence, while deleting dcuB and dcuC genes led to 15 and 11 % decrease of succinate titer, respectively. Deleting both dcuB and dcuC genes resulted in 90 % decrease of succinate titer. It was suggested that DcuB and DcuC were the main transporters for succinate efflux during glucose fermentation. These two genes were further modulated in combination by ribosomal binding site (RBS) libraries, leading to 34 % increase of succinate titer.
Materials and methods
Strains, medium, and growth conditions
Strains used in this study were listed in Table 1. During strain construction, cultures were grown aerobically at 30, 37, or 39 °C in Luria broth (per liter, 10 g Difco tryptone, 5 g Difco yeast extract, and 10 g NaCl). Ampicillin (100 mg L−1), kanamycin (25 mg L−1), and chloramphenicol (17 mg L−1) were used where appropriate.
Deletion of dcu genes in strain Suc-T110
The dcuA gene in strain Suc-T110 (Tan et al. 2013) was deleted using a two-step recombination method as described previously (Zhang et al. 2007; Jantama et al. 2008a). In the first recombination, cat-sacB cassette was amplified from pXZ-CS (Tan et al. 2013) using primer set dcuA-QC-cat-up/dcuA-QC-sacB-down and used to replace the coding region of dcuA gene. Fusion PCR was performed to prepare DNA fragment for the second recombination. The upstream and downstream regions (about 700 bp) of dcuA gene were amplified from E. coli ATCC 8739 genomic DNA using primer sets dcuA-QC-1/dcuA-QC-2 and dcuA-QC-3/dcuA-QC-4. These two fragments were then served as templates to perform fusion PCR using primer set dcuA-QC-1/dcuA-QC-4. The resulting DNA fragment (about 1,400 bp) was used to replace cat-sacB cassette by selection for resistance to sucrose. Cells containing sacB gene accumulated levan during incubation with sucrose and were killed. Surviving recombinants were highly enriched for loss of cat-sacB cassette (Zhang et al. 2007; Jantama et al. 2008a). The dcuB, dcuC, and dcuD genes were deleted in the same manner. All strains obtained were listed in Table 1, and all primers were listed in Table 2.
Modulating lacZ gene with RBS library
A one-step homologous recombination method was used to modulate lacZ gene with RBS library (Fig. 1). DNA fragment containing RBS library was amplified from genomic DNA of recombinant E. coli M1-93 using primer set lacI-up500/lacZ-RBSL-down and used for recombination. Recombinant E. coli M1-93 was selected from a previously constructed mRNA stabilizing region library, which had a regulatory part inserted in front of lacZ gene, and its strength was five times of induced E. coli lacZ promoter (Lu et al. 2012). The left homologous arm was 500 bp. The partial coding region of lacZ gene (from +1 to +50 relative to the translational start site of lacZ) of E. coli was used as the right homologous arm (Fig. 1b). The sequence of RBS library is CAGGAGRNNNNNN (Fig. 1a). After recombination, colonies were randomly picked for further PCR verification using primer set lacI-up500/lacZ-373. Twenty-nine correct colonies and wild-type strain ATCC 8739 were then picked and grown in LB medium for β-galactosidase assay.
Enzyme assays
Exponentially grown cells were harvested by centrifugation (7,000 g for 5 min, 4 °C). Cells were washed twice in 50 mM sodium phosphate buffer (pH 7.0), and the activity of β-galactosidase was measured as described by Miller (1992).
Modulating dcuB and dcuC genes with RBS library
A two-step homologous recombination method was used to modulate dcuB gene with RBS library. Firstly, cat-sacB cassette was amplified from pXZ-CS with primer set dcuB-cat-up-1/dcuB-sacB-down and used to insert before ATG translation start site of dcuB gene. DNA fragment containing RBS library, which was the same as the RBS library of lacZ gene, was amplified from genomic DNA of recombinant E. coli M1-93 using primer set dcuB-up-P-1/dcuB-RBSL-down and used for the second recombination. Colonies without chloramphenicol resistant were picked for further PCR verification using primer set Ap1-up/dcuB-down-200. Five right colonies were selected for further fermentation. The dcuC gene was modulated in a same manner.
Fermentation
Fresh colonies were picked from New Brunswick Scientific (NBS) mineral salts plates containing 20 g L−1 glucose, and inoculated into 250 ml flasks containing 100 ml NBS mineral salts medium with 50 g L−1 glucose, and grown at 37 °C and 100 rpm for 12 h. Cultures were then transferred to a 500 ml fermentation vessel containing 250 ml NBS mineral salts medium with 50 g L−1 glucose and 100 mM potassium bicarbonate. The initial OD550 was 0.1, and pH was maintained at 7.0 by automatic addition of a base containing 2.4 M potassium carbonate and 1.2 M potassium hydroxide.
Analysis
The dry weight of cells was calculated by measuring the optical density value at 550 nm (OD550). Organic acids and residual glucose in fermentation broth were measured by high-performance liquid chromatography (Zhang et al. 2009b).
Results
Effects of deleting dcu genes on succinate production
In order to investigate the roles of different Dcu transporters on succinate efflux during glucose fermentation, the four dcu genes of strain Suc-T110 were deleted individually. This parent strain was previously engineered for succinate production by deleting lactate dehydrogenase and pyruvate formate–lyase genes to inactivate competitive fermentation pathways, as well as inactivating PTS system and recruiting an alternative glucose utilization pathway (Galactose permease) to increase PEP supply. Furthermore, PEP carboxykinase was activated to increase energy supply (Tan et al. 2013). This strain produced 273 and 280 mM succinate after fermentation for 72 and 96 h, respectively (Table 3). Succinate yield of this strain was 1.12 mol/mol (Table 3).
Deleting dcuA or dcuD gene had nearly no influence on succinate titer and yield (Table 3). Succinate titer decreased 15 % (from 273 to 232 mM) and succinate yield decreased 15 % (from 1.12 to 0.95 mol/mol) after deleting dcuB gene. Succinate titer decreased 11 % (from 273 to 244 mM) and succinate yield decreased 10 % (from 1.12 to 1.01 mol/mol) after deleting dcuC gene. The dcuB and dcuC genes were further deleted simultaneously, resulting in strain JC-105. Cell mass, succinate titer, and succinate yield of strain JC-105 decreased significantly. This strain produced only 26 mM succinate with a yield of 0.41 mol/mol. Succinate titer and yield were 10 and 40 % of the parent strain Suc-T110, respectively (Table 3). It was thus suggested that DcuB and DcuC were the main two transporters for succinate efflux during glucose fermentation.
Strength variations of RBS library
RBS library, which had a sequence of CAGGAGRNNNNNN in front of the ATG translation start site, was selected in this work for modulating dcuB and dcuC genes to improve succinate efflux capability. In order to test the strength variations of the RBS library, it was firstly constructed in front of lacZ gene in the chromosome of E. coli ATCC 8739. After recombination, β-galactosidase activities of 29 colonies verified by PCR amplification and wild-type stain ATCC 8739 were measured. Strengths of regulatory parts in the RBS library distributed evenly (Fig. 2), which varied from 0.17 to 8.6 times of induced lacZ promoter. It was suggested that RBS library used in this work could lead to a wide range of strength variation.
Modulating dcuB and dcuC genes with RBS library
A regulatory part library, which included a promoter, a mRNA stabilizing region and a RBS library (Fig. 1a) was inserted in the ATG translation start site of dcuB and dcuC genes. The native promoters of dcuB and dcuC genes were kept. After modulating dcuB gene with RBS library, five strains verified by PCR were randomly picked for glucose fermentation (Fig. 3). Succinate titers varied from 235 to 295 mM and succinate yields varied from 1.12 to 1.25 mol/mol in these five strains. Strain Suc-T110-dcuB3, which had the highest succinate titer and yield, was renamed JC-205 (Table 4). Succinate titer increased 8 % compared to parent strain Suc-T110. Another strain Suc-T110-dcuB4, which produced 278 mM succinate with a yield of 1.15 mol/mol, was renamed JC-206 (Table 4).
Five strains verified by PCR in the dcuC library were also picked for glucose fermentation (Fig. 4). Succinate titers varied from 243 to 324 mM and succinate yields varied from 1.15 to 1.32 mol/mol in these five strains. Strain Suc-T110-dcuC2, which had the highest succinate titer and yield, was renamed JC-207 (Table 4). Succinate titer increased 19 % compared to parent strain Suc-T110. Another strain Suc-T110-dcuC3, which produced 311 mM succinate with a yield of 1.25 mol/mol, was renamed JC-208 (Table 4).
Modulating dcuB and dcuC genes in combination to improve succinate production
The dcuB and dcuC genes were further modulated in combination to improve succinate production. The dcuC gene of strains JC-205 and JC-206 were modulated with RBSL2 and RBSL3, resulting in strains JC-209, JC-210, JC-211, and JC-212 (Table 1). Succinate titer and yield increased in all four modulated strains compared to parent strains JC-205 or JC-206 (Table 4). The best strain JC-211, which had dcuB gene modulated with RBSL4 and dcuC gene modulated with RBSL2, produced 366 mM succinate with a yield of 1.41 mol/mol. Succinate titer of strain JC-211 was 32 % higher than the parent strain JC-206 and 34 % higher than unmodulated strain Suc-T110 (Table 4). On the other hand, succinate yield of strain JC-211 was 23 % higher than the parent strain JC-206 and 26 % higher than unmodulated strain Suc-T110 (Table 4).
Discussions
Modulating gene expression directly in chromosome by multiple regulatory parts with varied strengths had been demonstrated to be better than plasmid-based gene overexpression using inducible strong promoters (Shi et al. 2013; Tan et al. 2013; Zhao et al. 2013). However, it has been suggested that expression level of a specific gene depended on both the sequence of regulatory part and sequence of gene itself (Shi et al. 2013) since different gene sequences would have an influence on the secondary structures between the transcription and translation start sites (Carrier and Keasling 1999). Thus, regulatory parts having high strengths for one gene (such as lacZ gene) might have low strengths for other genes. Modulating gene expression with a regulatory part library would solve this problem since a wide range of strengths would be obtained. In this work, RBS library constructed in front of lacZ gene exhibited a strength variation of 50 times. The highest strength was 8.6 times of induced E. coli lacZ promoter, which was 72 % higher than commonly used T7 promoter (five times of induced E. coli lacZ promoter).
Product efflux system is very important for efficient production of target compounds. Although E. coli contains multiple Dcu carries for C4-dicarboxylates transportation, only DcuC had been reported previously to be involved in succinate efflux during glucose fermentation (Zientz et al. 1999). The main function of DcuB had been reported to catalyze C4-dicarboxylate exchange, for instance, fumarate uptake in exchange for succinate efflux (Six et al. 1994). In addition, DcuB had been implied in succinate efflux as well (Six et al. 1994). In this study, through deletion of four dcu genes either individually or in combination, it was found that both DcuB and DcuC were important for succinate efflux during glucose fermentation. Although deleting dcuB or dcuC gene individually led to only a small decrease of succinate production, deleting these two genes in combination had a 90 % decrease of succinate titer. It was suggested that DcuB and DcuC function as independent and mutually redundant succinate efflux transporters during glucose fermentation.
In a previous report, it was found that succinate efflux during glucose fermentation was not affected in an E. coli mutant deficient in DctA, DcuA, DcuB, DcuC, and DcuD (Janausch et al. 2001), suggesting that a carrier different from, or in addition to, the known Dcu and CitT carriers was used for succinate efflux. This was different with the finding in this work. It should be noted that only 1 mM succinate was produced in the previous E. coli mutant (Janausch et al. 2001). In contrast, the starting E. coli strain we used in this work produced 273 mM succinate in 72 h. The adverse results were suggested to be caused by the different succinate-producing level in these two strains. The unknown carrier can replace the succinate efflux function of Dcu carriers in a low intracellular succinate concentration, while it cannot replace the function of DcuB and DcuC when large amounts of succinate need to be excreted.
Furthermore, through modulating dcuB and dcuC genes in combination, succinate titer increased from 273 to 366 mM and succinate yield increased from 1.12 to 1.41 mol/mol. To the best of knowledge, this was the first report about improving succinate production through engineering succinate efflux system.
References
Carrier TA, Keasling JD (1999) Library of synthetic 5′ secondary structures to manipulate mRNA stability in Escherichia coli. Biotechnol Prog 15:58–64
Chatterjee R, Millard CS, Champion K, Clark DP, Donnelly MI (2001) Mutation of the ptsG gene results in increased production of succinate in fermentation of glucose by Escherichia coli. Appl Environ Microbiol 67:148–154
Golby P, Kelly DJ, Guest JR, Andrews SC (1998) Transcriptional regulation and organization of the dcuA and dcuB genes, encoding homologous anaerobic C4-dicarboxylate transporters in Escherichia coli. J Bacteriol 180:6586–6596
Janausch IG, Unden G (1999) The dcuD (former yhcL) gene product of Escherichia coli as a member of the DcuC family of C4-dicarboxylate carriers: lack of evident expression. Arch Microbiol 172:219–226
Janausch IG, Kim OB, Unden G (2001) DctA- and Dcu-independent transport of succinate in Escherichia coli: contribution of diffusion and of alternative carriers. Arch Microbiol 176:224–230
Jantama K, Haupt MJ, Svoronos SA, Zhang X, Moore JC, Shanmugam KT, Ingram LO (2008a) Combining metabolic engineering and metabolic evolution to develop nonrecombinant strains of Escherichia coli C that produce succinate and malate. Biotechnol Bioeng 99:1140–1153
Jantama K, Zhang X, Moore JC, Shanmugam KT, Svoronos SA, Ingram LO (2008b) Eliminating side products and increasing succinate yields in engineered strains of Escherichia coli C. Biotechnol Bioeng 101:881–893
Lee SJ, Lee DY, Kim TY, Kim BH, Lee J, Lee SY (2005) Metabolic engineering of Escherichia coli for enhanced production of succinic acid, based on genome comparison and in silico gene knockout simulation. Appl Environ Microbiol 71:7880–7887
Lin H, Bennett GN, San KY (2005) Fed-batch culture of a metabolically engineered Escherichia coli strain designed for high-level succinate production and yield under aerobic conditions. Biotechnol Bioeng 90:775–779
Lu J, Tang J, Liu Y, Zhu X, Zhang T, Zhang X (2012) Combinatorial modulation of galP and glk gene expression for improved alternative glucose utilization. Appl Microbiol Biotechnol 93:2455–2462
McKinlay JB, Vieille C, Zeikus JG (2007) Prospects for a bio-based succinate industry. Appl Microbiol Biotechnol 76:727–740
Miller JH (1992) A short course in bacterial genetics. Cold Spring Harbor Laboratory, New York, pp 71–74
Shi A, Zhu X, Lu J, Zhang X, Ma Y (2013) Activating transhydrogenase and NAD kinase in combination for improving isobutanol production. Metab Eng 16:1–10
Singh A, Cher Soh K, Hatzimanikatis V, Gill RT (2011) Manipulating redox and ATP balancing for improved production of succinate in E. coli. Metab Eng 13:76–81
Six S, Andrews SC, Unden G, Guest JR (1994) Escherichia coli possesses two homologous anaerobic C4-dicarboxylate membrane transporters (DcuA and DcuB) distinct from the aerobic dicarboxylate transport system (Dct). J Bacteriol 176:6470–6478
Stols L, Donnelly MI (1997) Production of succinic acid through overexpression of NAD+-dependent malic enzyme in an Escherichia coli mutant. Appl Environ Microbiol 63:2695–2701
Tan Z, Zhu X, Chen J, Li Q, Zhang X (2013) Activating phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxykinase in combination for improvement of succinate production. Appl Environ Microbiol 79:4838–4844
Tang J, Zhu X, Lu J, Liu P, Xu H, Tan Z, Zhang X (2013) Recruiting alternative glucose utilization pathways for improving succinate production. Appl Microbiol Biotechnol 97:2513–2520
Wang Q, Wu C, Chen T, Chen X, Zhao X (2006) Expression of galactose permease and pyruvate carboxylase in Escherichia coli ptsG mutant increases the growth rate and succinate yield under anaerobic conditions. Biotechnol Lett 28:89–93
Zhang X, Jantama K, Moore JC, Shanmugam KT, Ingram LO (2007) Production of l-alanine by metabolically engineered Escherichia coli. Appl Microbiol Biotechnol 77:355–366
Zhang X, Jantama K, Moore JC, Jarboe LR, Shanmugam KT, Ingram LO (2009a) Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc Natl Acad Sci U S A 106:20180–20185
Zhang X, Jantama K, Shanmugam KT, Ingram LO (2009b) Reengineering Escherichia coli for succinate production in mineral salts medium. Appl Environ Microbiol 75:7807–7813
Zhao J, Li Q, Sun T, Zhu X, Xu H, Tang J, Zhang X, Ma Y (2013) Engineering central metabolic modules of Escherichia coli for improving beta-carotene production. Metab Eng 17:42–50
Zientz E, Janausch IG, Six S, Unden G (1999) Functioning of DcuC as the C4-dicarboxylate carrier during glucose fermentation by Escherichia coli. J Bacteriol 181:3716–3720
Acknowledgments
This research was supported by grants from the Knowledge Innovation Project of the Chinese Academy of Sciences (KSCX2-EW-G-2), the National Basic Research Program of China (2011CBA00800), and the National High Technology Research and Development Program of China (2011AA02A203). Xueli Zhang was supported by the Hundred Talent Program of the Chinese Academy of Sciences.
Conflict of interests
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jing Chen and Xinna Zhu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Chen, J., Zhu, X., Tan, Z. et al. Activating C4-dicarboxylate transporters DcuB and DcuC for improving succinate production. Appl Microbiol Biotechnol 98, 2197–2205 (2014). https://doi.org/10.1007/s00253-013-5387-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5387-7