Abstract
Nonribosomal peptides (NRPs), a large family of natural products, possess numerous pharmaceutically significant bioactivities. However, many native microbial producers of NRPs are not cultivable or have low production yields making mass production infeasible. The recombinant production of natural products in a surrogate host has emerged as a strategy to overcome these limitations. De novo recombinant production of the NRP antibiotic valinomycin in an engineered Escherichia coli host strain was established with the necessary biosynthetic pathway constituents from Streptomyces tsusimaensis. In the present study, the initially modest valinomycin yields could be significantly increased from 0.3 up to 2.4 mg L−1 by switching from a batch to an enzyme-based fed-batch mode in shake flasks. A subsequent design of experiment-driven optimization of parallel fed-batch cultivations in 24-well plates with online monitoring of dissolved oxygen and pH led to valinomycin yields up to 6.4 mg L−1. Finally, repeated glucose polymer feeding to enzyme-based high cell density cultivations in shake flasks resulted in cell densities of OD600 >50 and a valinomycin titer of appr. 10 mg L−1. This represents a 33-fold improvement compared to the initial batch cultivations and is the highest concentration of a nonribosomal peptide which has been produced in E. coli without feeding of specific precursors so far to our knowledge. Also, such a small-scale optimization under fed-batch conditions may be generally applicable for the development and scale-up of natural product production processes in E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nonribosomal peptides (NRPs), belonging to a large class of peptide natural products, are mainly produced by various microorganisms like bacteria and fungi (Marahiel et al. 1997; Stack et al. 2007). These naturally originated compounds are of great interest since they possess a broad spectrum of biological activities and are used in many pharmaceutical applications (Felnagle et al. 2008). NRPs are biosynthesized in native producers via the assembly lines called nonribosomal peptide synthetases (NRPSs), which are composed of multiple catalytic modules (Finking and Marahiel 2004; Fischbach and Walsh 2006). Usually, each NRPS module contains three core domains responsible for the recognition, activation, and incorporation of one substrate monomer into the final peptide product and various optional tailoring domains for the modification of the immature NRP product (Hur et al. 2012).
The NRP antibiotic valinomycin is a cyclododecadepsipeptide containing a triple repeating unit of d-α-hydroxyisovaleryl-d-valyl-l-lactyl-l-valyl (Shemyakin et al. 1963). It has a wide range of significant bioactivities, such as antiviral (Wu et al. 2004), antifungal (Park et al. 2008), insecticidal (Heisey et al. 1988), antimicrobial (Tempelaars et al. 2011), and anticancer activity (Pettit et al. 1999). Several Streptomyces strains have been reported to produce valinomycin synthesized by a large multifunctional NRPS (Matter et al. 2009). However, these natural Streptomyces hosts grow slowly and can become viscous under high cell density conditions making large-scale production of valinomycin infeasible and costly. Since the gene cluster of the valinomycin NRPSs has been isolated and characterized (Cheng 2006; Magarvey et al. 2006), the heterologous production of valinomycin in a robust and easy to manipulate host like Escherichia coli has become a putative strategy to produce valinomycin and analogs thereof.
In recent years, E. coli has been used extensively to produce bioactive natural products, including large multi-modular enzyme-derived compounds NRPs (Watanabe et al. 2006), polyketides (PKs) (Pfeifer et al. 2001; Zhang et al. 2010), and NRP/PK hybrids (Mutka et al. 2006). However, it is challenging to produce these natural products in high levels using E. coli, which limits industrial production and drug development. The antitumor agent NRP echinomycin was reported to be totally biosynthesized in E. coli with a microgram per liter level productivity (Watanabe et al. 2006). While for heterologous production of the anticancer NRP/PK epothilones C and D in E. coli, the yields were reported lower than 1 μg L−1 (Mutka et al. 2006). Therefore, it is necessary to develop a feasible approach for the optimization and overproduction of medically relevant agents in E. coli.
We have recently realized the reconstituted biosynthesis of the valinomycin in E. coli as a whole-cell biocatalyst (unpublished results). E. coli was able to provide the three necessary precursors l-valine, α-ketoisovaleric acid (Kiv), and pyruvate for the de novo biosynthesis of valinomycin from its own primary metabolism (Fig. 1). Valinomycin production was shown to correlate with E. coli cell growth and it was hypothesized that valinomycin formation was limited by the precursor supply from the primary metabolism during batch cultivations. In this study, we used an enzyme-based glucose delivery system to enhance valinomycin production under fed-batch conditions in small scale. This fed-batch-like cultivation system could provide a controllable growth environment and nutrients for high cell density cultivation. It is effective to improve the expression yields of active recombinant proteins (Krause et al. 2010; Ukkonen et al. 2011). A design of experiment (DoE)-guided optimization was implemented in parallel in milliliter-scale 24-well plates, followed by glucose polymer feeding in shake flask fed-batch cultivation. We could show that small-scale fed-batch cultivations can easily reach high cell densities and valinomycin titers could be improved up to milligram per liter levels compared to the low levels (microgram per liter) obtained in batch cultivations. Moreover, the optimized parameters obtained from the small-scale screenings could be utilized to rationally scale valinomycin production in a laboratory bioreactor.
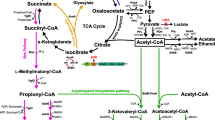
The biosynthetic pathway of valinomycin in the host E. coli. KR ketoreductase, E epimerase, ilvBN acetohydroxy acid synthase I, ilvC acetohydroxy acid isomeroreductase, ilvD dihydroxy acid dehydratase, ilvE branched chain amino acid aminotransferase, Pyr pyruvate, l -Lac lactate, α-Kiv α-ketoisovalerate, d -Hiv d-hydroxyisovalerate, l -Val l-valine, d -Val d-valine
Materials and methods
Bacterial strains and plasmids
For generation of the E. coli strain BJJ01, the Bacillus subtilis PPTase sfp gene under control of a weak tryptophanase promoter was integrated into the xylA gene locus of E. coli BL21Gold (Agilent Technologies) by λ Red recombineering (Sharan et al. 2009). Before integration, the sfp gene was fused to a chloramphenicol resistance cassette. The first integration step was followed by a second recombination to remove the chloramphenicol resistance gene afterwards according to the method described in Sharan et al. (2009). Plasmids pCTUT7-Vlm1 and pKS01-Vlm2 contained genes vlm1 (appr. 10 kb) and vlm2 (appr. 8 kb), respectively, for valinomycin synthetase co-expression (for details, see Supplementary Fig. S1). Both plasmids were constructed via the Gateway® cloning technology with the LR reaction (Invitrogen Life Technologies). Each plasmid had a pCTU promoter, which is a lac promoter derivative adapted to the consensus sequence for strong expression in E. coli (Kraft et al. 2007). The plasmids are stabilized by insertion of chloramphenicol and ampicillin resistance cassettes into pCTUT7-Vlm1 and pKS01-Vlm2, respectively. In addition, pKS01-Vlm2 also contained the plasmid-stabilizing parB locus (Gerdes 1988).
Batch cultivations
For all experiments, cells from freshly grown overnight plates were used to inoculate main cultures to a starting OD600 of 0.1. The medium for batch cultivation was Terrific Broth (TB) (Tartof and Hobbs 1987). Cultivations were performed in 500 mL baffled Ultra Yield FlasksTM (Thomson Instrument Company, USA) containing 100 mL TB with 17 mg L−1 chloramphenicol and 50 mg L−1 ampicillin. The flasks were sealed by air-permeable membranes (AirOtopTM Enhanced Seals, Thomson Instrument Company, USA). After inoculation, cultures were incubated on an orbital shaker (amplitude, 25 mm; Infors HT, Switzerland) at 200 rpm and 30 °C. When OD600 reached 0.6–0.8, protein expression was induced with 20 μM isopropyl-β-d-thiogalactopyranoside (IPTG) followed by 36 h cultivation for valinomycin production.
Fed-batch type cultivations
The fed-batch-like EnBase® cultivations were performed with EnPresso® B medium (BioSilta, Oulu, Finland) according to the instructions of the supplier with small modifications due to the long production phase for valinomycin. Briefly, 100 mL medium in 500 mL Ultra Yield Flasks were supplemented with 10 μL antifoam 204 (Sigma), 17 mg L−1 chloramphenicol, and 50 mg L−1 ampicillin. Then, the glucose-releasing biocatalyst was added to a concentration of 0.3 U L−1 as a first dose shortly before inoculation. Afterwards, the flasks were sealed by the AirOtop Seals and the cultivation was initiated in the shaker at 200 rpm and 30 °C. After overnight cultivation (15 h), boosting nutrients (Booster, BioSilta) and the second dose of biocatalyst (0.6 U L−1) were added to the culture for further 96 h of cultivation.
DoE experimental design
The DoE-guided optimization was conducted in parallel 24-well plates (PreSens Precision Sensing GmbH, Regensburg, Germany) using EnPresso B fed-batch medium. The PreSens 24-well plate system allows noninvasive online measurement of oxygen and pH during the cultivation process. Three key parameters, nutrient boosting (yes/no), biocatalyst concentration relating to glucose releasing rate (0 to 6 U L−1 as the second dose), and culture volume correlating to oxygen transfer rate (0.5 to 1.5 mL), were investigated and optimized in relation to the product titer based on the DoE D-Optimal design created with the software MODDE 8.0.2 (Umetrics, Umeå, Sweden) (Table 1).
These DoE-generated 20 experimental runs were carried out as follows. Overnight cultivation was performed in shake flasks in the same manner as described above in the section “Fed-batch type cultivations.” In the morning, the shake flask culture was distributed into the 24-well plates. For each well, boosting nutrients, a second biocatalyst dose, and culture volume were performed as shown in Table 1. Afterwards, the 24-well plate was covered with the Duetz System sandwich cover and clamp system (Enzyscreen, Er Haarlem, The Netherlands) (Duetz et al. 2000). The following cultivation lasted for 48 h in the shaker (amplitude, 50 mm, Kühner, Basel, Switzerland) at 250 rpm under 30 °C and, meanwhile, online dissolved oxygen (DO) and pH values were monitored (SensorDish Reader, PreSens).
The results of the experimental design were evaluated with MODDE. In order to improve the model, the insignificant factors were deleted stepwise.
Effect of glucose feeding rate on valinomycin production
In the enzyme-based glucose delivery cultivation system, the glucose feeding rate is mediated by the applied biocatalyst concentration in the medium. In order to obtain the optimal biocatalyst dosage, a further screening was conducted in 24-well plates with EnPresso B medium. Overnight cultivation was performed as described above. After boosting (Booster, BioSilta), 1 mL culture per well was distributed into the 24-well plates. Different amounts of biocatalyst were added per well with 1.5, 3, 9, 15, or 20 U L−1 as the second dose. The subsequent cultivation was continued for 48 h at 30 °C and 250 rpm. Oxygen and pH values were recorded online (SensorDish Reader, PreSens).
Effect of glucose polymer feeding on valinomycin production
The EnPresso B medium was used and overnight cultivation was performed like before. At the boosting point, additional biocatalyst (9 U L−1 as the second dose) was added to the culture. Then, 12.5 mL of the culture was transferred into a 125-mL baffled shake flask (PreSens) with integrated sensors for the measurement of dissolved oxygen and pH. One flask worked as a control without glucose polymer feeding. The second flask was fed with 30 g L−1 glucose polymer (obtained from BioSilta) after 12 h. The third flask was fed twice with 30 g L−1 glucose polymer after 12 and 24 h, respectively.
Cell growth determination
To monitor the cell growth in shake flasks, OD600 values were measured at different time intervals during the cultivation with a UV/Visible spectrophotometer (Ultrospec 3300, Amersham Biosciences, Germany). For parallel 24-well plate samples, 5 μL culture of each well was mixed with 145 μL of 0.9 % NaCl resulting in a 30-fold dilution in 96-microwell plates. The automatic measurement of OD600 was performed using a robotic platform (Hamilton Robotics, Switzerland) with a Synergy Mx microplate reader (BioTek Instruments, USA) at 600 nm. All OD600 values were recalculated for a light path length of 1 cm according to calibration curves. All measurements were determined in triplicate.
Valinomycin extraction and quantitation
Samples of 1 mL culture were collected for valinomycin extraction. After centrifugation at 16,000×g for 3 min, the medium supernatant was transferred into a 15-mL tube and extracted with 2 mL ethyl acetate. Pellets were resuspended in 2 mL methanol and sonicated for 3 min (30 s on/off, sonotrode with 7 mm diameter, 30 % amplitude) (UP200S, Hielscher Ultrasonics, Teltow, Germany). After centrifugation (16,000×g, 15 min), the organic fractions from the medium and pellet extracts were combined and dried by vacuum rotary evaporator (IKA-Werke GmbH, Staufen, Germany). The residues were resuspended in 1 mL of methanol for valinomycin analysis.
For valinomycin detection, 1 μL of each sample was injected into an Agilent 6460 Triple Quadrupole LC/MS System equipped with an Eclipse Plus C18 column (RRHD 1.8 μm, 2.1 × 50 mm). The buffers A (H2O + 0.1 % formic acid) and B (acetonitrile + 0.1 % formic acid) were used to elute analytes with a flow rate of 0.3 mL min−1 through a linear gradient elution from 5 to 100 % B over 2.5 min, followed by a 100 % B wash for 7.5 min and finishing with a linear gradient back to 5 % B over 2 min. Before every experimental analysis, a calibration curve was generated from a series of valinomycin standard (Sigma) with concentrations of 10, 100, 1,000, 2,000, and 4,000 μg L−1. All measurements were performed in triplicate.
Results
Valinomycin production under batch and fed-batch cultivation conditions
We compared valinomycin production under batch conditions in TB medium with the production in the enzyme-controlled fed-batch type glucose delivery EnBase system in shake flasks. The two valinomycin synthetase subunits Vlm1 (370 kDa) and Vlm2 (284 kDa) were co-expressed and appeared dominantly in soluble form under both cultivation modes (Fig. 2b). While in TB best expression was reached with 20 μM IPTG inducer concentration, the highest valinomycin synthetase expression in the EnBase system was obtained without addition of IPTG, simply by derepression under glucose limitation (data not shown). This way of derepression of valinomycin synthetase without the need for IPTG would be very beneficial in larger production scales.
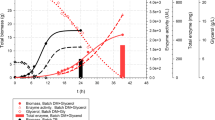
Valinomycin formation and valinomycin synthetase expression with batch and fed-batch cultivations in shake flasks. a Cells were cultivated in Terrific Broth (batch) or in EnPresso B medium (fed-batch), respectively. b SDS-PAGE analysis of the expression of Vlm1 and Vlm2. 1 Vlm1 and Vlm2 were co-expressed in Terrific Broth with 20 μM IPTG induction for 5 h of cultivation; 2 negative control with empty plasmids; 3 Vlm1 and Vlm2 were co-expressed in EnPresso B medium without IPTG induction for 24 h cultivation. Vlm1 (370 kDa) and Vlm2 (284 kDa) are indicated by arrows. P = insoluble protein, S = soluble protein, M = protein molecular weight marker
In the TB batch cultivation, IPTG was added to induce Vlm1 and Vlm2 expression when OD600 reached 0.6–0.8 followed by 36 h cultivation for valinomycin formation. As shown in Fig. 2a, the cells keep growing exponentially for appr. 4 h after induction until an OD600 of appr. 7 is reached. After 8 h, cells enter into a stationary phase at an OD600 of appr. 8.5. After 24 h, cells probably start to die as we conclude through the remarkable drop of the OD600. The final volumetric valinomycin titer reached 300 μg L−1, while most of the product (225 μg L−1) was produced within 4 h after induction, which is consistent with the exponential growth phase. Only 25 % of the total valinomycin was accumulated in the subsequent 32 h presumably due to decreased cell growth and thus precursor limitation.
To prolong cell growth and obtain high cell density, a controlled continuous glucose diet was realized by the EnPresso B medium. The OD600 of the culture reached 9 after 15 h at the end of overnight cultivation (see Fig. 2a). After nutrient boosting, the culture continuously grew for more than 20 h up to an OD600 of appr. 20, which is more than the double of the OD600 that is reached in the TB cultivation. Meanwhile, valinomycin formation was remarkably enhanced along with the extended cells growth period. The final titer of 2.4 mg L−1 of valinomycin represented an eightfold increase without any optimization compared to the titer obtained in batch TB cultivation.
DoE optimization of fed-batch type production
One principle of the EnBase fed-batch cultivation is that glucose only gets available to the cells after it is gradually released by a biocatalyst from the solved glucose polymer. If the concentration of the biocatalyst is too low, cells starve for glucose resulting in low cell densities. If the concentration of the biocatalyst is too high, high metabolic activities and overflow metabolism may lead to oxygen limitation and medium acidification, which deteriorates the cultivation. Therefore, the biocatalyst concentration, i.e., the glucose release rate, had to be optimized. DO and pH were monitored and evaluated as key parameters during the cultivation because of their influence on cell growth and recombinant protein expression in E. coli (Ukkonen et al. 2011).
Biocatalyst concentration, culture volume (as a parameter to affect the oxygen transfer rate), and booster amount were selected as optimization criteria for valinomycin production in a D-Optimal design approach with the final OD600 values and volumetric valinomycin titers as responses (Table 1). A quadratic model with interactions was fitted to the measured values. The regression coefficients for both models were high (R 2 = 0.96 for OD600 and R 2 = 0.97 for valinomycin production, respectively) and both models were significant (α < 0.05). It is obvious from the effect plots (Fig. 3a, b), which result from the analysis of variance, that boosting causes the highest effect on cell growth and valinomycin production. If booster was added to the culture, both cell density and valinomycin production increased. The added complex nutrient booster contains amino acids, vitamins, and cofactors and thus supports the efficient synthesis of proteins (Vlm1 and Vlm2) and supplies further precursors for valinomycin biosynthesis. Culture volume caused the second highest effect on both parameters. This effect is even higher with boosting than without, which can be concluded from the high value for the interaction between both parameters (7 in Fig. 3a, b). Since a regression analysis is difficult with qualitative factors, boosting was eliminated from the model and a quadratic model is established using the results of the experiments with boosting. The stepwise elimination of the insignificant effects led to a reduced quadratic model, which only contained the culture volume as an influencing factor. The two models have again a good regression coefficient (R 2 = 0.84 for OD600 and R 2 = 0.98 for valinomycin production) and both models are significant. The lack of fit is not significant for the OD600, which means the model error is lower than the pure error and shows a good fit of the model to the experimental data. In contrast, the lack of fit for the valinomycin production is significant, which is probably caused by the high reproducibility in the center points of the experimental plan (Table 1). The variety is likely higher with other settings of the experimental plan, which results in the mentioned lack of fit. The effect of the culture volume, which is related to the oxygen transfer rate, on cell growth, and valinomycin production, is shown in Fig. 3c, d and suggested taking the smallest culture volume, which was tested. The effect of the biocatalyst concentration might be judged as insignificant because of the small range which has been investigated. Therefore, the range was increased in the next optimization step.
Results of the design of experiments with boosting, biocatalyst concentration, and culture volume as influence parameters on cell density and valinomycin production performed in 24-well plates. Upper graphs: Effect plots for the influence of 1, volume; 2, boosting; 3, (volume)2; 4, biocatalyst; 5, (biocatalyst)2; 6, (biocatalyst × volume); 7, (boosting × volume); 8, (biocatalyst × boosting) on a OD600 (R 2 = 0.96) and b valinomycin titer (micrograms per liter) (R 2 = 0.95). Lower graphs: Response prediction plot with 95 % confidence interval for the reduced quadratic model fitted to the results obtained with boosting for c OD600 (model equation: OD600 = 24.9 + 13.0 × volume − 12.0 × (volume)2, R 2 = 0.84) and d valinomycin titer (micrograms per liter) (model equation: valinomycin = 13,988 – 19,866 × volume + 7,548 × (volume)2, R 2 = 0.98). All models are significant (α < 0.05) and the models for OD600 have no significant lack of fit (α > 0.05)
The stability of the pH was obviously not influenced by boosting. The online data showed that a very stable pH level is maintained in EnPresso B medium, although the values (appr. 7.0–7.5) with boosting were higher compared to those without boosting (appr. 6.5–7.0) (Supplementary Fig. S2). These pH values are close to the optimal pH of 7.0 for E. coli growth, demonstrating the fed-batch-like medium could buffer the culture system well without external pH control. In contrast, the pH gradually increased from 7.0 to 8.5 in TB batch cultivations (data not shown).
In the fed-batch type cultivation process, the glucose-releasing biocatalyst was added twice to the culture to maximize the controlled feeding of glucose. At the inoculation point, biocatalyst was added at a concentration of 0.3 U L−1. The initial low amount of biocatalyst could provide a consistent cultivation environment with slowly released glucose for the cells to reach a reasonable density before the production phase. To optimize the biocatalyst concentration, the second addition was investigated in a range from 0 to 6 U L−1. The results revealed that higher biocatalyst concentrations correlate with higher valinomycin titers, while there was no obvious influence on cell density (Table 1).
The total volume of a well in the 24-well plate was 3.3 mL and working volumes from 0.5 to 1.5 mL were tested. According to the online data (Supplementary Fig. S2) of the boosting group, the change of the DO content over the time in cultures with the same volume was similar. Along with an increase of the culture volume from 0.5 to 1.5 mL, a prolonged period of oxygen limitation appeared. This oxygen limitation had a negative impact on the cell growth and on valinomycin accumulation. The DO increase in the late phase of the cultivation is probably due to the exhaustion of the glucose polymer substrate, leading to the stop of cell growth and less oxygen being consumed. In other words, a lower culture volume and therefore a higher oxygen transfer rate led to a higher cell density (Fig. 3c) and an increased valinomycin titer (Fig. 3d).
The optimized parameters determined from the DoE experiment were addition of nutrients, a biocatalyst concentration of 6 U L−1, and a culture volume of 0.5 mL. These conditions lead to a 3-fold improvement of the final OD600 compared to the batch cultivation in TB medium (Fig. 4). More importantly, the final volumetric valinomycin titer dramatically increased to 6.4 mg L−1, which is >20-fold higher than that in the initial batch fermentation. In addition, DO and pH were shown to remain stable at nearly 100 % and around 7.2, respectively, during the 48-h production process (Fig. 4).
Effect of glucose feeding rate on valinomycin production
The investigated range of the biocatalyst concentration (0 to 6 U L−1) was too small to see a significant effect on valinomycin production. Since the raw data suggested a positive correlation between faster glucose release and valinomycin production, we further tested a broader range of biocatalyst concentrations. The preculture was initiated with 0.3 U L−1 biocatalyst in a 500-mL Ultra Yield flask. The overnight preculture was then distributed into the 24-well plate (PreSens) with 1 mL culture per well and biocatalyst concentrations between 1.5 and 20 U L−1 (the second dose).
Figure 5a, b shows the dynamics of online DO and pH in each well with different biocatalyst concentrations during the cultivation. With less biocatalyst (1.5 and 3 U L−1), less glucose is released and thus cells used complex nutrients (e.g., amino acids) as carbon source for growth. Therefore, deamination of amino acids generated ammonia and resulted in a pH increase. As the specific activity of the biocatalyst strongly depends on the pH with a maximum activity at a pH of 4 and only a residual activity of less than 20 % at pH 7 (information from BioSilta), the glucose release rate decreases with an increasing pH. After almost 10 h, complex nutrients were probably exhausted and cell growth was mainly based on the slowly released glucose. Therefore, cells needed less oxygen for respiration and DO increased accordingly. With a biocatalyst concentration of 9 U L−1, the oxygen limitation was prolonged to 15 h and the pH increase was delayed compared to the lower biocatalyst concentrations (1.5 and 3 U L−1). The highest biocatalyst concentrations (15 and 20 U L−1) resulted in faster glucose release and a medium acidification. The decreasing pH, by contrast, increased the activity of the biocatalyst. Therefore, the glucose polymer was rapidly depleted after 8 or 10 h, respectively. Afterwards, under the acidic conditions, cell growth was significantly impaired and DO was increased.
Effect of glucose feeding rate on valinomycin production: online measurement of a oxygen and b pH, c cell growth curves, and d valinomycin titers. Numbers in a, b, and c represent the amounts (units per liter) of biocatalyst. Cultivation conditions: 1 mL EnPresso B medium per well in 24-well plate with nutrient boosting
The cell growth curves are shown in Fig. 5c. With low biocatalyst concentrations (1.5 and 3 U L−1), the final OD600 values were around 30, which is 3-fold higher than the cultivations with high biocatalyst concentrations (15 and 20 U L−1). The final valinomycin titers are presented in Fig. 5d. The results indicate that, within the range of 1.5 to 9 U L−1 of biocatalyst, more biocatalyst leads to a higher volumetric valinomycin yield. The highest valinomycin titer of 2.4 mg L−1 was obtained with 9 U L−1. However, above 9 U L−1, the volumetric yield of valinomycin was sharply reduced.
Effect of glucose polymer feeding on valinomycin production
The curves for the DO (Fig. 5a) and for the cell growth (Fig. 5c) suggested that the carbon source polymer for glucose release was exhausted with a biocatalyst concentration of 9 U L−1 after appr. 15 h (DO increase and no change in cell density). Therefore, we tested if continued glucose release by supply of additional polymer to the medium would further enhance cell growth and valinomycin formation.
Therefore, parallel shake flask experiments were performed (see Fig. 6). Before the first feeding point (12 h), all three flasks had the same DO in the medium (Fig. 6a). The DO decreased in the initial batch phase (until 5 h) under glucose excess from 70 to 5 %. Afterwards, the DO increased to 60 % in the following 2 h indicating the transition to glucose-limited growth. In the flasks where extra polymer was added to the medium (at 12 h), the DO suddenly decreased to almost 0 %, but recovered fast to a stable level. This short disturbance in the DO is probably because the glucose polymer contains some degraded free glucose that the cells consume. A comparison of the three DO profiles clearly revealed that the growth could be maintained by additional feeding of the polymer. With polymer feeding, the pH slightly decreased compared to the control but still remained close to pH 7 (Fig. 6b). OD600 values indicate that polymer feeding was beneficial for cell growth as assumed (Fig. 6c). Without polymer feeding, cells stopped growing after 12 h with a final OD600 of 24. Glucose polymer feeding at two times markedly improved cell density up to an OD600 of 55. Correspondingly, the volumetric valinomycin titer increased to 10 mg L−1 (Fig. 6d), which is a 33-fold increase compared to TB batch production. In addition, the specific production of valinomycin with twice polymer feeding reached 182 μg L−1 OD600 −1, indicating a 5.2-fold improvement compared to the initial batch TB cultivation with a specific yield of 35 μg L−1 OD600 −1.
Discussion
NRPs are a large group of microbial secondary metabolites that have a wide spectrum of important pharmacological activities (Felnagle et al. 2008). Although more and more of the gene clusters of NRPSs are identified and sequenced (Finking and Marahiel 2004; Fischbach and Walsh 2006; Marahiel et al. 1997), only a few of them have been heterologously expressed in E. coli for the total biosynthesis of relevant NRPs (Watanabe et al. 2006). In the present work, we report an optimization process for enhanced production of valinomycin in E. coli through a purely physiological approach, without any gene optimization, which also could offer a reasonable route for the production of other NRPs. The highest valinomycin titer reached was appr. 10 mg L−1, which is comparable to the productivity of several native Streptomyces producers (Matter et al. 2009). Therefore, we believe that E. coli is a suitable surrogate host for heterologous valinomycin production. Further arguments are (a) much shorter cultivation times (2 days) compared to Streptomyces strains (6 days) to reach similar valinomycin titers, (b) E. coli tolerates expression of the large foreign valinomycin synthetase which is correctly folded with biological functionality, and (c) all the three precursors for valinomycin biosynthesis (pyruvate, α-ketoisovalerate, and l-valine) are native parts of the intracellular E. coli metabolite pool. Also, (d) E. coli cultivations can be easily scaled up to high cell densities in a bioreactor.
Previous reports demonstrated that the fed-batch-like EnBase cultivation system can efficiently promote expression yields of small proteins (<50 kDa) (Krause et al. 2010; Šiurkus et al. 2010). Also, the dimeric Fab antibody fragments were successfully expressed recently by the same strategy (Ukkonen et al. 2013). Here, we report that the EnBase strategy also benefits the production of valinomycin synthetase (Vlm1 370 kDa and Vlm2 284 kDa) in E. coli, a very large dimeric protein, and even the resultant antibiotic product valinomycin. The specific rate of valinomycin production is only appr. 90 μg g−1 h−1 in batch cultivations. Therefore, for a high valinomycin yield, it is important to keep the cell's production system active over a long time, which is not possible in a batch-type shake flask. Therefore, the task of EnBase here is to provide a prolonged controlled production phase. With the EnBase technology, cell growth could be maintained over appr. 50 h and, importantly, valinomycin synthesis continued over the whole time period. Also, during the whole cultivation process, a stable pH level between 6.5 and 7.5, depending on the specific cultivation conditions, could be maintained (Figs. 5b and 6b and Supplementary Fig. S2). In contrast, the pH gradually increased in TB batch cultivations initially from 7.0 to 8.5, which exceeds the optimal pH range of E. coli growth (data not shown). This finding is in agreement with a previous report, suggesting EnBase medium is a good self-buffered system to cultivate E. coli cells with different kinds of recombinant proteins (Krause et al. 2010).
Interestingly, in TB batch cultivations, valinomycin was notably accumulated within the exponential growth phase, accounting for 75 % of the total yield (Fig. 2a). In contrast, in EnBase fed-batch type cultivations, valinomycin was mainly produced in the glucose-limited phase after nutrients boosting (Figs. 2a and 4). We assume the difference can be attributed to the different cultivation modes. In the TB batch mode, most nutrients are consumed in the exponential growth phase for cell growth and valinomycin formation. The following stationary phase is characterized by nutrient starvation, and consequently, no further valinomycin can be produced. In contrast, in the EnBase fed-batch mode, the complex booster added during the glucose limitation phase could be directly used for anabolic reactions including the synthesis of valinomycin, while the cells obtained their energy mainly from the released glucose. Therefore, E. coli cells have enough energy not only to cover their maintenance requirements, but also for valinomycin biosynthesis.
According to the DoE results, addition of booster had a significant positive effect on the valinomycin yield (Table 1). Although E. coli cells can also grow up to high cell density without the boosted nutrients, valinomycin yields were much lower in this case. The booster supplies amino acids, vitamins, and cofactors to support efficient protein expression and probably provides additional precursors (e.g., l-valine) for valinomycin biosynthesis. Thus, a quadratic model was established using only the results with boosting. According to the model, only the culture volume had a significant influence on the OD600 and valinomycin production. The biocatalyst concentration had no significant influence probably due to the small biocatalyst concentration range (0 to 6 U L−1 as the second dose) that was investigated. In EnBase, the glucose feeding rate depends on the biocatalyst concentration. Our results indicated that a higher biocatalyst concentration gave rise to higher valinomycin titers. When the biocatalyst concentrations were increased from 1.5 to 9 U L−1 (the second dose), the specific valinomycin yields were improved by appr. 2-fold. This suggests that E. coli cells only need a certain amount of glucose for self-maintenance during the production phase. A surplus of glucose may provoke an accumulation of pyruvate. Further, a higher concentration of pyruvate may provide a higher flux into the l-valine synthesis route which includes the third precursor α-ketoisovalerate (Fig. 1). Consequently, more biocatalyst leads to more precursors for valinomycin formation. However, if the biocatalyst concentration is higher than 9 U L−1, cell growth and valinomycin titer are drastically decreased (Fig. 5c, d) due to accumulation of acetic acid as a side product and acidification of the cellular cytoplasm impairing the cultivation and the recombinant protein production (Han et al. 1992; Jensen and Carlsen 1990; Kleman and Strohl 1994). In summary, compared to the batch cultivation, balanced glucose polymer feeding dramatically improved the volumetric (33-fold) and even specific (5.2-fold) yields of valinomycin, probably due to a higher flux of glucose into the precursor metabolic pathways.
Although we have earlier shown that a shift to anaerobic conditions may provoke a higher flux into the branched chain amino acid pathway by the accumulation of pyruvate (Soini et al. 2008; Soini et al. 2011), a high glucose feeding rate and oxygen limitation did not increase the valinomycin yield (data not shown). The maintenance of aerobic conditions during the whole cultivation was very important for a high valinomycin yield. In the multiwell plates, a lower culture volume allowed a better oxygen transfer and thus contributed to higher cell density and valinomycin titers (Fig. 3c, d).
In conclusion, the NRP antibiotic valinomycin production was significantly enhanced to 10 mg L−1 through small-scale high cell density fed-batch cultivation, which is 33-fold higher compared to the yield which was detected in rich medium-based batch cultivations. Since the EnBase fed-batch system enables scalable cultivation from microwell plate to pilot plant scale bioreactors (Glazyrina et al. 2012; Šiurkus et al. 2010), the optimized parameters achieved from the current small-scale screenings could be used to rationally guide the scaling of the valinomycin process into the bioreactor. Moreover, we present here a feasible approach and an example to optimize and enhance heterologous production of other complex natural products in E. coli.
References
Cheng Y-Q (2006) Deciphering the biosynthetic codes for the potent anti-SARS-CoV cyclodepsipeptide valinomycin in Streptomyces tsusimaensis ATCC 15141. ChemBioChem 7(3):471–477
Duetz WA, Rüedi L, Hermann R, O’Connor K, Büchs J, Witholt B (2000) Methods for intense aeration, growth, storage, and replication of bacterial strains in microtiter plates. Appl Environ Microbiol 66(6):2641–2646
Felnagle EA, Jackson EE, Chan YA, Podevels AM, Berti AD, McMahon MD, Thomas MG (2008) Nonribosomal peptide synthetases involved in the production of medically relevant natural products. Mol Pharmaceut 5(2):191–211
Finking R, Marahiel MA (2004) Biosynthesis of nonribosomal peptides. Annu Rev Microbiol 58:453–488
Fischbach MA, Walsh CT (2006) Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: logic, machinery, and mechanisms. Chem Rev 106(8):3468–3496
Gerdes K (1988) The parB (hok/sok) locus of plasmid R1: a general purpose plasmid stabilization system. Nat Biotechnol 6(12):1402–1405
Glazyrina J, Krause M, Junne S, Glauche F, Storm D, Neubauer P (2012) Glucose-limited high cell density cultivations from small to pilot plant scale using an enzyme-controlled glucose delivery system. New Biotechnol 29(2):235–242
Han K, Lim HC, Hong J (1992) Acetic acid formation in Escherichia coli fermentation. Biotechnol Bioeng 39(6):663–671
Heisey RM, Huang J, Mishra SK, Keller JE, Miller JR, Putnam AR, D’Silva TDJ (1988) Production of valinomycin, an insecticidal antibiotic, by Streptomyces griseus var. flexipertum var. nov. J Agric Food Chem 36(6):1283–1286
Hur GH, Vickery CR, Burkart MD (2012) Explorations of catalytic domains in non-ribosomal peptide synthetase enzymology. Nat Prod Rep 29(10):1074–1098
Jensen EB, Carlsen S (1990) Production of recombinant human growth hormone in Escherichia coli: expression of different precursors and physiological effects of glucose, acetate, and salts. Biotechnol Bioeng 36(1):1–11
Kleman GL, Strohl WR (1994) Acetate metabolism by Escherichia coli in high-cell-density fermentation. Appl Environ Microbiol 60(11):3952–3958
Kraft M, Knüpfer U, Wenderoth R, Kacholdt A, Pietschmann P, Hock B, Horn U (2007) A dual expression platform to optimize the soluble production of heterologous proteins in the periplasm of Escherichia coli. Appl Microbiol Biotechnol 76(6):1413–1422
Krause M, Ukkonen K, Haataja T, Ruottinen M, Glumoff T, Neubauer A, Neubauer P, Vasala A (2010) A novel fed-batch based cultivation method provides high cell-density and improves yield of soluble recombinant proteins in shaken cultures. Microb Cell Factories 9:11
Magarvey NA, Ehling-Schulz M, Walsh CT (2006) Characterization of the cereulide NRPS α-hydroxy acid specifying modules: activation of α-keto acids and chiral reduction on the assembly line. J Am Chem Soc 128(33):10698–10699
Marahiel MA, Stachelhaus T, Mootz HD (1997) Modular peptide synthetases involved in nonribosomal peptide synthesis. Chem Rev 97(7):2651–2674
Matter AM, Hoot SB, Anderson PD, Neves SS, Cheng Y-Q (2009) Valinomycin biosynthetic gene cluster in Streptomyces: conservation, ecology and evolution. PLoS ONE 4(9):e7194
Mutka SC, Carney JR, Liu Y, Kennedy J (2006) Heterologous production of epothilone C and D in Escherichia coli. Biochemistry 45(4):1321–1330
Park CN, Lee JM, Lee D, Kim BS (2008) Antifungal activity of valinomycin, a peptide antibiotic produced by Streptomyces sp. strain M10 antagonistic to Botrytis cinerea. J Microbiol Biotechnol 18(5):880–840
Pettit GR, Tan R, Melody N, Kielty JM, Pettit RK, Herald DL, Tucker BE, Mallavia LP, Doubek DL, Schmidt JM (1999) Antineoplastic agents. Part 409: isolation and structure of montanastatin from a terrestrial actinomycete. Bioorg Med Chem 7(5):895–899
Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla C (2001) Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science 291(5509):1790–1792
Sharan SK, Thomason LC, Kuznetsov SG, Court DL (2009) Recombineering: a homologous recombination-based method of genetic engineering. Nat Protoc 4(2):206–223
Shemyakin MM, Aldanova NA, Vinogradova EI, Feigina MY (1963) The structure and total synthesis of valinomycin. Tetrahedron Lett 4(28):1921–1925
Šiurkus J, Panula-Perälä J, Horn U, Kraft M, Rimšeliene R, Neubauer P (2010) Novel approach of high cell density recombinant bioprocess development: optimisation and scale-up from microlitre to pilot scales while maintaining the fed-batch cultivation mode of E. coli cultures. Microb Cell Factories 9:35
Soini J, Falschlehner C, Liedert C, Bernhardt J, Vuoristo J, Neubauer P (2008) Norvaline is accumulated after a down-shift of oxygen in Escherichia coli W3110. Microb Cell Factories 7:30
Soini J, Ukkonen K, Neubauer P (2011) Accumulation of amino acids deriving from pyruvate in Escherichia coli W3110 during fed-batch cultivation in a two-compartment scale-down bioreactor. Adv Biosci Biotechnol 2:336–339
Stack D, Neville C, Doyle S (2007) Nonribosomal peptide synthesis in Aspergillus fumigatus and other fungi. Microbiology 153(5):1297–1306
Tartof KD, Hobbs CA (1987) Improved media for growing plasmid and cosmid clones. Bethesda Res Lab Focus 9:12
Tempelaars MH, Rodrigues S, Abee T (2011) Comparative analysis of antimicrobial activities of valinomycin and cereulide, the Bacillus cereus emetic toxin. Appl Environ Microbiol 77(8):2755–2762
Ukkonen K, Vasala A, Ojamo H, Neubauer P (2011) High-yield production of biologically active recombinant protein in shake flask culture by combination of enzyme-based glucose delivery and increased oxygen transfer. Microb Cell Factories 10:107
Ukkonen K, Veijola J, Vasala A, Neubauer P (2013) Effect of culture medium, host strain and oxygen transfer on recombinant Fab antibody fragment yield and leakage to medium in shaken E. coli cultures. Microb Cell Factories 12:73
Watanabe K, Hotta K, Praseuth AP, Koketsu K, Migita A, Boddy CN, Wang CCC, Oguri H, Oikawa H (2006) Total biosynthesis of antitumor nonribosomal peptides in Escherichia coli. Nat Chem Biol 2(8):423–428
Wu C-Y, Jan J-T, Ma S-H, Kuo C-J, Juan H-F, Cheng Y-SE, Hsu H-H, Huang H-C, Wu D, Brik A, Liang F-S, Liu R-S, Fang J-M, Chen S-T, Liang P-H, Wong C-H (2004) Small molecules targeting severe acute respiratory syndrome human coronavirus. Proc Natl Acad Sci U S A 101(27):10012–10017
Zhang H, Wang Y, Wu J, Skalina K, Pfeifer BA (2010) Complete biosynthesis of erythromycin A and designed analogs using E. coli as a heterologous host. Chem Biol 17(11):1232–1240
Acknowledgments
This work was supported by fellowships for JL and JJ from the Berlin International Graduate School of Natural Sciences and Engineering of the Cluster of Excellence “Unifying Concepts in Catalysis,” funded by the Deutsche Forschungsgemeinschaft and coordinated by the Technische Universität Berlin. JJ was further supported by a fellowship from the HaVo-Stiftung. We kindly would like to thank M. Nicolas Cruz-Bournazou for supporting the 24-well plate cultivations.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jian Li and Jennifer Jaitzig contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 178 kb)
Rights and permissions
About this article
Cite this article
Li, J., Jaitzig, J., Hillig, F. et al. Enhanced production of the nonribosomal peptide antibiotic valinomycin in Escherichia coli through small-scale high cell density fed-batch cultivation. Appl Microbiol Biotechnol 98, 591–601 (2014). https://doi.org/10.1007/s00253-013-5309-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5309-8