Abstract
κ-Carrageenases exhibit apparent distinctions in gene sequence, molecular weight, enzyme properties, and posttranslational processes. In this study, a new κ-carrageenase gene named cgkZ was cloned from the marine bacterium Zobellia sp. ZM-2. The gene comprised an open reading frame of 1,638 bp and encoded 545 amino acids. The natural signal peptide of κ-carrageenase was used successfully for the secretory production of the recombinant enzyme in Escherichia coli. A posttranslational process that removes an amino acid sequence of about 20 kDa from the C-terminal end of κ-carrageenase was first discovered in E. coli. An increase in enzyme activity by 167.3 % in the presence of 5 mM DTT was discovered, and Na+ at a certain concentration range was positively correlated with enzyme activity. The κ-carrageenase production of E. coli was 9.0 times higher than that of ZM-2. These results indicate the potential use of the enzyme in the biotechnological industry.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
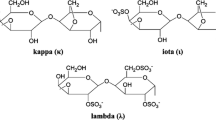
Carrageenans are gel-forming linear sulfated galactans extracted from the extracellular matrix of red marine algae. They consist of linear chains of galactopyranose residues linked by alternating α-1,3 and β-1,4 linkages. Based on the number and position of sulfate substitutions, as well as the presence of a 3,6-anhydro bridge in α-l,4-linked galactose residues, carrageenans are mainly classified into three types: κ-, ι-, and λ-carrageenans (Barbeyron et al. 2000). Carrageenans are widely used as thickening and gelling agents in structural and functional applications and generally regarded safe by the United States Food and Drug Administration (Zhou et al. 2008). Sulfated oligosaccharides obtained from marine algae have diverse biological and physiological activities, including antitumor (Hiroishi et al. 2001), anticoagulation (Alban et al. 2002), anti-inflammation (Arfors and Ley 1993), anti-thrombosis (Suzuki et al. 1991), and viral inactivation (Caceres et al. 2000) activities, depending on the degree of sulfate esterification of the molecules (Liu et al. 2000). Carrageenans, which are important sources of sulfated oligosaccharides, have drawn considerable research interest (Mou et al. 2003). Chemical degradation methods, such as acid hydrolysis, are usually so severe that liable and valuable native constituents do not keep intact during the depolymerization of carrageenan. Degrading carrageen with a specific enzyme called carrageenase, which features high substrate specificity and mild reaction conditions, is thus a promising alternative to chemical degradation (Mou 2003).
κ-Carrageenase (EC 3.2.1.83) belongs to a family 16 of the glycoside hydrolases (GH 16). It specifically cleaves the internal β (1–4) linkages of carrageenans and yields a series of homologous even-numbered oligosaccharides (Mou 2003). Carrageenases have been obtained from several marine bacteria, such as Alteromonas (Michel et al. 2000), Cytophaga (Barbeyron et al. 1998; Potin et al. 1991), Pseudoalteromonas (Michel et al. 2001a, b), Pseudomonas (Øtgaard et al. 1993), and Vibrio (Araki et al. 1999). In addition, the structure of κ-carrageenase from Pseudoalteromonas carrageenovora has been determined (Mark et al. 2009). κ-Carrageenases of different organisms have obvious distinctions in terms of primary structure, enzymatic characteristics, and productivity. κ-Carrageenase genes have been cloned from Zobellia galactanivorans (Potin et al. 1991), Alteromonas carrageenovora (Barbeyron et al. 1994), Pseudoalteromonas tetraodonis (Kobayashi et al. 2012), Pseudoalteromonas porphyrae (Liu et al. 2011), and Cytophaga drobachiensis (Barbeyron et al. 1998). κ-Carrageenase genes in these species are likely to reflect not only the requirement of bacterium- and time-specific expression of different genes but also functional differences among individual isozymes. Very few carrageenase genes have been studied through heterologous expression, partly because of the lack of an efficient and generally applicable system for the heterologous production of carrageenase gene products. For instance, the κ-carrageenase gene from P. tetraodonis has been heterologously expressed in Escherichia coli but the final yield of the soluble enzyme is rather low, which is mainly due to protein interactions between the hydrophobic regions of the proteins (Kobayashi et al. 2012; Choi and Lee 2004).
We found and fully analyzed a new κ-carrageenase gene cloned from the marine bacterium ZM-2. To analyze the function of the natural signal peptide in the recombinant strain, two expression plasmids, HTa-cgkZ-SIG− (encoding κ-carrageenase without signal peptide) and HTa-cgkZ (encoding κ-carrageenase with signal peptide), were constructed and heterologously expressed in E. coli. A recombinant strain capable of extracellular production of recombinant proteins was obtained, and its degradation products were detected by electrospray ionization–mass spectrometry (ESI-MS). Compared with the natural strain, the productivity of extracellular κ-carrageenase by the recombinant bacterium was evidently increased. The recombinant enzyme was purified and its properties were further studied.
Materials and methods
Strains, plasmids, and media
ZM-2 (CCTCC no. M2013256) is now preserved in China Center for Type Culture Collection, Wuhan, China, and used as the original strain of the κ-carrageenase gene (cgkZ). E. coli BL21 (DE3) and E. coli DH5α were cultured in Luria-Bertani (LB) medium containing 100 μg/mL ampicillin, 10 % bacto-tryptone, 5 % yeast extract, and 10 % NaCl. Two plasmids, pUCm-T (Sangon, Shanghai China) and pProEX-HTa (Yellow Sea Fisheries Research Institute, Chinese Academy of Fishery Science), were used as cloning and expression vectors, respectively. The κ-carrageenase-producing bacterial strain ZM-2 was cultured in modified 2216E medium (Mou 2003).
Sequencing and phylogenetic analysis of 16S rDNA
ZM-2 was inoculated into modified 2216E medium and aerobically grown on a shaker (32 °C, 170 rpm) for approximately 24 h. Genomic DNA, as the template for polymerase chain reaction (PCR), was extracted and purified from the culture using a genome DNA extraction kit (Sangon, Shanghai China). The gene was amplified with the universal primers 27F/1,492R (Table 1) in a DNA thermal cycler (Applied Biosysterms 2720) and then sequenced. A phylogenetic tree was constructed by MEGA 5.0 based on the DNA alignment using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Cloning and sequencing of κ-carrageenase
Three pairs of primers (PF1/PR1, PF2/PR2, and PF3/PR3; Table 1) were designed using Primer-BLAST (http://www.ncbi.nlm.nih.gov/tools/primer-blast/) with reference to the complete genome sequence of Z. galactanivorans strain DsijT (GenBank accession no. FP476056), which contains a full κ-carrageenase gene. PCR was conducted in a thermal cycler using the genomic DNA of ZM-2 as a template and pfu DNA polymerase (Sangon, Shanghai China). The PCR conditions were as follows: 5 min at 94 °C, followed by 30 cycles of 40 s at 94 °C, 45 s at 57 °C, and 2 min at 72 °C. The DNA fragment amplified by the primer pair PF2/PR2 was sequenced and analyzed using open reading frame (ORF) Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/), SignalP 4.0 (http://www.cbs.dtu.dk/Services/SignalP/), and NEBcutter V2.0 (http://tools.neb.com/NEBcutter2/index.php). To amplify the pure κ-carrageenase gene, two forward primers, KC-PF2 (BamH1, Table 1) and KC-PF3 (BamH1, Table 1), which share the same reverse primer KC-PR (2/3) (XhoI, Table 1), were designed. The κ-carrageenase gene with a signal peptide sequence (cgkZ) was amplified with the primer pair KC-PF3/KC-PR(2/3); the primer pair KC-PF2/KC-PR(2/3) was used to amplify the κ-carrageenase gene without a signal peptide sequence (cgkZ-SIG−). The target genes were ligated into the T-cloning vectors (pUCm-T) and sequenced to further determine the accuracy of the open reading frame of the κ-carrageenase gene. The cgkZ gene was translated into an amino acid sequence by EMBOSS Transeq (http://www.ebi.ac.uk/Tools/st/emboss_transeq/). A phylogenetic tree of the deduced amino acid sequence was constructed using MEGA 5.0. Further analysis of this amino acid sequence was performed using ProtParam (http://web.expasy.org/protparam/) and ProtScale (http://web.expasy.org/protscale/).
Heterologous expression of the recombinant enzyme
The target genes, cgkZ and cgkZ-SIG−, were ligated into the expression plasmid pProEX-HTa and then transferred to BL21 (DE3) competent cells. Transformed E. coli, BL21-HTa-cgkZ, and BL21-HTa-cgkZ-SIG− were cultured in LB medium containing 100 μg ampicillin/mL at 23 °C and 120 rpm for 2 h. Afterwards, the expression of the target gene was induced by 1 mM isopropyl-β-thiogalactoside. Cultivation was continued at 23 °C and 120 rpm for 24 h.
Purification of recombinant κ-carrageenase
Supernatant and precipitate cells of the culture medium, which were separated by centrifugation (10,000 rpm, 5 min), were used for the purification of extracellular and intracellular enzymes, respectively. Precipitated cells were resuspended in lysis buffer containing 20 mM sodium phosphate, 0.5 M NaCl, 5 mM imidazole, 0.2 mg/mL lysozyme, 20 μg/mL DNase, and 1 mM MgCl2 and then disrupted by ultrasonication. Cell debris was removed by centrifugation, and the cell extract solution was brought to 40 % (w/v) saturation with solid ammonium sulfate and centrifuged once more. Then, the supernatant was brought to 80 % (w/v) saturation with solid ammonium sulfate and kept at 4 °C overnight. The sediment was redissolved and dialyzed against distilled water to desalt. The protein was purified further by loading into a Ni sepharose 6FF column (GE Healthcare, USA). His-tagged target protein was eluted with imidazole at concentrations ranging from 50 to 150 mM. Active fractions were pooled, dialyzed against 20 mM potassium phosphate buffer (pH 7.4), and then supplemented with 1 mM DTT (DL-dithiothreitol) for storage. The extracellular enzyme was also purified according to the procedures described above. To detect the purified enzyme, 12 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed.
Assay of enzyme activity
κ-Carrageenase activity was assayed using the 3,5-dinitrosalicylic acid method (Miller 1959). The enzymatic hydrolysis reaction was conducted in 20 mM sodium phosphate buffer (pH 6.0) containing 0.5 % (w/v) κ-carrageenan (average molecular mass, 37.3 kDa) for 10 min. One unit of enzyme (U) was defined as the amount of protein needed to release 1 μmol reducing sugar (measured as d-galactose) from κ-carrageenan per minute.
Characterization of the recombinant κ-carrageenase
The optimum temperature for enzyme activity was measured in standard conditions at seven temperatures ranging from 30 to 55 °C. The thermal stability of recombinant κ-carrageenase was determined by detecting the residual activity of the enzyme that had been pre-incubated at different temperatures.
The optimum pH of the enzyme was determined by assessing its activity at different pH. Three buffers, including 100 mM Na2HPO4-citric acid buffer (pH 3.0, 4.0, and 5.0), 100 mM sodium phosphate buffer (pH 6.0, 7.0, and 8.0), and 100 mM Gly-NaOH buffer (pH 9.0, 10.0, and 11.0), were used. pH stability was tested after 2 h pre-incubation of the extracellular enzyme at pH values ranging from 5.0 to 10.0 at 20 °C.
To discover the effects of ions and chemical reagents on enzyme activity, the enzyme assay was performed in the presence of 5 mM Na+ K+, Li+, Mn+, Mg2+, Ba2+, Cu2+, Pb2+, Ca2+, NH4+, EDTA, SDS, DTT, 0.5 % (v/v) tritonX-100, and 0.5 % (v/v) Tween-80. Enzyme activity was measured at 39 °C and pH 6.0. Reaction systems without addition of ions and chemical reagents were used as controls.
Kinetic parameters were determined by measuring initial velocities at κ-carrageenan concentrations ranging from 0.5 to 10 mg/mL under optimal temperature and pH conditions. The enzyme solution, including extracellular and intracellular enzymes, was prepared from the same volume of culture broth and used to determine the maximum velocity. The Michaelis constant (K m) and V max were determined using Lineweaver–Burk double reciprocal plots.
Identification of hydrolytic products of recombinant κ-carrageenase
The purified enzyme was mixed with 0.5 % κ-carrageenan and kept at 39 °C for approximately 12 h. Then, hydrolytic products were precipitated by alcohol, freeze-dried (FD-1A-50 vacuum freezer dryer, Xi A DP Biological Technology, China), and detected using electrospray ionization mass spectrometry. Oligosaccharide samples were dissolved in acetonitrile/1 mM NH4HCO3 (1:1, v/v) and injected into Micromass Q-TOF and Q-TOF Ultima instruments (Waters, Manchester, UK) in negative-ion mode. ESI-MS conditions were as follows: N2 was used as both the drying gas and nebulizing gas at flow rates of approximately 1,000 and 80 L/h, respectively. The cone voltage was set to 50 V, and the electrospray capillary was set to 3 kV. The source was operated at a temperature of 100 °C, and the desolvation temperature was 180 °C (Ekeberg et al. 2001).
Accession numbers
The nucleotide sequences of 16S rDNA and κ-carrageenase gene cgkZ of Zobellia sp. ZM-2 have been deposited in GenBank with accession numbers KC503904 and KC503903, respectively.
Results
Isolation and identification of ZM-2
The κ-carrageenan degrading strains were isolated from decayed seaweed collected from the Yellow Sea, in China. Based on the sole carbon source culture and clearing zone screening on κ-carrageenan plates, the strain showing the highest activity was selected for isolation of the κ-carrageenase gene. According to its morphological, physiological, biochemical characteristics, as well as phylogenetic analysis (Fig. 1) of its 16S rDNA sequence, this strain was assigned to the genus Zobellia and named Zobellia sp. ZM-2.
Cloning and analysis of the full κ-carrageenase gene
The DNA fragment that was amplified by the primer pair PF2/PR2 contained a single ORF 1,638 bp long and displayed 87 % similarity with existing κ-carrageenase encoding genes (GenBank accession nos. FP476052 and AF007559). The deduced amino acid sequence of κ-carrageenase was composed of 545 aa, with a signal peptide of 29 aa. Signal IP 4.0 analysis showed that the most probable cleavage site of the signal peptide is between Gly29 and Gln30. Meanwhile, ProtScale analysis suggested that a strong helical transmembrane motif is located at the region of amino acids 1–28. The molecular mass (Mw) and isoelectric point of the mature enzyme deduced from its amino acid sequence by ProtParam were 58.5 kDa and 8.76, respectively. According to conserved domain analysis based on the NCBI database, the deduced protein was classified as belonging to family GH 16. Twelve active sites (Thr108-Cys137-Ser139-Trp141-Glu159-Asp161-Glu164-Asp182-Asn184-Lys210-Ser259-Gly261) and three catalytic sites (Glu159-Asp161-Glu164; Fig. 2) were found within 545 aa (Johansson et al. 2004; Michel et al. 2001a, b). Protein blast of the deduced amino acid sequence of the cgkZ gene showed that the protein obtained shared 87 % homology with the κ-carrageenase from Z. galactanivorans (GenBank accession no. YP 004734701). The next closest match (85 % shared identity) was a κ-carrageenase precursor (GenBank accession no. AAC27890) of Z. galactanivorans. Low similarities were obtained with the κ-carrageenase amino acid sequences of Cellulophaga lytica (GenBank accession no. YP 004263603; 46 %), Rhodopirellula baltica (GenBank accession no. ZP 12091460; 45 %), Rhodopirellula europaea (GenBank accession no. ZP 21769815; 46 %), Coraliomargarita akajimensis (GenBank accession no. YP003547637; 40 %), P. carrageenovora (GenBank accession no. P43478; 39 %), and P. tetraodonis (GenBank accession no. BAJ61957; 39 %). The polygenetic tree of the deduced amino acid sequence of the cgkZ gene (Fig. 3) manifested its relationship with κ-carrageenases from other microorganisms.
Comparison of predicted κ-carrageenase amino acid sequence of gene cgkZ with eight published amino acid sequences deduced from other κ-carrageenase genes cloned from Z. galactanivorans (GenBank accession no. CAZ94309), P. tetraodonis (GenBank accession no. BAJ61957), R. baltica (GenBank accession no. ZP 12091460), C. akajimensis (GenBank accession no. YP 003547637), Pseudoalteromonas sp. LL1 (GenBank accession no. ADD92366), Z. galactanivorans (GenBank accession no. AAC27890), Z. galactanivorans (GenBank accession no. YP 004734701), and P. carrageenovora (GenBank accession no. P43478). Eleven columns that were marked with white stars indicated active sites (active site Lys210 not shown in this figure), among which three black stars represented catalytic sites (E-D-E). The yellow columns indicated completely identical amino acid zones
Heterologous expression, productivity, and purification of the recombinant enzyme
The recombinant protein was mostly expressed in soluble form under the growth and induction conditions described above. Both the recombinant strain BL21-HTa-cgkZ-SIG− and BL21-HTa-cgkZ showed apparent and almost-equivalent intracellular enzyme activities. However, compared with BL21-HTa-cgkZ-SIG−, BL21-HTa-cgkZ displayed significant extracellular enzyme activity. The recombinant enzyme produced by BL21-HTa-cgkZ was further studied. Transformed E. coli BL21-HTa-cgkZ was propagated in LB broth at 23 °C for 24 h. Productivity of recombinant κ-carrageenase in 1 mL of culture broth was 1.35 U in supernatant and 1.63 U in cell extract. Hence, the total productivity of the recombinant enzyme may be considered as 2.98 U/mL culture broth, 9.0 times higher than that of ZM-2 (0.33 U). Comparative analysis of the intracellular proteins of the experimental and control groups by SDS-PAGE indicated that the Mw of recombinant κ-carrageenase was approximately 45 kDa (Fig. 4), which is in accordance with the Mw of the purified enzyme. Meanwhile, the extracellular κ-carrageenase had the same Mw with the intracellular enzyme. The intracellular recombinant enzyme was purified 19-fold to a specific activity of 107.3 U/mg, and the overall protein yield was approximately 67.4 %. Meanwhile, the extracellular enzyme was purified 23-fold to a specific activity of 74.8 U/mg, and the overall protein yield was 47 % (Table 2).
SDS-PAGE analysis of recombinant κ-carrageenase produced by BL21-HTa-cgkZ. A Lines 1 and 3 were intracellular protein components induced with IPTG; lines 2 and 4 were intracellular protein components without induction; line 5 indicated extracellular protein components induced with IPTG. Line M represented protein markers. The bands marked with circles indicate the recombinant enzyme at 45 kDa. B purified His-tagged protein produced by BL21-HTa-cgkZ
General properties of recombinant κ-carrageenase
The optimal temperature of the recombinant κ-carrageenase was 36–42 °C; the highest enzyme activity was obtained at 39 °C. This result is nearly consistent with that of the natural enzyme from Zobellia sp. ZM-2 (Fig. 5a). Thermal stability analysis of enzyme showed that it was stable after incubation at 35 °C for 3 h with retention of its maximum activity of 95 %. When incubated at 40 °C for 60 min, approximately 50 % of the enzyme activity remained. However, approximately 80 % of the activity was lost after incubation of the enzyme at 45 °C for 15 min (Fig. 5b).
Characterization of the recombinant κ-carrageenase. a The effect of pH on the enzyme activity. b Thermal stability of recombinant κ-carrageenase. After the enzyme was pre-incubated at different temperatures, the remaining activity was detected at 39 °C. c Optimal pH and pH stability of κ-carrageenase as measured in the following buffers: Na2HPO4–citric acid buffer (pH 3.0, 4.0, and 5.0), sodium phosphate buffer (pH 6.0, 7.0, and 8.0), and Gly-NaOH buffer (pH 9.0, 10.0, and 11.0)
The effect of pH on enzyme activity is shown in Fig. 5c. Maximum enzyme activity was observed at pH 6.0 in sodium phosphate buffer. The enzyme was stable from pH 6.0–8.0; over 85 % of the total activity remained after incubation at pH 6.0–8.0 and 20 °C for 120 min. When the pH was decreased to 5.0 or increased to 9.0, approximately 65 % of the initial activity was observed. At pH 10.0, the recombinant enzyme almost completely lost its activity.
The enzyme activity was measured in the presence of various chemicals under standard assay conditions. Ions such as NH4+, Li+, Mn2+, Mg2+, and Ba2+ appeared to slightly impair enzyme activity, whereas K+ and Ca2+ slightly stimulated the enzyme. Partial activation (12.3–18.3 % enhancement) was observed in the presence of TritonX-100, 5 mM Na+, and 150 mM Na+. More apparent stimulation (32.9–167.3 %) was induced by 100 mM Na+, Tween-80, and DTT. In contrast, enzyme activity was strongly inhibited by Cu2+, Pb2+, EDTA, and SDS (Table 3). The Michaelis constant (K m) and V max of recombinant κ-carrageenase in the culture broth were determined using Lineweaver–Burk double reciprocal plots. K m and V max values were estimated to be 0.84 mg/mL (0.023 mM) and 2.92 U/mL, respectively.
Analysis of hydrolytic products
The main depolymerized end-products were collected after sufficient hydrolysis and repeated fractionation by ethanol precipitation with different volume ratio of ethanol to reaction solution (3:1 and 6:1). According to ESI-MS spectra, carrageenan oligosaccharides, including tetrasaccharides, hexasaccharides, octasaccharides, and decasaccharides, were released after hydrolysis by the recombinant enzyme. The first three oligosaccharides were detected in hydrolytic products separated by ethanol precipitation (3:1; Fig. 6a), whereas decasaccharides were detected in hydrolytic products prepared by ethanol precipitation (6:1; Fig. 6b). These oligosaccharides had repeating units consisting of β-d-galactopyranose-4-O-sulfate (G4s) residues and 3,6-anhydro-galactopyranose (An) residues (Liu et al. 2011; Lu et al. 2009).
ESI-MS of κ-carrageenase hydrolytic fragment. a Hydrolytic products separated by ethanol precipitation (3:1). b Hydrolytic products prepared by ethanol precipitation (6:1). ①, An–G4s–An–G4sCl–An–G4sNa–Na; ②, An–G4s–An–G4s–An–G4s–An–G4s–Na; ③, An–G4s–An–G4s–An–G4s–Na; ④, An–G4sCl–An–G4sCl–An–G4sCl–Na; ⑤, An–G4s–An–G4s–Na; ⑥, An–G4s–An–G4s–An–G4sNa–Na; ⑦, An–G4s–An–G4s–An–G4s–An–G4sCl–An–G4sCl–An–G4sNa–Na. A n stands for 3,6-anhydro-α-d-galactopyranose, G 4S stands for β-d-galactopyranose with 4-O-sulfo group
Discussion
We have cloned a new κ-carrageenase-encoding gene, cgkZ, from the newly isolated marine bacterium Zobellia sp. ZM-2. The maximum similarity of cgkZ with existing κ-carrageenase genes was 87 %. The deduced κ-carrageenase was 545 aa in length, with a theoretical Mw of 61.9 kDa, close to that of the κ-carrageenase from C. drobachiensis (Barbeyron et al. 1998). Protein blast combined with phylogenetic analysis also indicated that the κ-carrageenase studied in this paper is a new enzyme of family GH 16. Active sites of glycoside hydrolases commonly encompass two amino acids, Glu and Asp, which are extremely conserved in every glycoside hydrolase family. Although different carrageenases have notable distinctions in their primary sequences, they usually feature a common catalytic motif. The result in Fig. 2 shows that the deduced protein has the characteristic motif E(I/L/V)D(I/V/A/F)(V/I/L/M/F)(0,1)E (E-I-D-V-V-E in Fig. 4) (Planas et al. 1992; Juncosa et al. 1994; Krah et al. 1998). This organization is characteristic of the catalytic site of family GH 16, which features two glutamic acid residues separated either by three amino acids or by four residues (Barbeyron et al. 1998).
To facilitate overexpression, the predicted natural signal peptide was generally removed or substituted by a secretion signal peptide before insertion into the expression plasmid (Kim et al. 2010; Kaewthai et al. 2010). In this paper, the natural signal peptide successfully achieved secretory production of recombinant κ-carrageenase in E. coli. This signal peptide was composed of 29 aa (MTKLKFNGKIRRTALSCLFYLFYLGLVYG), most of which were hydrophobic amino acids. Although an additional 28 aa, including His-tag sequence and the other 22 aa located upstream the signal peptide, it seemed that the N-terminal tag had no negative effect on the function of signal sequence. To date, no general rule in selecting a proper signal sequence for a given recombinant protein to guarantee its successful secretion has been made (Choi and Lee 2004). Thus, this natural signal peptide sequence may be a good option for constructing secretory expression vectors.
The theoretical Mw of the deduced enzyme of gene cgkZ was 61.9 kDa, whereas that of the recombined protein determined by SDS-PAGE electrophoresis was 45 kDa. After removal of the signal peptide and fusion of His-tag sequences to the recombinant product, the Mw of the mature κ-carrageenase from ZM-2 was estimated to be 40 kDa, which corresponds to the κ-carrageenase produced by C. drobachiensis. This experimental phenomenon indicates the posttranslational removal of a large portion of the C-terminal end of the protein, a process that is likely required for crossing the outer membrane (Barbeyron et al. 1998). Interestingly, the posttranslational process of κ-carrageenase from ZM-2 can also occur in E. coli. κ-Carrageenases from different organisms vary in terms of Mw (Liu et al. 2011). For instance, κ-carrageenases secreted by the marine Pseudoalteromonas-like bacterium WZUC10 has a molecular mass of 45 kDa (Zhou et al. 2008). The Mw of κ-carrageenase encoded by cgkA gene in the marine bacterium A. carrageenovora (ATCC 43555) is 44.4 kDa (Barbeyron et al. 1994). Pseudomonas elongata yields a κ-carrageenase with an approximate Mw of 128 kDa (Khambhaty et al. 2007a, b). Three isozymes (Mw of 39, 58, and 100 kDa) from Cytophaga sp. have also been reported (Sarwar et al. 1987).
Based on ESI-MS analysis of enzymolysis products separated by different volumes of ethanol, we concluded that the main products of recombinant κ-carrageenase hydrolyzing κ-carrageenan are tetrasaccharides and hexasaccharides. ESI-MS spectra also showed that the main component of hydrolytic products separated by ethanol precipitation (3:1; Fig. 6a) was hexasaccharide, while tetrasaccharide was the main product of hydrolytic products separated by ethanol precipitation (6:1; Fig. 6b). This demonstrated that κ-carrageenan oligosaccharides with different polymerization degrees could be roughly separated by fractional precipitation with ethanol. In addition, octasaccharides and decasaccharides were detected in the hydrolytic products. Such findings are in accordance with the hydrolytic products of the natural κ-carrageenase from Zobellia sp. ZM-2 (data not shown). Generally speaking, κ-carrageenases produced by different organisms degrade κ-carrageenan to yield distinct oligosaccharides. κ-Carrageenases secreted by P. carrageenovora yield κ-neocarratetraose-sulfate and κ-neocarrabiose-sulfate as end-products (McLean and Williamson 1979; Weigl and Yaphe 1966). The enzyme from Cytophaga sp. MCA-2 breaks down κ-carrageenan into κ-neocarratetraose and κ-neocarrahexaose (Lu et al. 2009). κ-Neocarratetraose-sulfate and larger oligosaccharides with repeating β-d-G4S-(1→4)-α-d-An structures have been obtained from enzymolysis products of κ-carrageenases from Pseudoalteromonas-like bacterium, WZUC10 (Zhou et al. 2008).
Heavy metal ions such as Cu2+ and Pb2+ strongly inhibited the activity of recombinant κ-carrageenase, mainly because these metals combine with –SH groups of the cysteine residue (Cys137) at the active site. This finding indicates that Cys137 is necessary for the recombinant enzyme exerting enzymatic activity or maintaining integrity of protein conformation. Based on this consideration, the addition of DTT improves enzyme activity, because thiols play a role in protecting Cys137 in a reduction state to become active (Yao et al. 2012). Consequently, Cys137 is indispensable for the activity of κ-carrageenase, and DTT could serve as a protective agent during purification and storage of recombinant κ-carrageenases.
Lineweaver–Burk plots in this study showed that the K m value of the recombinant enzyme for κ-carrageenan was 0.842 mg/mL (0.023 mM). This value is smaller than those of most reported κ-carrageenases, such as those from P. porphyrae (4.4 mg/mL) (Liu et al. 2011), Vibrio sp. CA-1004 (3.3 mg/mL; 16), and P. elongata (MTCC 5261; 6.66 mg/mL) (Khambhaty et al. 2007a, b). The smaller K m value of the recombinant enzyme indicates higher affinity for κ-carrageenan.
In conclusion, structural and phylogenetic analyses indicate that the κ-carrageenase-encoding gene of Zobellia sp. ZM-2 is a new gene. The function of the natural signal peptide on the extracellular transport of the recombinant enzyme in E. coli indicates its potential for the construction of secretory expression vectors. Further studies on the signal peptide sequence will yield more precise information on its structure–function relationships. Moreover, a posttranslational process was discovered in E. coli. To the best of our knowledge, this study is the first to report the discovery of a posttranslational process of κ-carrageenase in a recombinant strain. The recombinant enzyme has high affinity to κ-carrageenan, and its productivity is 9.0 times higher than that of the original strain. All of these characteristics indicate the potential use of the enzyme in the biotechnological industry
References
Alban S, Schauerte A, Franz G (2002) Anticoagulant sulfated polysaccharides: Part I. Synthesis and structure–activity relationships of new pullulan sulfates. Carbohydr Polym 47:267–276
Araki T, Higashimoto Y, Morishita T (1999) Purification and characterization of kappa-carrageenase from a marine bacterium, Vibrio sp. CA-1004. Fish Sci 65:937–942
Arfors KE, Ley K (1993) Sulfated polysaccharides in inflammation. J Lab Clin Med 121:201–202
Barbeyron T, Henrissat B, Kloareg B (1994) The gene encoding the kappa-carrageenase of Alteromonas carrageenovora is related to −1,3-1,4-glucanase. Gene 139:105–109
Barbeyron T, Gerard A, Potin P, Henrissat B, Loareg B (1998) The kappa-carrageenase of the marine bacterium Cytophaga drobachiensis. Structural and phylogenetic relationships within family-16 glycoside hydrolases. Mol Biol Evol 15:528–537
Barbeyron T, Gurvan MG, Philippe PP, Bernard HB, Bernard KB (2000) ι-Carrageenases constitute a novel family of glycoside hydrolases, unrelated to that of κ-carrageenases. J Biol Chem 27:35499–35505
Caceres PJ, Carlucci MJ, Damonte EB, Matsuhiro B, Zuniga EA (2000) Carrageenans from Chilean samples of Stenogramme interrupta (Phyllophoraceae): structural analysis and biological activity. Phytochemistry 53:81–86
Choi JH, Lee SY (2004) Secretory and extracellular production of recombinant proteins using Escherichia coli. Appl Microbiol Biotechnol 64:625–635
Ekeberg D, Knutsen SH, Sletmoena M (2001) Negative-ion electrospray ionisation–mass spectrometry (ESI–MS) as a tool for analysing structural heterogeneity in kappa-carrageenan oligosaccharides. Carbohydr Res 334:49–59
Hiroishi S, Sugie K, Yoshida T, Morimoto J, Taniguchi Y, Imai S, Kurebayashi J (2001) Antitumor effects of Marginisporum crassissimum (Rhodophyceae), a marine red alga. Cance Lett 167: 145–150
Johansson P, Brumer H 3rd, Baumann MJ, Kallas AM, Henriksson H, Denman SE, Teeri TT, Jones TA (2004) Crystal structures of a poplar xyloglucan endotransglycosylase reveal details of transglycosylation acceptor binding. Plant Cell 16:874–886
Juncosa M, Pons J, Dot T, Querol E, Planas A (1994) Identification of active site carboxylic residues in Bacillus licheniformis 1,3-1,4-beta-d-glucan 4-glucanohydrolase by site-directed mutagenesis. J Biol Chem 269:14530–14535
Kaewthai N, Harvey AJ, Hrmova M, Brumer H, Ezcurra I, Teeri TT, Fincher GB (2010) Heterologous expression of diverse barley XTH genes in the yeast Pichia pastoris. Plant Biotechnol 27:251–258
Khambhaty Y, Mody K, Jha B, Gohel V (2007a) Statistical optimization of medium components for κ-carrageenase production by Pseudomonas elongata. Enzym Microb Technol 40:813–822
Khambhaty Y, Mody K, Jha B (2007b) Purification and characterization of κ-carrageenase from a novel γ-proteobacterium, Pseudomonas elongate (MTCC 5261) syn. Microbulbifer elongates comb. nov. Biotechnol Bioprocess Eng 12:668–675
Kim HT, Lee S, Lee D, Kim HS, Bang WG, Kim KH, Choi IG (2010) Overexpression and molecular characterization of Aga50D from Saccharophagus degradans 2–40: an exo-typeβ-agarase producing neoagarobiose. Appl Microbiol Biotechnol 86:227–234
Kobayashi T, Uchimura K, Koide O, Deguchi S, Horikoshi K (2012) Genetic and biochemical characterization of the Pseudoalteromonas tetraodonis alkaline κ-carrageenase. Biosci Biotechnol Biochem 76:506–511
Krah M, Misselwitz R, Politz O, Thomsen KK, Welfle H, Borriss R (1998) The laminarinase from thermophilic eubacterium Rhodothermus marinus. Eur J Biochem 257:101–111
Liu JM, Haroun-Bouhedja F, Boisson-Vidal C (2000) Analysis of the in vitro inhibition of mammary adenocarcinoma cell adhesion by sulphated polysaccharides. Anticancer Res 20:3265–3271
Liu GL, Li Y, Chi Z, Chi ZM (2011) Purification and characterization of κ-carrageenase from the marine bacterium Pseudoalteromonas porphyrae for hydrolysis of κ-carrageenan. Process Biochem 46:265–271
Lu XZ, Yan C, Wu QQ, Gu YC, Han F, Yu WG (2009) Cloning, expression and characterization of a new agarase-encoding gene from marine Pseudoalteromonas sp. Biotechnol Lett 37:1565–1570
Mark P, Baumann MJ, Eklöf JM, Gullfot F, Michel G, Kallas AM, Teeri TT, Brumer H, Czjzek M (2009) Analysis of nasturtium TmNXG1 complexes by crystallography and molecular dynamics provides detailed insight into substrate recognition by family GH16 xyloglucan endo-transglycosylases and endo-hydrolases. Proteins 75:820–836
McLean MW, Williamson FB (1979) κ-Carrageenase from Pseudomonas carrageenovora. Eur J Biochem 93:553–558
Michel G, Flament D, Barbeyron T, Vernet T, Kloareg B, Dideberg O (2000) Expression, purification, crystallization and preliminary X-ray analysis of the iota-carrageenase from Alteromonas fortis. Acta Crystallogr, Sect D: Biol Crystallography 56:766–768
Michel G, Chantalat L, Duee E, Barbeyron T, Henrissat B, Kloareg B, Dideberg O (2001a) The κ-carrageenase of P. carrageenovora features a tunnel shaped active site: a novel insight in the evolution of Clan-B glycoside hydrolases. Structure 9:513–525
Michel G, Chantalat L, Duee E, Barbeyron T, Henrissat B, Kloareg B, Dideberg O (2001b) The κ-carrageenase of P. carrageenovora features a tunnel-shaped active site: a novel insight in the evolution of Clan-B glycoside hydrolases. Structure 6:513–525
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Mou HJ (2003) Studies on chemical and biological properties of κ-neocarratetraose and κ-neocarrahexaose by enzyme depolymerization. Ph.D. thesis, Ocean university of China
Mou HJ, Jiang XL, Guan HS (2003) A κ-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity. J Appl Phycol 15:297–303
Øtgaard K, Wangen BF, Knutsen SH, Aasen IM (1993) Large-scale production and purification of κ-carrageenase from Pseudomonas carrageenovora for applications in seaweed biotechnology. Enzyme Microb Technol 15:326–333
Planas A, Juncosa M, Lloberas J, Querol E (1992) Essential catalytic role of Glu134 in endo-β-1,3-1,4-d-glucan 4-glucanohydrolase from B. licheniformis as determined by site-directed mutagenesis. FEBS Lett 308:141–145
Potin P, Sanseau A, Gall Y, Rochas C, Kloareg B (1991) Purification and characterization of a new κ-carrageenase from a marine Cytophaga-like bacterium. Eur J Biochem 201:241–247
Sarwar G, Matayoshi S, Oda H (1987) Purification of κ-carrageenase from a marine Cytophaga species. Microbiol Immunol 31:869–877
Suzuki N, Kitazato K, Takamatsu J, Saito H (1991) Antithrombotic and anticoagulant activity of depolymerized fragment of the glycosaminoglycan extracted from Stichopus japonicus Selenka. Thromb Haemost 65:369–373
Weigl J, Yaphe W (1966) The enzymic hydrolysis of carrageenan by Pseudomonas carrageenovora: purification of a kappa-carrageenase. Can J Microbiol 12:939–947
Yao Z, Zhang C, Lu F, Bie X, Lu Z (2012) Gene cloning, expression, and characterization of a novel acetaldehyde dehydrogenase from Issatchenkia terricola strain XJ-2. Appl Microbiol Biotechnol 5:1999–2009
Zhou MH, Ma JS, Li J, Ye HR, Huang KX, Zhao XW (2008) A κ-Carrageenase from a newly isolated Pseudoalteromonas-like bacterium, WZUC10. Biotechnol Bioproc Eng 13:545–551
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 41076087), Program for New Century Excellent Talents in University (NCET-10-0719), and Program for Changjiang Scholars and Innovative Research Team in University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Z., Li, G., Mo, Z. et al. Molecular cloning, characterization, and heterologous expression of a new κ-carrageenase gene from marine bacterium Zobellia sp. ZM-2. Appl Microbiol Biotechnol 97, 10057–10067 (2013). https://doi.org/10.1007/s00253-013-5215-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5215-0