Abstract
The rising trend of bioflavour synthesis by microorganisms is hindered by the high manufacturing costs, partially attributed to the cost of the starting material. To overcome this limitation, in the present study, dilute-acid hydrolysate of orange peel was employed as a low-cost, rich in fermentable sugars substrate for the production of flavour-active compounds by Saccharomyces cerevisiae. With this purpose, the use of immobilized cell technology to protect cells against the various inhibitory compounds present in the hydrolysate was evaluated with regard to yeast viability, carbon and nitrogen consumption and cell ability to produce flavour active compounds. For cell immobilization the encapsulation in Ca alginate beads was used. The results were compared with those obtained using free-cell system. Based on the data obtained immobilized cells showed better growth performance and increased ability for de novo synthesis of volatile esters of "fruity" aroma (phenylethyl acetate, ethyl hexanoate, octanoate, decanoate and dodecanoate) than those of free cells. The potential for in situ production of new formulations containing flavour-active compounds derive from yeast cells and also from essential oil of orange peel (limonene, α-terpineol) was demonstrated by the fact that bioflavour mixture was found to accumulate within the beads. Furthermore, the ability of the immobilized yeast to perform efficiently repeated batch fermentations of orange peel hydrolysate for bioflavour production was successfully maintained after six consecutive cycles of a total period of 240 h.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Flavour plays a crucial role in the acceptance of many food products. Flavour compounds comprise one quarter of the global market with earnings reaching up to US$20.3 billion in 2009 (Natural colors and flavors market 2011). Traditionally, these molecules are either produced via chemical synthesis or extracted directly from plants (Longo and Sanromán 2006; Dubal et al. 2008). During the last decades, the rising demand of modern consumers for natural food ingredients has stimulated wide interest in the development of alternative routes for the production of natural flavourings based on biotechnology. According to the latest EC legislation (EU Regulation No 1334 2008), natural flavours include biotechnology-derived products using microorganisms, plant cell cultures and enzyme catalysed reactions (Longo and Sanromán 2006; Dubal et al. 2008; Saerens et al. 2010).
Industrial-scale application of microbial technology for bioflavour production has been hindered by high manufacturing costs, the formation of undesired by-products due to the complex pathways and the toxicity of the substrates used or the aroma compounds produced (Schrader 2007). For the development of economically competitive microbial processes, the use of expensive substrates, the low product yields and the high product recovery costs must be overcome. Some agro-industrial residues with negligible or even no-cost offer excellent possibilities to be used as substrates for flavour production by microorganisms. Currently, many research groups have developed successful procedures using these non-conventional media (e.g., cassava bagasse, sugarcane bagasse, apple pomace, soybean, coffee husk) in the microbial production of flavour compounds (Longo and Sanromán 2006). This trend is in line with our efforts to exploitate the whole residue after extraction of juice from orange fruit (hereafter referred to as orange peel) for the production of volatile esters of "fruity" aroma by using a commercial wine yeast strain (Mantzouridou and Paraskevopoulou 2012). This waste attracted our interest considering that in the juice-making industries large volumes of citrus peel (mainly from oranges) are produced, accounting for about 50 % of the total fruit weight. Its management revolves around a million tons per year and it costs U$10.00/ton (Rossi et al. 2009). High sugar levels along with insoluble polysaccharides (cellulose, hemicellulose, pectin) make orange peel a potential substrate for microbial bioprocesses. Previously, it was demonstrated that the polysaccharide-rich fractions did not contribute to the de novo synthesis of bioflavour (Mantzouridou and Paraskevopoulou 2012). This finding was motivation to carry out experiments for the hydrolysis of these carbohydrates into simple sugars and the full exploitation of the waste in the direction of flavour production.
Most research on the exploitation of orange waste focuses on ethanol production. Among others, yield is highly dependent on the successful hydrolysis of cellulose, hemicellulose and pectin polymers into fermentable sugars. According to the available literature, dilute acid-based hydrolysis and/or enzymatic hydrolysis is commonly applied for sugar production from lignocellulosic materials (Taherzadeh and Karimi 2007). Optimization of the hydrolysis conditions based on the material is strongly recommended (Oberoi et al. 2010). Recent economical analysis of a citrus waste biorefinery strengthens the prospect for ethanol production after dilute acid-based hydrolysis of this waste (Lohrasbi et al. 2010).
The main obstacle of orange peel hydrolysate use is the presence of d-limonene (found in the peel oil at a percentage higher than 90 %, w/w), which along with the extremely toxic to yeast hydrolysis by-products (such as carboxylic compounds, furans, phenolic compounds), are expected to cause negative interference in the bioprocess performance (Pourbafrani et al. 2007; Taherzadeh and Karimi 2007; Liu et al. 2013). An effective way to address the substrate toxicity is the use of immobilized cells technology (Pourbafrani et al. 2007; Taherzadeh and Karimi 2007; Purwadi and Taherzade 2008). Immobilization offers a wide range of advantages with the most important being the protection effect against inhibitory substances and contaminants as well as higher production rates and the easy purification of the product (cell-free product) (Nedovic et al. 2010; Westman et al. 2012). It involves enclosing of cells inside a selective and porous membrane so as to permit the influx of oxygen, nutrients and other essential growth factors and at the same time to exclude strong inhibitors present in the toxic medium to be fermented. Immobilizing the microbial cells also allows for their reuse in repeated batch cycles further reducing the total cost of the process. Additionally, several studies upon the application of immobilized cells technology to beer and wine making have resulted in satisfactory flavour profiles in the final products (Tataridis et al. 2005; Willaert and Nedovic 2006; Vilela et al. 2013). From the various techniques available, yeast cells entrapped within calcium alginate beads are commonly used due to the very mild conditions applied, the low cost and ease of use (Nedovic et al. 2010).
The aim of this study was to examine the production of flavour-active compounds by means of immobilized cells of the commercial wine yeast strain Vitilevure MT (Saccharomyces cerevisiae var. cerevisiae) in alginate beads, grown in dilute-acid hydrolysate of orange peel. The parameters such as cell viability, nutrient assimilation and stability of the immobilized particles in batch and repeated batch fermentation were assessed in order to evaluate the convenience of the immobilized cells for growth in orange peel hydrolysate. Gas chromatography (GC-MS/GC-FID) analysis was employed to monitor changes in the composition of flavour-active compounds inside and outside the beads during the fermentation processes. The results were compared with those from cultivation of free cells in the hydrolysate. The effects of encapsulation on the viability of the cells and flavour production from orange peel hydrolyzate were specifically noticed. The potential for in situ production of new formulations containing bioflavour is also discussed.
Materials and methods
Materials
Washington Navel oranges were obtained from the local market. After the removal of the juice, the peel (white mesocarp and orange-yellow exocarp) was sliced into small pieces, dried to a constant weight at 105°C in a hot air oven (KT 500, W.C Heraeus Hannau, Germany) and grounded with an electric mill (Braun 4240, Germany). Fresh oranges were employed in all experiments.
Culture media contained d-glucose monohydrate supplied by Panreac (Quimica, Barcelona, Spain), soy peptone supplied by Lab M Ltd (Lancashire, UK), and yeast extract, KH2PO4, MgSO4 and (NH4)2SO4 supplied by Merck (Darmstadt, Germany).
Medium viscosity sodium alginate (MW = 80,000–120,000) extracted from Macrocystis pyrifera (A2033; Sigma-Aldrich, Munich, Germany) was used as a hydrophilic polymer to form encapsulating matrix for immobilization of yeast cells. The content of guluronic acid of the commercial sample was approximately 61 % (w/w). Calcium chloride dihydrate (Merck, Germany) was used as crosslinking agent.
Standard compounds employed (isoamyl acetate, phenylethyl acetate, ethyl hexanoate, ethyl octanoate, ethyl decanoate, ethyl dodecanoate, limonene, α-terpineol, 5-hydroxymethyl-furfural [HMF]), were from Fluka (Munich, Germany). All other common reagents and solvents were of the appropriate purity from various suppliers.
Hydrolysis of orange peel
The orange peel was subjected to a two stage dilute acid hydrolysis, performed as optimized by Oberoi et al. (2010) for ethanol production. At first, 12 g of dried orange peel powder were treated with 100 ml H2SO4 (0.5 w/v) at 121°C for 15 min. The hydrolysate was recovered after filtration through a double layered cheese cloth. The residual solid was subjected to a second hydrolysis step using the same conditions as used for the first one, but for a longer time period (30 min). The hydrolysates obtained from both stages were combined and further analyzed for sugars, nitrogen and aroma compounds.
Microorganism and inoculum preparation
The commercial wine yeast strain Vitilevure MT (S. cerevisiae var. cerevisiae), in the form of active dry powder, used in this study was provided by a Greek wine industry (Tsantalis S.A., Chalkidiki, Greece) and was maintained at 4°C till use. Inoculum was prepared as described by Mantzouridou and Paraskevopoulou (2012). Briefly, the commercial active dry yeast strain was first activated by suspension in water (1:10, w/v; 35–37°C) and then by periodical stirring inside a water bath for 15–20 min. Inoculum was prepared by transferring 2 ml of the cell suspensions in 100 ml of a liquid medium with the following composition (g/l): glucose (20), soy peptone (10) and yeast extract (5). The culture was grown aerobically in a shaking incubator at 30°C/200 min−1 to a final OD600 value of approximately 2. Then, yeast cells were collected by centrifugation (3,000 × g, 10 min), washed and resuspended in a 9 g/l NaCl sterile saline solution at about 1 × 109 colony forming units (cfu)/ml.
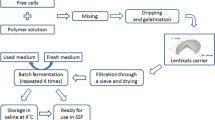
Immobilization of S. cerevisiae in alginate beads
Alginate microbeads were produced with the following procedure: sodium alginate solution was prepared by dissolving 1.3 g of Na alginate powder to 100 ml of deionised water. The prepared Na alginate solution was autoclaved, cooled down at room temperature and gently mixed with yeast suspension at a ratio of 4:1 (v/v). Spherical microbeads with a mean diameter of ~800 μm were formed by the extrusion of the polymer/cell suspension mixture, through a blunt stainless steel sterile needle (22 gauge), to a 20 g/l CaCl2 solution under aseptic conditions. The distance between the needle tip and the collecting solution was 2.5 cm, while the flow rate of the polymer solution was 70 cm3/h. During the addition of the above mixture to the collecting solution gentle stirring was applied by a magnetic stirrer, which continued for 30 min more (Manojlovic et al. 2006). The beads were allowed to harden in fresh CaCl2 solution for 1 h to complete the gelation process and then washed twice with sterile saline solution to remove the excess of calcium ions. The beads were stored in sterile saline solution at 4°C until use (Nedovic et al. 2001).
Batch fermentation experiments
The fermentation medium was comprised of the orange peel hyrolysate obtained by the two stage dilute acid hydrolysis, neutralized (pH 5.5) and enriched with the following nutrients (g/l): yeast extract (0.4), (NH4)2SO4 (1), KH2PO4 (1), MgSO4 (5). Experiments were carried out under semi-anaerobic conditions (Mantzouridou and Paraskevopoulou 2012) at 25°C by allowing 60 ml of the above medium to stand in hydrophobic cotton-stopped Erlenmeyer flasks (100 ml). The flasks containing the fermentation medium were autoclaved at 121°C for 15 min and after cooling were inoculated with free and immobilized cells so as to achieve the same initial number of yeast cells in both cases (approximately 6.04 × 107 cfu/ml). Fermentation kinetic studies were conducted at 25°C for 5 days. At specific time intervals (0, 12, 24, 48, 72, 96 and 120 h), the fermentation broth was removed from the flasks and subjected to further analysis. All fermentation experiments were carried out in duplicate (CV % = 7.15 % for ethyl octanoate, n = 5).
Repeated batch fermentation experiments
The experiment was performed during six consecutive cycles using immobilized yeast cells under the same process conditions as in the batch fermentation. Each cycle lasted 48 h, except for the first two cycles, which lasted 24 h to achieve adaptation of the biocatalyst in the fermentation environment. At the end of each cycle, immobilized cells were separated from the exhausted medium and replaced in fresh hydrolysate.
Yeast enumeration
Total number of free viable yeast cells was determined by plating diluted samples of fermentation broth (peptonized water) on Yeast Peptone Dextrose (YPD) agar. Cultures were incubated for 48 h at 30 °C to determine yeast population. To count the viable cell numbers in the Ca alginate beads, yeast cells were released from the gel beads by complete disruption of particles in sterile 2 % w/v Na citrate (Sigma-Aldrich) solution with the aid of a shaking incubator for 15 min (volume ratio, 5:1 v/v). Suspended cells were then enumerated by standard plate counts as described above. The results were expressed in log10 cfu/ml.
Determination of residual carbon and nitrogen content
Residual sugars and nitrogen consumption were determined in the filtered aliquots of the fermentation broth spectrophotometrically (Mantzouridou et al. 2006).
Isolation of volatiles
Flavour-active compounds were extracted from the fermentation broth (liquid with free cells, 15 ml) as well as from the disrupted beads (30 ml) with equal volume of dichloromethane (shaking for about 2 h at 20°C). The organic fractions were dried over anhydrous Na2SO4 and concentrated firstly to 2 ml in a Vigreux column and then under a slow nitrogen stream up to a final volume of 500 μl. The crude extracts were kept at −18°C until further analysis.
Qualitative and quantitative analysis of volatiles
Final extracts were analysed both qualitatively and quantatively as described by Mantzouridou and Paraskevopoulou (2012). An Agilent 6890A gas chromatograph (USA) equipped with MSD 5973 mass spectrometer (MS) as well as an Agilent 6890A gas chromatograph equipped with a flame ionization detector (FID) were used. Volatile compounds were separated on a HP-FFAP column (25 m × 0.20 mm i.d., film thickness 0.30 μm) with helium as carrier gas (2 ml/min). The oven temperature was kept at 40 °C for 2 min and then raised to 230 °C at a rate of 10 °C/min (4 min). The injector and the transfer line temperature was set at 230°C and 240°C, respectively. The injection volume was 2 μl (split ratio 100:1). The MS was operated in the EI mode at 70 eV, scanning the range 35–350 m/z at a scan rate of 2 scans/s and the ion source temperature was 230 °C. The identification of the compounds were conducted by comparing their retention times and mass spectra with those of the standard compounds. Their concentration was measured by comparison with calibration curves made with the reference compounds under the same conditions.
Statistics
Mean values of four independent measurements are shown in Table 1 and in the figures. Error bars represent the standard deviation (SD) of the mean value.
Results
Growth characteristics of S. cerevisiae in dilute-acid hydrolysate of orange peel using free and encapsulated-cell system
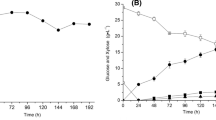
The time profiles of the viable cell number, residual total sugars and nitrogen during batch cultivation of encapsulated and freely suspended S. cerevisiae cells in the dilute-acid hydrolysate of orange peel are presented in Fig. 1 and 2. During the first 24 h of fermentation the entrapped in Ca alginate beads viable cells exhibited a high growth rate which resulted in a size population of 10.3 log10 cfu/ml. A slower growth rate and eventually a 1.1-log reduction in the number of free viable cells compared to the encapsulated ones were observed during the same period (24 h) (Fig. 1). Although soluble sugars (62.8 g/l) were assimilated rapidly in around 12 h both by the free and encapsulated cells, the percentage of sugars consumed by the latter was higher than the respective value for the free cells (84 % and 78 % of total sugars, respectively) (Fig. 2a). A trend similar to that of soluble sugars was also observed for nitrogen consumption. Specifically, after 12 h, 73.9 % and 64.1 % of total nitrogen was consumed by encapsulated and free cells, respectively (Fig. 2b). In all cases, cell growth was accompanied by a significant drop in pH value of the medium (data not shown). Noticeably, after the 24 h, free yeast cells ceased growing and a progressive loss of viability was observed until the end of cultivation (120 h). However, inside the beads cell viability remained practically constant up to 72 h, increased thereafter until 96 h and remained high for the next 24 h, approaching 3 log units more than the corresponding value for the free cells at the end of the cultivation period (Fig. 1). Moreover, in the experiment with free cells considerable amounts of residual sugars and nitrogen were still available in the flasks after 120 h (11.2 and 0.48 g/l, respectively) (Fig. 2a, b). In the case of immobilized cells, the cell count outside the beads increased up to 48 h and remained constant thereafter (Fig. 1). This may result from the combination of the number of viable cells leaked out of the beads and the maintenance of cell viability in the toxic hydrolysate.
Bioflavour production in dilute-acid hydrolysate of orange peel using free and encapsulated cell system
The evolution of individual volatile esters produced by Vitilevure MT growing in dilute acid hydrolysate of orange peel (Figs. 3 and 4a–d) are discussed below with regard to the effect of immobilization technology applied to yeast cells. As it is obvious by comparing data in Figs. 3 and 4a–d, different production rates of esters and yields have been observed in free and encapsulated-cell systems. In particular, by the use of immobilized cells, phenylethyl acetate showed increased total maximum production (calculated as the sum of the concentrations detected inside and outside the beads) of 2,585 μg/l at 96 h, while the maximum production of the above ester from free cells, reached after 72 h, was almost 2.5 times lower (1,048 μg/l) (Fig. 3). The production of ethyl esters was also enhanced with the use of immobilized cells (Fig. 4a–d). As shown in Fig. 4a, ethyl hexanoate was synthesized only by immobilized cells after 48 h of fermentation. The total maximum observed concentration of 2,723 μg/l at 72 h was rather high when compared to those reported during beer and wine fermentation by immobilized yeast (Mallouchos et al. 2002; Willaert and Nedovic 2006) and also in orange pulp based medium (Mantzouridou and Paraskevopoulou 2012), in chemically defined medium (Saerens et al. 2008) and in wine (Swiegers et al. 2005) using free cells. Regarding ethyl octanoate and decanoate, their maximum concentrations between immobilised on Ca alginate and free yeast cells differed slightly: for immobilised 1,654 and 2,420 μg/l (total production), respectively, and for free 1,194 and 2,024 μg/l, respectively (Fig. 4b, c). In case of ethyl decanoate, it should be noted that, its total maximum production was reached faster by using entrapped than free cells (48 vs. 72 h, respectively). The difference for ethyl dodecanoate was more evident. Specifically, entrapped cells exhibited accelerated synthesis of the above ester reaching 4,407 μg/l at 12 h, whereas, only 1,986 μg/l was produced by free cells after 72 h of the bioprocess (Fig. 4d).
Kinetics of ethyl esters production by free (blue filled circle; all symbols are connected by line) and immobilized cells of S. cerevisiae inside the beads (orange filled square) and in the liquid medium (green filled triangle) in orange peel hydrolysate: a ethyl hexanoate, b ethyl octanoate, c ethyl decanoate and d ethyl dodecanoate
Repeated batch fermentation
From the results obtained so far, entrapment of yeast cells in Ca alginate beads were found to be a suitable strategy for the production of flavour active compounds in dilute-acid hydrolysate of orange peel. On such a ground, it was challenging to further investigate the ability of the immobilized yeast to perform efficiently repeated batch fermentations of dilute-acid hydrolysate of orange peel. The experiment was performed during six consecutive cycles that were completed in a total period of 240 h. After each 48 h cycle of fermentation (except for the first two cycles, which lasted 24 h to achieve adaptation of the biocatalyst in the fermentation environment), the fermented broth was replaced by fresh medium, and the concentration of flavour-active compounds was determined in the replaced medium and inside the beads.
As shown in Fig. 5, the number of viable cells in Ca alginate beads increased significantly during the first cycle of fermentation. Enumeration of the immobilized viable cells directly after immobilization indicated that the biocatalyst contained 7.78 log10 cfu/ml. At the end of the first fermentation cycle (24 h), the immobilized viable cells increased to 10.62 log10cfu/ml and remained almost stable until the end of the second fermentation cycle (48 h). Further incubation of the immobilized cells during the third cycle resulted in a rapid decrease in viability to a value of 8.84 log10 cfu/ml. However, high densities of immobilized cells at the end of the third cycle demonstrated stable numbers of viable cells during the subsequent cycles (4–6) (Fig. S1). As it is depicted in Fig. 5, the number of yeast cells leaked into the broth was low and stable during cycles 1–4, while higher values were detected for cycles 5 and 6. Similarly, Rakin et al. (2009) reported that Ca alginate beads disintegrated after 4–5 days of repeated batch fermentation of corn meal hydrolysates. This was attributed to the intensive growth of cells and CO2 evolution inside the matrix causing instability of Ca alginate in acidic conditions during the fermentation. In our case, the sugar consumption at the end of each batch ranged from 77 % to 88 % (w/w) of the initial sugar concentration indicating no significant mass transfer limitations from substrate (Fig. 6). The fact that this consumption can be attributed partially to the liberated cells, especially in the last two cycles that their population started to increase, should not be precluded.
The concentrations of flavour-active compounds produced at the end of the third, fourth, fifth and sixth cycles are shown in Fig. 7a, b. At the end of the third fermentation cycle, the total ethyl hexanoate concentration (inside and outside the beads) was 62 % of the maximum value achieved. The latter was observed at the end of the fourth cycle, mainly retained inside the beads (82.2 %), and was 17.3 % lower than that observed in the single-stage fermentation (2,251 vs. 2,723 μg/l, respectively); as the total fermentation time was longer (144 than 72 h), this resulted in a lower ethyl hexanoate productivity. During the subsequent cycles, the total ethyl hexanoate concentration progressively decreased up to zero value at the end of the sixth cycle. At the end of the third cycle (48 h), ethyl octanoate reached its total maximum concentration, which was more than 2-fold higher than the respective value attained after 72 h of single-stage batch fermentation (3,809 vs. 1,654 μg/l, respectively). This is also an interesting result because it demonstrates an important increase in the productivity of the process with respect to ethyl octanoate. Nevertheless, the high flavour load led to a strong release of ethyl octanoate (82.3 %) into the liquid medium. Ethyl octanoate level dropped to 1,000 and increased back to 1,700 μg/l at the end of the fifth and sixth cycles, respectively. The decreasing trend in the production level of the above compounds during the repeated batch operation mode may be a result of the residual metabolic by-products in the cells, such as ethanol (Legras et al. 2010). The total maximum concentrations of ethyl decanoate (1,735 μg/l attained at the end of the third cycle), ethyl dodecanoate and phenylethyl acetate (4,900 and 1,840 μg/l, respectively, attained at the end of the sixth cycle) (Fig. 7a, b) were not so different with regard to the respective values obtained in the single-batch fermentation (2,420, 4,440 and 2,585 μg/l, respectively) (Figs. 3 and 4a–d). However, productivity values were low for all of them, given that maximum production of these compounds was reached faster in the latter case (Fig. 4a–d). The above results can be explained by the decreased availability of key precursors during the initial cycles of repeated batch operation, where high cellular growth of the yeast cells was observed.
Kinetics of volatile ester production (a) inside the beads and (b) in the liquid medium by S. cerevisiae immobilized cells in orange peel hydrolysate during repeated batch fermentation. ethyl hexanoate (red filled diamond ; all symbols are connected by line connected by line), ethyl octanoate (violet filled square), ethyl decanoate (orange filled triangle), phenylethyl acetate (yellow multiplication symbol) and ethyl dodecanoate (violet multiplication symbol)
Discussion
One of the challenges associated with the growth of S. cerevisiae in orange peel hydrolysate is the presence of the essential oil of the raw material with a very pronounced antimicrobial activity. Previous findings showed an increase of the lag period before yeast growth by about 35 % in a liquid medium containing 300 ppm of orange essence and stronger toxicity at higher concentrations (Belletti et al. 2004). As shown in Table 1, the main compounds identified in the volatile fraction of orange peel hydrolysate by GC-MS analysis were d-limonene, α-terpineol and terpin hydrate. The content of limonene in the orange peel hydrolysate was found to be 168 mg/l. This value corresponded to 2378 mg/kg dry orange peel and was lower than that of the raw material (4,200 mg/kg dry orange peel, 95 % of the main volatile compounds) (data not shown). Obviously, limonene's loss, caused by evaporation or transformation, takes place during the dilute-acid hydrolysis process. α-Terpineol was present in the hydrolysate at a value of 100 mg/l. The presence of terpin hydrate, which was not traced in the volatile fraction of orange peels (data not shown), may be attributed to the hydration of terpineol and limonene under acidic conditions (Perkin 1904).
The results in Table 1 also revealed the formation of the antimicrobial compound HMF as a consequence of the acid hydrolysis of the lignocellular raw material. In particular, HMF was detected at relatively low concentration (22.22 mg/l) with regard to its toxic level to yeast metabolism (>500 mg/l) (Oberoi et al. 2010). However, the above compound is expected to act synergistically with those of the essential oil and the other toxic compounds formed, such as furfural and acetic acid, increasing, thus, the overall antimicrobial effect of the substrate (Alriksson 2006). Furfural and HMF act as inhibitors for many crucial enzymes, responsible for the primary catabolism of carbon, such as dehydrogenase and aldehyde dehydrogenase (Alriksson 2006; Taherzadeh and Karimi 2007). Additionally, furfural and HMF can be further degraded to formic and levulinic acid, which repress the growth of yeasts cells. The antimicrobial action of the acids (formic, acetic, levulinic) can be attributed to alterations of intracellular pH (Alriksson 2006).
Overall, the better growth performance of immobilized cells as compared to free cells in the toxic hydrolysate shown here strengthen previous findings suggesting increased survival of encapsulated yeast cells in toxic hydrolysates (Pourbafrani et al. 2007; Purwadi and Taherzade 2008). Our results led us to collect quantitative data for the toxic compounds of the hydrolysate in the internal micro-environment of the beads at the end of the exponential growth phase (24 h) by applying GC-FID analysis. Among terpenoid compounds, limonene and α-terpineol were present in the beads at 3.28 and 10.93 mg/l, respectively. Considering the levels of the above compounds outside the beads (27.9 and 23.08 mg/l, respectively) and in the free-cell system (29.56 and 34.92 mg/l, respectively) two important observations can be made. Firstly, around 82 % of limonene present in the orange peel hydrolysate at the beginning of fermentation was evaporated. A similar observation was made by Wilkins et al. (2007), who found loss of 60.4 % of the limonene during solid state fermentation of orange peel waste for ethanol production by yeast. These authors marked the minor role of S. cerevisiae in the transformation products of limonene under limited oxygen supply. The 66 % loss of α-terpineol may be due to evaporation or biotransformation to terbin hydrate by yeast cells (King and Dickinson 2000). Secondly, only 10.5 % and 32.15 % of the available amount of limonene and α-terpineol passed through the hydrophilic membrane of the beads, probably to their hydrophobic character. This finding is in line with results from the study of Pourbafrani et al. (2007), who showed the protective effect of yeast cells encapsulation on Ca alginate membrane against inhibition by d-limonene in fermentation of orange wastes to ethanol. In the case of HMF, low concentration of this compound inside the beads compared to the respective value outside the beads (4.5 vs. 22.78 mg/l, respectively) and in the free cells system (10.11 mg/l) may be attributed to the in situ detoxification capacity of highly concentrated encapsulated cells (Talebnia and Taherzadeh 2006).
The central finding of our study was the feasibility of batch fermentation of dilute acid hydrolysate of orange peel for the de novo synthesis of bioflavour by Ca alginate immobilized yeast cells. Up to now, most research on the exploitation of orange waste hydrolysate via microbial fermentation focuses on ethanol production (Pourbafrani et al. 2007; Oberoi et al. 2010). Our results demonstrate that esters were quantitatively the major group of flavour active compounds produced throughout the fermentation process using free and encapsulated yeast cells. In total, four esters were found as newly formed compounds in both systems, namely phenylethyl acetate ("rose-like, honey, fruity" aroma), ethyl octanoate ("sour apple, pineapple" aroma), ethyl decanoate ("grape-like" aroma) and ethyl dodecanoate ("sweet, cream like" aroma). Moreover, ethyl hexanoate responsible for an "anise, apple-like, strawberry" aroma was only detected in fermented orange pulp hydrolysate by encapsulated cells. Inability of free cells to synthesize ethyl hexanoate is probably correlated with the inhibitory effect of liberated inositol from phytic acid (inositol hexakisphosphate) and its hydrolysates by the action of yeast phytases (Kaur et al. 2007). The inhibitory mechanism is through the reduction of hexanoic acid production by repressing FASI (β-subunit of fatty acid synthetase gene) expression (Furukawa et al. 2003). The protective role of immobilization using Ca alginate beads against inositol can be explained by the binding affinity of the inositol polyphosphates for Ca2+ present in the medium or after solubilization from the gel. This affinity has been found to be depended on the number of phosphate substituents and their stereochemistry (Luttrell 1993) and is essential considering that it may play a key role in disrupting the gel due to the breaking of the ion bonds between alginate and Ca (Margaritis and Kilonzo 2005). In this study, an obvious advantage can be withdrawn by using Ca alginate encapsulation system when cheap agro-residue from orange fruit (i.e., orange peel) rich in phytic acid (i.e., waste) (Mittal et al. 2012) are to be used as a fermentation substrate for bioflavour production.
The results in Figs. 3 and 4 revealed strong ester concentration and yield fluctuations over time that may depend on the availability of precursors and also the activity of the enzymes involved in ester synthesis and subsequent hydrolysis, and/or evaporation rates (Saerens et al. 2008). The increased production of phenylethyl acetate by encapsulated S. cerevisiae is in line with previous demonstrations on acetate ester production versus immobilized cell technology reported in the literature. More specifically, isoamyl acetate formation was doubled by cells immobilized on stainless-steel fibre cloth thanks to the increased expression level of the yeast alcohol acetyl transferase AATase I gene ATF1 through the Ras/cAMP/PKA nutrient signalling pathway activation in the microenvironment inside the beads (Shen et al. 2003a). Additionally, oxygen transfer limitation within different carriers (stainless steel fibre cloth, DEAE cellulose) resulted in the reduced fatty acid synthesis and degree of unsaturation by immobilized cells stimulating the formation of acetate esters either by excess of acyl-CoA and/or induced AATase synthesis at low levels of oxygen and unsaturated fatty acids (Van Iersel et al. 1999; Shen et al. 2003b). The positive effect of limited oxygen supply on phenylethyl acetate production from orange pulp-containing medium using free cells of Vitilevure MT has been previously reported by Mantzouridou and Paraskevopoulou (2012). In particular, under limited oxygen supply, phenylethyl acetate showed increased maximum concentration of 7,441 μg/l. In the current study, lower value of the above compound can be explained by loss of precursor phenylalanine present in the raw material due to some degree of degradation during hydrolysis or amino acid transfer limitation within the alginate beads (Willaert and Nedovic 2006). Nevertheless, the value is within the range or even higher than those previous reported in literature with free cells of S. cerevisiae strains, e.g., 0 to 18,500 μg/l in grape and orange wines (Swiegers et al. 2005; Fan et al. 2009), 51.6 to 60.2 μg/l in fermented beverages (Díaz-Montaño et al. 2008), 250 μg/l in chemically defined media (Rojas et al. 2001) and, also, with immobilized yeast cells in grape must fermentation (200 μg/l) (Mallouchos et al. 2002).
Considering that ethyl ester synthesis by yeasts is dependent mainly on the availability of medium-chain fatty acids (MCFAs), a substrate rich in carbon such as orange peel hydrolysate will provoke the synthesis of MCFAs via the enhancement of fatty acid synthesis. The primary effect of oxygen limitation in the micro-environment of the beads is the resulting increase of the MCFA ethyl ester synthesis by encapsulated cells as this is expected to restrict unsaturated fatty acid synthesis causing long-chain saturated fatty acid accumulation and subsequently acetyl-CoA carboxylase inhibition. As a result, the intracellular pool of medium-chain fatty acyl-CoAs increases, which leads in increased MCFA ethyl ester synthesis (Saerens et al. 2010). In the case of ethyl octanoate and decanoate, their formation is related to the detoxifying mechanism for their acids that act as inhibitors for the cell growth (Legras et al. 2010). Overproduction of ethyl dodecanoate could also be attributed to the above mechanism.
In general, a trend of flavour molecules to be accumulated preferentially inside the micro-beads was observed. More specifically, when the highest amounts of volatiles production was achieved, percentages of 63.26 %, 85.97 %, 76.15 %, 77.82 % and 81.0 % for ethyl hexanoate, octanoate, decanoate, dodecanoate and phenyl ethyl acetate, respectively, were detected inside the beads in comparison to those outside the beads (Figs. 3 and 4a–d). Based on the above findings it seems that smaller molecules with higher volatility and hydrophilicity are mostly released from the beads (i.e., the case of ethyl hexanoate). Also, the increased concentration of the larger molecules with lower volatility and hydrophilicity stimulates their release (i.e., the case of ethyl dodecanoate). Limonene and α-terpineol were also detected inside the beads at levels of 18.1 and 3.7 mg/l, respectively, whereas no HMF was found after 72 h of fermentation.
These results highlight the potential for in situ production of formulations containing a mixture of flavour-active compounds. These aroma-containing beads could be employed as flavour additives or enhancers in various food preparations, such as bakery products, dairy products, etc., accompanied by prolonged release in the food matrix during storage and/or consumption. Moreover, their easy handling and storage ability along with a reasonable cost of natural aroma obtainment are some of the major expected benefits from this approach.
The economic feasibility of the process is strengthened by the efficient use of the biocatalyst during repeated batch fermentation of hydrolysate for bioflavour production. In all cases except for ethyl hexanoate, the cumulative amount of esters produced in the liquid medium after six consecutive cycles of the total period of 240 h exceeded the respective values produced by encapsulated yeast cells in batch fermentation. In particular, the accumulation of phenylethyl acetate (2,624 μg/l) at the end of the 6th cycle was slightly higher than the maximum total production in single-batch mode (2,585 μg/l) (Figs. 3 and 8). Also, by the repeated batch fermentation, ethyl decanoate (2,998 μg/l) and ethyl dodecanoate (6,939 μg/l) surpassed the maximum total production in single batch mode by 19.3 % and 36.5 %, respectively (Figs. 4c,d and 8). In the case of ethyl octanoate, a significant built up in the liquid medium accounted for a 76.5 % increase compared to the respective value by the single batch fermentation (7,025 vs. 1,654 μg/l in single batch) (Figs. 4b and 8). The strong release of the above compounds into the liquid medium, especially after the sixth fermentation cycle, could be attributed to the degradation of the micro-beads (Rakin et al. 2009). To fully benefit from this technology in the direction of the in situ production of food-grade formulations containing bioflavour, improvement of the stability of alginate micro-capsules in continuous fermentation system should be considered in future research.
Cumulative ester production in the liquid medium by S. cerevisiae immobilized cells in orange peel hydrolysate during repeated batch fermentation. Ethyl hexanoate (red filled diamond; all symbols are connected by line), ethyl octanoate (violet filled square), ethyl decanoate (ornage filled triangle), phenylethyl acetate (yellow multiplication symbol) and ethyl dodecanoate (violet multiplication symbol)
References
Alriksson B (2006) Ethanol from lignocellulose: alkali detoxification of dilute-acid spruce hydrolysates. Licentiate thesis, Faculty of Technology and Science Biochemistry. Karlstad University Studies, Sweden
Belletti N, Ndagijimana M, Sisto C, Guerzoni ME, Lanciotti R, Gardini F (2004) Evaluation of the antimicrobial activity of citrus essences on Saccharomyces cerevisiae. J Agric Food Chem 52:6932–6938
Díaz-Montaño DM, Délia ML, Estarrón-Espinosa M, Strehaiano P (2008) Fermentative capability and aroma compound production by yeast strains isolated from Agave tequilana Weber juice. Enzyme Microb Technol 42:608–616
Dubal SA, Tilkari YP, Momin SA, Borkar IV (2008) Biotechnological routes in flavour industries. Adv Biotechnol 3:20–30
EU Regulation No 1334 (2008) Official J EU 354/34
Fan G, Lu W, Yao X, Zhang Y, Wang K, Pan S (2009) Effect of fermentation on free and bound volatile compounds of orange juice. Flav Fragrance J 24:219–225
Furukawa K, Yamada T, Mizoguchi H, Hara S (2003) Increased ethyl caproate production by inositol limitation in Saccharomyces cerevisiae. J Biosci Bioeng 95:448–454
Kaur P, Kunze G, Satyanarayana T (2007) Yeast phytases: present scenario and future perspectives. Crit Rev Biotechnol 27:93–109
King A, Dickinson JR (2000) Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast 16:499–506
Legras JL, Erny C, Le Jeune C, Lollie M, Adolphe Y, Demuyter C, Delobel P, Blondin B, Karst F (2010) Activation of two different resistance mechanisms in Saccharomyces cerevisiae upon exposure to octanoic and decanoic acids. Appl Environ Microbiol 76:7526–7535
Liu J, Zhu Y, Du G, Zhou J, Chen J (2013) Response of Saccharomyces cerevisiae to d-limonene-induced oxidative stress. Appl Microbiol Biotechnol. doi:10.1007/s00253-013-4931-9
Lohrasbi M, Pourbafrani M, Niklasson C, Taherzadeh MJ (2010) Process design and economic analysis of a citrus waste biorefinery with biofuels and limonene as products. Bioresour Technol 101:7382–7388
Longo MA, Sanromán MA (2006) Production of food aroma compounds: microbial and enzymatic methodologies. Food Technol Biotechnol 44:335–353
Luttrell B (1993) The biological relevance of the binding of calcium ions by inositol phosphates. J Biol Chem 268:1521–1524
Mallouchos A, Komaitis M, Koutinas A, Kanellaki M (2002) Investigation of volatiles evolution during the alcoholic fermentation of grape must using free and immobilized cells with the help of solid phase miroextraction (SPME) headspace sampling. J Agric Food Chem 50:3840–3848
Manojlovic V, Djonlagic J, Obradovic B, Nedovic V, Bugarski B (2006) Investigations of cell immobilization in alginate: rheological and electrostatic extrusion studies. J Chem Technol and Biotechnol 81:505–510
Mantzouridou F, Paraskevopoulou A (2012) Volatile bio-ester production from orange pulp-containing medium using Saccharomyces cerevisiae. Food Bioprocess Technol. doi:10.1007/s11947-012-1009-0
Mantzouridou F, Tsimidou MZ, Roukas T (2006) Performance of crude olive pomace oil and soybean oil during carotenoid production by Blakeslea trispora in submerged fermentation. J Agric Food Chem 54:2575–2581
Margaritis A, Kilonzo PM (2005) Production of ethanol using immobilised cell bioreactor systems. In: Nedović V, Willaert R (eds) Applications of cell immobilisation biotechnology. Springer, Netherlands, pp 375–405
Mittal A, Singh G, Goyal V, Yadav A, Aggarwal NK (2012) Production of phytase by acido-thermophilic strain of Klebsiella sp. DB-3FJ711774.1 using orange peel flour under submerged fermentation. Innov Rom Food Biotechnol 10:18–27
Natural colors and flavors market by types, applications and geography: global trends and forecasts, 2011–2016. Available at: www.marketsandmarkets.com/Market-Reports/natural-colors-flavors-market-676.html. Accessed May 2012
Nedovic V, Obradovic B, Leskošek-Cukalovic I, Trifunovic O, Pešic R, Bugarski B (2001) Electrostatic generation of alginate microbeads loaded with brewing yeast. Process Biochem 37:17–22
Nedovic VA, Manojlovic V, Bugarski B, Willaert R (2010) State of the art in immobilized/encapsulated cell technology in fermentation processes. In: Encapsulation technologies for active food Ingredients and food processing. Springer, London, pp 119–146
Oberoi HS, Vadlani PV, Madl RL, Saida L, Abeykoon JP (2010) Ethanol production from orange peels: two-stage hydrolysis and fermentation studies using optimized parameters through experimental design. J Agric Food Chem 58:3422–3429
Perkin WH Jr (1904) Experiments on the synthesis of the terpenes: Part I. Synthesis of terpin, inactive terpineol, and diterpene. J Chem Soc 85:656–671
Pourbafrani M, Talebnia F, Niklasson C, Taherzadeh MJ (2007) Protective effect of encapsulation in fermentation of limonene-contained media and orange peel hydrolyzate. Int J Mol Sci 8:777–787
Purwadi R, Taherzadeh MJ (2008) The performance of serial bioreactors in rapid continuous production of ethanol from dilute-acid hydrolyzates using immobilized cells. Biores Technol 99:2226–2233
Rakin M, Mojovic L, Nikolic S, Vukasinovic M, Nedovic V (2009) Bioethanol production by immobilized Sacharomyces cerevisiae var. ellipsoideus cells. Afr J Biotechnol 8:464–471
Rojas V, Gil JV, Piñaga F, Manzanares P (2001) Studies on acetate ester production by non-Saccharomyces wine yeasts. Int J Food Microbiol 70:283–289
Rossi SC, Vandenberghe LPS, Pereira BMP, Gago FD, Rizzolo JA, Pandey A (2009) Improving fruity aroma production by fungi in SSF using citric pulp. Food Res Int 42:484–486
Saerens SM, Delvaux F, Verstrepen KJ, Van Dijck P, Thevelein JM, Delvaux FR (2008) Parameters affecting ethyl ester production by Saccharomyces cerevisiae during fermentation. Appl Environ Microbiol 74:454–461
Saerens SM, Delvaux FR, Verstrepen KJ, Thevelein JM (2010) Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Technol 3:165–177
Schrader J (2007) Microbial flavour production. In: Berger RG (ed) Flavours and fragrances – chemistry, bioprocessing and sustainability. Springer, Heidelberg, pp 507–574
Shen HY, Moonjai N, Verstrepen KJ, Delvaux F, Delvaux FR (2003a) Immobilization of Saccharomyces cerevisiae induces changes in the gene expression levels of HSP12, SSA3 and ATF1 during beer fermentation. J Am Soc Brew Chem 61:175–181
Shen HY, Moonjai N, Verstrepen KJ, Delvaux F, Delvaux FR (2003b) Impact of attachment immobilization on yeast physiology and fermentation performance. J Am Soc Brew Chem 61:79–87
Swiegers JH, Bartowsky EJ, Henschke PA, Pretorius IS (2005) Yeast and bacterial modulation of wine aroma and flavour. Aust J Grape Wine Res 11:139–173
Taherzadeh MJ, Karimi K (2007) Acid-based hydrolysis processes for ethanol from lignocellulosic materials: a review. Biogeosciences 2:472–499
Talebnia F, Taherzadeh MJ (2006) In situ detoxification and continuous cultivation of dilute-acid hydrolyzate to ethanol by encapsulated S. cerevisiae. J Biotechnol 125:377–384
Tataridis P, Ntagas P, Voulgaris I, Nerantzis ET (2005) Production of sparkling wine with immobilized yeast fermentation. Electron J Sci Technol 1:1–21
Van Iersel MFM, Brouwer PE, Rombouts FM, Abee T (1999) Influence of yeast immobilization on fermentation and aldehyde reduction during the production of alcohol free beer. Enzyme Microb Tech 26:602–607
Vilela A, Schuller D, Mendes-Faia A, Côrte-Real M (2013) Reduction of volatile acidity of acidic wines by immobilized Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol 97:4991–5000
Westman JO, Ylitervo P, Franzén CJ, Taherzadeh MJ (2012) Effects of encapsulation of microorganisms on product formation during microbial fermentations. Appl Microbiol Biotechnol 96:1441–1454
Wilkins MR, Widmer WW, Grohmann KL (2007) Simultaneous saccharification and fermentation of citrus peel waste by Saccharomyces cerevisiae to produce ethanol. Process Biochem 42:1614–1619
Willaert R, Nedovic VA (2006) Primary beer fermentation by immobilised yeast—a review on flavour formation and control strategies. J Chem Technol Biotechnol 81:1353–1367
Acknowledgements
This study was supported by BIOFLAVOUR, COST Action FA0907 (www.bioflavour.insa-toulouse.fr).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 21 kb)
Rights and permissions
About this article
Cite this article
Lalou, S., Mantzouridou, F., Paraskevopoulou, A. et al. Bioflavour production from orange peel hydrolysate using immobilized Saccharomyces cerevisiae . Appl Microbiol Biotechnol 97, 9397–9407 (2013). https://doi.org/10.1007/s00253-013-5181-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5181-6