Abstract
The release of products from microbial cells is an essential process for industrial scale production of bio-based chemicals. However, traditional methods of cell lysis, e.g., mechanical disruption, chemical solvent extraction, and immobilized enzyme degradation, account for a large share of the total production cost. Thus, an efficient cell lysis system is required to lower the cost. This review has focused on our current knowledge of two cell lysis systems, bacteriophage holin–endolysin system, and lipid enzyme hydrolysis system. These systems are controlled by conditionally inducible regulatory apparatus and applied in microbial production of fatty acids and polyhydroxyalkanoates. Moreover, toxin–antitoxin system is also suggested as alternative for its potential applications in cell lysis. Compared with traditional methods of cell disruption, the inducible cell lysis systems are more economically feasible and easier to control and show a promising perspective in industrial production of bio-based chemicals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Metabolic pathway engineering has driven the development of commercially viable processes for production of bio-based chemicals (Millard et al. 1996). So far, a variety of bio-based chemicals including aromatic compounds, carbohydrates, organic acids, alcohols, and other secondary metabolites has been produced by microbial fermentation. Of all these bio-products, some are easy to be released from the host cells, such as amino acids (Berry 1996; Ikeda and Katsumata 1999; Ito et al. 1990), succinic acid (Millard et al. 1996), lactic acid (Zeikus 1980), ethanol (Moniruzzaman and Ingram 1998), glycerol (Albertyn et al. 1994; Ben-Amotz and Avron 1979; Steinbüchel and Müller 1986), and 1,3-propanediol (Biebl et al. 1992). However, others like fatty acids and polyhydroxyalkanoates (PHAs) cannot be released from the cell.
Fatty acids are important precursors for biodiesel synthesis and can be converted to biodiesel by esterification with methanol or ethanol. For fatty acid production on a large scale, the most efficient way is to cultivate and extract fatty acids from oil-rich cyanobacteria and microalgae which can directly convert solar energy and CO2 into fuels (Peralta-Yahya and Keasling 2010). PHAs are a family of structurally diverse polyesters accumulated by many bacteria and can be applied in the fields of bioplastics, fine chemicals, implant biomaterials, medicines, and biofuels (Chen 2009). To extract fatty acids and PHAs from their hosts, cell disruption is often a necessary step (Mendes-Pinto et al. 2001). The mechanical methods for cell disruption, like high-speed agitator bead mill and high-pressure homogenizers, are popular for industrial production due to their convenience without chemical interaction between cells and media (Zhang et al. 1999). Ultrasonication is one of the favorite physical methods employed for cell disruption especially at a laboratory scale (Feliu et al. 1998; Ho et al. 2006). It can lyse a wide range of bacteria cells according to the power of acoustic waves and the physical strength of the cell walls (Choonia and Lele 2011). Iida et al. (2008) combined ultrasonication and mechanical homogenization for baker's yeast cell disruption and obtained a better protein release than either of them. Of all these physical methods, grinding in liquid nitrogen is the quickest method that only needs 2 min to process 500 ml microalgae cells for the lipids release (Zheng et al. 2011). There are also some other physical methods of cell disruption such as bead-beater pulsed electric field and laser treatment in oil extraction from microalgae (McMillan et al. 2013; Sheng et al. 2011). However, low energy efficiency owing to the dissipation by generating high temperature is the most obvious disadvantage of these methods.
Chemical methods including organic solvent extraction, alkali or detergent treatment of cells, also have been widely applied for intracellular substances extraction (Numanoğlu and Sungur 2004). For instance, chloroform was usually used to extract PHAs from dry biomass. Combined with acetone pretreatment and ethanol precipitation, chloroform extraction recovered poly(β-hydroxybutyrate) (PHB) with a purity of 95 % from lyophilized Cupriavidus necator cells (Ramsay et al. 1994). For large-scale production of poly(3-hydroxyoctanoate) (PHO), the precipitation solvent containing 70 % (v/v) methanol and ethanol with a ratio of 1:1 was employed and led to 94 % recovery and 99 % purity of PHO (Elbahloul and Steinbuchel 2009). Organic solvent extraction is also used to isolate lipids from cyanobacteria and microalgae; furthermore, cell membrane electroporation can improve the penetration of organic solvent through cell barrier and extracting efficiency (Hamilton and Sale 1967; Weaver and Chizmadzhev 1996). Besides mechanical and chemical methods, enzyme digestion is another useful way to disrupt the bacterial cells, but it is rather expensive when dealing with large amount of samples (Fu et al. 2010).
The product recovery from bacterial cells using traditional methods has been claimed to contribute to 20–30 % of the total production cost (Gudin and Thepenier 1986). Compared with bacteria, the cost of microalgae downstream processes is even higher and accounts for 70–80 % of the total production cost because the cell walls containing hemicellulose and saccharides are much thicker than that of bacteria (Molina Grima et al. 2003). To cut down the production cost, strains secreting the fermentation products automatically were designed and constructed by genetic modification. When tesA gene encoding thioesterase from Escherichia coli was introduced to Synechocystis sp. PCC 60803, the cellular acyl-acyl carrier proteins (ACPs) were converted into free fatty acids, relieving the inhibition of fatty acid synthesis by long-chain acyl-ACP and resulting in overproduction of cellular free fatty acids which promoted the fatty acid secretion to culture medium (Liu et al. 2011b; Fell 1997). Furthermore, weakened cell walls had a synergistic effect on fatty acid secretion (Liu et al. 2011b). In addition, the production of extracellular fatty acids had also been achieved in E. coli by TesA overexpression and deleting fadL gene to inhibit the β-oxidation pathway or re-absorbance of extracellular fatty acids (Liu et al. 2012). But the fatty acid-secreting strains still had some disadvantages, such as low growth rate and increased cell fragility which even caused cell damage at low cell density (Liu et al. 2011b). Simplified PHA extraction process based on osmotic lysis in the presence of alkali/detergent was performed using a novel moderately halophilic bacteria strain Halomonas sp. SK5 which could grow and accumulate PHA granules only in high-salinity environment (Rathi et al. 2013). In the presence of distilled water, the osmotic pressure difference between inside and outside cell will cause cell rupture and PHA release. This method resulted in approximately 90–100 % recovery of PHA with purity as high as 90 %; however, the use of high-salinity medium and alkali/detergent brought some economic and environmental issues. Thus, current work has focused on developing economically feasible mechanisms to release valuable bio-products by lysing host cells in a genetically-regulated manner.
Up to now, two strategies using bacteriophage holin–endolysin and lipid enzyme, respectively, have been tested for controllable cell lysis. The cell lysis systems were controlled by conditionally inducible regulatory apparatus and could be expressed with the presence of specific inducer or environmental condition, such as isopropyl β-d-thiogalactoside (IPTG), arabinose, xylose, and CO2 depletion. Moreover, toxin–antitoxin system is also suggested as alternative for its potential applications in cell lysis.
Holin–endolysin system
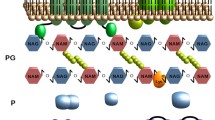
The lysis process of E. coli phage λ has been regarded as the model to study the holin–endolysin lysis system (Fig. 1) (São-José et al. 2007). When separated from antiholin which has an inhibitory effect on the function of holin (Young 2002), the hydrophobic domain of holin monomer will insert into cell membrane and then oligomerize to form higher-order assembled holins (Grundling et al. 2000), developing into a hole large enough for about 500-kDa proteins to get though the cell membrane (Savva et al. 2008; Wang et al. 2003). Then accumulated endolysin in cytoplasm can be released into the periplasm, degrading the peptidoglycan in the cell wall (Loessner 2005). Four enzyme activities are associated with the endolysin: the glucosaminidase hydrolyzing the glycosyl–oxygen bond (Drulis-Kawa et al. 2012), the transglycosylase attacking the same bond but forming a muramic acid product (Blackburn and Clarke 2000), the amidase hydrolyzing the amide bond in the oligopeptide cross-linking chains (Low et al. 2005), and the endopeptidase attacking the cross-linking peptide bonds (Donovan et al. 2006). In addition, Rz/Rz1 complex of phage λ can promote the fusion of inner membrane (IM) and outer membrane (OM), thus pushing the OM away from the murein layer and eliminating the last barrier (Young 1992; Berry et al. 2010; Berry et al. 2008).
The cell lysis process of phage λ holin–endolysin system. 1 Transcription: the genes of holin, endolysin, and Rz/Rz1 transcribe into corresponding messenger RNA (mRNA); 2 translation: the holin and antiholin is co-expression, forming holin–antiholin complex; 3 the antiholins are degraded by specific proteinase, and functional holins are released; 4 holins accumulate in the cell membrane, forming the hole for 500-kDa protein to get through; 5 the endolysin and Rz and Rz1 are released into periplasm and Rz and Rz1 are located to the IM and OM, respectively; 6 enzymolysis: the endolysin hydrolyzes the peptidoglycan, degrading the cell wall; and 7 the Rz and Rz1 proteins link the IM and OM, transmitting mechanical stress from IM and forcing the disruption of OM
To reduce the cost of PHB recovery from engineered E. coli strain, an autolysis system was developed using the lysis cassette of phage λ and the promoter of Salmonella typhimurium magnesium transporter gene mgtB (P mgtB ), which is activated only in the Mg2+-depletion condition (Zhang et al. 2009). During fermentation, magnesium sulfate was added into the culture medium to inhibit the expression of lysis genes and promote the PHB accumulation. When the cells were harvested and resuspended in water or low ionic strength buffer, the promoter P mgtB was activated and started the expression of phage λ lysis genes, resulting in immediate cell lysis (Zhang et al. 2009). Additionally, Resch et al. (1998) integrated the lysis gene E of bacteriophage ΦX174 downstream of the phage λ PR promoter into the plasmid pSH2, controlled by the thermosensitive repressor CI857 encoded by the same plasmid. The protein E can complement phage λ S and R dysfunction for host cell lysis (Roof and Young 1993) and can introduce a transmembrane tunnel in the cell envelope complex with a diameter of 40–200 nm (Witte and Lubitz 1989; Witte et al. 1990). When the growth temperature was upshifted from 28 to 42 °C, the gene E was expressed with inactivation of CI857 repressor, leading to the collapse of cell membrane and the release of PHB granules.
Inducible holin–endolysin lysis system to release PHA was also achieved in other hosts. For example, the holin and endolysin genes of Bacillus amyloliquefaciens phage were inserted into the amyE locus of chromosomal DNA of Bacillus megaterium, a PHB-producing strain (Hori et al. 2002). The expression of holin–endolysin system was controlled by the regulatory system P xylA -XylR, which is induced by xylose but inhibited by glucose (Rygus et al. 1991; Dahl et al. 1995). For PHB accumulation, the recombinant strain was grown in a medium containing glucose as carbon source in the presence of xylose. When the glucose in culture medium was exhausted, cell disruption was spontaneously induced, releasing intracellular PHB into culture broth (Hori et al. 2002). To construct a self-disruptive strain of medium-chain-length PHAs producer Pseudomonas putida KT2440, two strategies were applied simultaneously: the holin gene ejh and endolysin gene ejl from the pneumococcal bacteriophage EJ-1 (Díaz et al. 1996) integrated into the bacterial chromosome DNA and the tolB gene mutant exhibiting outer membrane integrity defect and lysis hypersensitivity. The expression of genes ejh and ejl was under the control of Pm-XylS monocopy expression system, and 3-methylbenzoate was used as inducer molecule to promote cell lysis (Martinez et al. 2011).
The holin–endolysin system was also used in microbial fatty acid production. A series of Synechocystis sp. PCC 6803 strains containing controllable lysis systems were designed and constructed in three strategies (Fig. 2) (Liu and Curtiss 2009). In strategy 1, the lysis genes from Salmonella phage P22 (13 15 19) and phage λ (S R Rz), respectively, were inserted into the chromosome of Synechocystis PCC 6803 downstream of the promoter P nrsB , which is activated by addition of Ni2+. The P22 lysis cassette could lyse the strains much faster after induction of Ni2+ than that of phage λ. In strategy 2, the P22 endolysin and the auxiliary lysis factor genes 19 and 15 were overexpressed under a constitutive promoter P psbAII , while expression of the holin gene 13 was regulated by P nrsB . Before Ni2+ induction, the lysis enzymes were accumulated in the cytosol. When Ni2+ was added into the culture, the holin protein was synthesized, thus helping the accumulated lysis enzymes to cross the cytoplasmic membrane and collapse the cell wall. As expected, strain SD123 constructed using strategy 2 exhibited a significantly faster lysis rate than strain SD121 in strategy 1. Strategy 3 was to incorporate the lysis genes from λ with P22 lysis genes. The endolysin and the auxiliary lysis factor genes, P22 19 15, and λ R Rz, were inserted downstream of the promoter P psbAII . At the same time, the holin genes, P22 13, and λ S, were controlled by the P nrsB promoter. The strategy 3 strain SD127 performed successful inducible cell lysis; however, its growth rate was slower than strain SD123. Due to the fact that all strains constructed using three strategies grew much slower than the wild-type strain, which might be caused by the basal transcription of P nrsB promoter before Ni2+ induction (López-Maury et al. 2002), further studies should focus on screening for a strain with both higher growth rate and faster lysis rate.
The strategies used in Green Recovery process of Synechocystis sp. PCC 6803. The lysis cassette (shaded area) was inserted downstream of P nrsB by two-step double crossover homologous exchanges, deleting the nrsB and nrsA fragments in the chromosome at the same time. nrsR and nrsS, nickel-sensing and -responding genes; nrsA, nrsB, nrsC, and nrsD, nickel-resistance genes; P psbAII, a constitutive promoter of Synechocystis gene psbAII; P nrsB , the nickel inducible promoter; 13 holin, 19 endolysin, and 15 auxiliary lysis enzyme are the cell lysis genes from Salmonella phage P22; S holin, R endolysin, and Rz auxiliary lysis enzyme are the lysis genes from E. coli phage λ; TT transcriptional terminator from cyanophage Pf-WMP4
Lipid enzyme system
As been well known, the cell membrane is generally comprised of proteins and membrane lipids, such as glycerides, phospholipids, and glycolipids, and the lipolytic enzymes can hydrolyze the carboxylic ester bonds to release fatty acids from acylglycerols. For example, galactolipase catalyzes the hydrolysis of galactolipids by removing one or two fatty acids (Helmsing 1969), and phospholipase B presenting both activities of phospholipase A1 and phospholipase A2 cleaves acyl chains from both the sn-1 and sn-2 positions of a phospholipid (Kohler et al. 2006). So cell lysis could be achieved using some proper lipid enzyme genes.
In cyanobacterium Synechocystis sp. PCC 6803 strain, Green Recovery strategy utilizing lipolytic enzymes was employed to convert membrane lipids into fatty acids and destroy the cell (Liu et al. 2011a). As CO2 is the raw material of photosynthesis, CO2-containing air was used to bubble the culture medium and the concentration of CO2 is controlled exactly. To induce the expression of lipid enzymes efficiently and economically after fatty acid accumulation, the promoter P cmp activated by CO2 limitation (McGinn et al. 2003) was selected to control the lipid enzyme genes. When lowering the CO2 concentration in the culture medium to near zero level by aeration with CO2-free air for 30 min, the lipolytic enzyme genes started to express, increasing cell membrane permeability and releasing fatty acids. Three lipolytic enzymes were tested for their ability to hydrolyze membrane lipids, including the bacterial lipase from Staphylococcus hyicus (Shl) (Rosenstein and Gotz 2000), the modified fungal phospholipase from Fusarium oxysporum (Fol) (Rapp 1995), and the guinea pig lipase (Gpl) from the digestive juice of guinea pig (Andersson et al. 1996). Although Gpl was reported to show the highest galactolipase activity (Andersson et al. 1996), three strains carrying various lipid enzymes produced fatty acids at a similar level, and the Gpl resulted in a membrane damage rate much lower than Shl and Fol (Liu et al. 2011a). Green Recovery strategy is clearly an efficient and effective method for lipid recovery from biomass; however, there is still a problem in this system. The lipase synthesis requires adequate light to provide the energy while the concentrated cultures create a self-shading environment, making that Green Recovery had to be applied on unconcentrated cultures, which lowers efficiency. To solve this problem, thermorecovery system was developed, in which genes encoding thermophilic lipases were inserted into Synechocystis chromosome under the control of both the CO2-depletion-inducible promoter P cmp and the constitutive promoter P trc (Liu and Curtiss 2012). During growth in an unconcentrated culture with plenty of light at ambient temperature, the thermophilic lipase was synthesized and accumulated due to the basal activity of promoter P trc without affecting cell growth. Then the CO2 limitation pretreatment boosted the synthesis of thermophilic lipase, which was activated by the subsequent temperature elevation of concentrated culture. In this study, the lipase Fnl from Fervidobacterium modosum Rt17-B1 (Yu et al. 2010) released the most fatty acids. Compared with Green Recovery, the biomass volume to be processed in thermorecovery was significantly reduced because of light-independence, resulting in the cost-decrease. It is expected that the efficiency of thermorecovery could be further improved by introducing additional thermophilic lipases with different substrate specificities to recover a greater fraction of the total membrane lipids.
Toxin–antitoxin system
For the possible applications in cell lysis, toxin–antitoxin system is suggested as an alternative to construct a self-disruption strain. Comparing with the holin–endolysin and lipid enzyme systems, toxin–antitoxin systems show a quite different mechanism of cell lysis, that can kill the host cells or cause apoptosis by inhibiting the replication of DNA, digesting mRNA, restraining the synthesis of proteins and so on (Chang et al. 1989; Sandvig and van Deurs 1992). In the cell death process, one key event is increasing of cell membrane permeability (Tsujimoto and Shimizu 2007), which will contribute to the release of intracellular substrates.
Genes encoding toxins and antitoxins are widespread in most prokaryotic chromosomes (Hayes 2003). The toxin–antitoxin system is generally comprised of two components: a comparable stable toxin protein targeting different cellular processes and inducing cell growth arrest or death and a labile antitoxin counteracting the action of toxin protein. The antitoxin protein is usually degraded by a specific intracellular protease such as Lon or ClpPA (Buts et al. 2005; Short et al. 2012; van Melderen et al. 1996). To construct an inducible cell lysis system, a conditionally inducible regulatory device is needed to prevent the expression of antitoxin or to elevate the toxin level in the cell.
Inducible regulatory apparatus
Due to the fact that the lysis systems above can cause growth inhibition or cell death, the expression of the cell lysis systems must be controlled by conditionally inducible regulatory apparatus strictly. We can choose the suitable promoter according to the different host cells and end products. Besides the conditionally inducible regulatory apparatus discussed above, some other regulatory devices were also used to control the cell lysis systems (Table 1). In cheese fermentation strain Lactococcus lactis, the promoter P nisA induced by antimicrobial peptide nisin, was employed to control the expression of the lytic genes lytA (lysin) and lytH (holin) from lactococcal bacteriophage ΦUS3. The nisin-induced cell lysis led to releasing of intracellular proteolytic and esterolytic enzymes, accelerating cheese ripening and contributing to the flavor development (de Ruyter et al. 1997). The regulatory systems having been used to control the cell lysis process also include PBAD-AraC system sensing l-arabinose (Guzman et al. 1995; Lim et al. 2012), P lac -LacI system responsible to lactose and its derivative IPTG (Lubitz et al. 1981; Henrich et al. 1982 ), and chloride-inducible system P gad -GadR (Sanders et al. 1997). In addition, some tight regulatory systems, like tetracycline-inducible system P tetA -TetR (Skerra 1994; Wirtz et al. 1999), are also the alternatives to control the cell lysis.
However, there are still some problems in the currently used regulatory devices. First, the addition of inducers is mandatory and brings some economic and environmental concerns. IPTG is relatively expensive and has toxic effect on cell growth (Baneyx 1999), and the use of nickel increases production cost and causes environmental pollution as well as the high-salinity medium to activate the promoter P gad . So the cell lysis systems need to be regulated economically and environment-friendly. In this issue, the CO2-limitation-inducible promoter in fatty acids recovery and the Mg2+-depletion sensing promoter in PHA production are the best choices for regulation of the cell lysis systems. Second, some chemical induction systems cause rapid accumulation of target proteins within a short period (van den Berg et al. 1999), resulting in the inclusion bodies without native biological activities. Therefore, the appropriate expression intensity is another criterion for selecting the regulatory apparatus.
Differently from that the cell lysis systems are controlled by inducible regulatory devices in most cases, automatic cell lysis also can be realized by downstream operation or chemical reagent. Yu et al. (2000) constitutively expressed the phage λ lysis genes with an S amber mutation (S - RRz) in a recombinant E. coli strain producing PHB. By introduction of the S amber mutation, the encoding of the S gene was unable to damage the cell membrane, and the endolysin R and auxiliary lysis factor Rz were synthesized and accumulated in the cytoplasm without restraining the growth of the host strain. At the end of fermentation, both EDTA and high-temperature treatment could cause the cell membrane damage, releasing R Rz to the periplasm and resulting in the cell wall degradation (Yu et al. 2000; Yu et al. 2003).
Conclusion
Conditionally inducible cell lysis system is an efficient and economical strategy to simplify the downstream purification and extraction process of some bio-based chemicals that fail to release from the cells. At present, the holin–endolysin system and the lipid enzyme system have been successfully used to release fatty acids and PHAs from their host cells. Another system, the toxin–antitoxin system, is discussed about its potential use in cell lysis. It is believed that more lysis systems with different mechanisms and suitable regulatory apparatus will be used in this field in the future. Currently, the usage of conditionally inducible lysis systems is still restricted on the laboratory pilot scale. The principle task is to exploit the applications of conditionally inducible cell lysis systems in industrial production of bio-based chemicals for cost reduction.
References
Albertyn J, Hohmann S, Thevelein JM, Prior BA (1994) GPD1, which encodes glycerol-3-phosphate dehydrogenase, is essential for growth under osmotic stress in Saccharomyces cerevisiae, and its expression is regulated by the high-osmolarity glycerol response pathway. Mol Cell Biol 14(6):4135–4144
Andersson L, Carrière F, Lowe ME, Nilsson Å, Verger R (1996) Pancreatic lipase-related protein 2 but not classical pancreatic lipase hydrolyzes galactolipids. BBA-Lipids Lipid Metab 1302(3):236–240
Baneyx F (1999) Recombinant protein expression in Escherichia coli. Curr Opin Biotechnol 10(5):411–421
Ben-Amotz A, Avron M (1979) Osmoregulation in the halophilic algae Dunaliella and Asteromonas. Basic Life Sci 14:91–99
van den Berg B, Ellis RJ, Dobson CM (1999) Effects of macromolecular crowding on protein folding and aggregation. EMBO J 18(24):6927–6933
Berry A (1996) Improving production of aromatic compounds in Escherichia coli by metabolic engineering. Trends Biotechnol 14(7):250–256
Berry J, Savva C, Holzenburg A, Young R (2010) The lambda spanin components Rz and Rz1 undergo tertiary and quaternary rearrangements upon complex formation. Protein Sci 19(10):1967–1977
Berry J, Summer EJ, Struck DK, Young R (2008) The final step in the phage infection cycle: the Rz and Rz1 lysis proteins link the inner and outer membranes. Mol Microbiol 70(2):341–351
Biebl H, Marten S, Hippe H, Deckwer W-D (1992) Glycerol conversion to 1, 3-propanediol by newly isolated clostridia. Appl Microbiol Biotechnol 36(5):592–597
Blackburn NT, Clarke AJ (2000) Assay for lytic transglycosylases: a family of peptidoglycan lyases. Anal Biochem 284(2):388–393
Buts L, Lah J, Dao-Thi MH, Wyns L, Loris R (2005) Toxin-antitoxin modules as bacterial metabolic stress managers. Trends Biochem Sci 30(12):672–679
Chang MP, Bramhall J, Graves S, Bonavida B, Wisnieski B (1989) Internucleosomal DNA cleavage precedes diphtheria toxin-induced cytolysis. Evidence that cell lysis is not a simple consequence of translation inhibition. J Biol Chem 264(26):15261–15267
Chen GQ (2009) A microbial polyhydroxyalkanoates (PHA) based bio- and materials industry. Chem Soc Rev 38:2434–2446
Choonia HS, Lele S (2011) β-Galactosidase release kinetics during ultrasonic disruption of Lactobacillus acidophilus isolated from fermented Eleusine coracana. Food Bioprod Process 89(4):288–293
Díaz E, Munthali M, Lünsdorf H, Höltje JV, Timmis KN (1996) The two-step lysis system of pneumococcal bacteriophage EJ-1 is functional in gram-negative bacteria: triggering of the major pneumococcal autolysin in Escherichia coli. Mol Microbiol 19(4):667–681
Dahl MK, Schmiedel D, Hillen W (1995) Glucose and glucose-6-phosphate interaction with Xyl repressor proteins from Bacillus spp. may contribute to regulation of xylose utilization. J Bacteriol 177(19):5467–5472
Donovan DM, Lardeo M, Foster–Frey J (2006) Lysis of staphylococcal mastitis pathogens by bacteriophage phi11 endolysin. FEMS Microbiol Lett 265(1):133–139
Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B, Delattre A-S, Lavigne R (2012) Learning from bacteriophages-advantages and limitations of phage and phage-encoded protein applications. Curr Protein Peptide Sci 13(8):699–722
Elbahloul Y, Steinbuchel A (2009) Large-scale production of poly(3-hydroxyoctanoic acid) by Pseudomonas putida GPo1 and a simplified downstream process. Appl Environ Microbiol 75(3):643–651
Feliu J, Cubarsi R, Villaverde A (1998) Optimized release of recombinant proteins by ultrasonication of E. coli cells. Biotechnol Bioeng 58(5):536–540
Fell D (1997) Understanding the control of metabolism. Portland Press, Portland
Fu CC, Hung TC, Chen JY, Su CH, Wu WT (2010) Hydrolysis of microalgae cell walls for production of reducing sugar and lipid extraction. Bioresour Technol 101(22):8750–8754
Grundling A, Blasi U, Young R (2000) Genetic and biochemical analysis of dimer and oligomer interactions of the lambda S holin. J Bacteriol 182(21):6082–6090
Gudin C, Thepenier C (1986) Bioconversion of solar energy into organic chemicals by microalgae. Adv Biotechnol Process 6:73–100
Guzman LM, Belin D, Carson MJ, Beckwith J (1995) Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol 177(14):4121–4130
Hamilton W, Sale A (1967) Effects of high electric fields on microorganisms: II Mechanism of action of the lethal effect. BBA-Gen Subjects 148(3):789–800
Hayes F (2003) Toxins-antitoxins: plasmid maintenance, programmed cell death, and cell cycle arrest. Science 301(5639):1496–1499
Helmsing P (1969) Purification and properties of galactolipase. BBA-Enzymol 178(3):519–533
Henrich B, Lubitz W, Plapp R (1982) Lysis of Escherichia coli by induction of cloned ΦX174 genes. Mol Gen Genet 185(3):493–497
Ho CW, Chew TK, Ling TC, Kamaruddin S, Tan WS, Tey BT (2006) Efficient mechanical cell disruption of Escherichia coli by an ultrasonicator and recovery of intracellular hepatitis B core antigen. Process Biochem 41(8):1829–1834
Hori K, Kaneko M, Tanji Y, Xing XH, Unno H (2002) Construction of self-disruptive Bacillus megaterium in response to substrate exhaustion for polyhydroxybutyrate production. Appl Microbiol Biotechnol 59(2–3):211–216
Iida Y, Tuziuti T, Yasui K, Kozuka T, Towata A (2008) Protein release from yeast cells as an evaluation method of physical effects in ultrasonic field. Ultrason Sonochem 15(6):995–1000
Ikeda M, Katsumata R (1999) Hyperproduction of tryptophan by Corynebacterium glutamicum with the modified pentose phosphate pathway. Appl Environ Microbiol 65(6):2497–2502
Ito H, Sato K, Enei H, Hirose Y (1990) Improvement in microbial production of L-tyrosine by gene dosage effect of aroL gene encoding shikimate kinase. Agric Biol Chem 54(3):823–824
Kohler GA, Brenot A, Haas-Stapleton E, Agabian N, Deva R, Nigam S (2006) Phospholipase A2 and phospholipase B activities in fungi. BBA-Mol Cell Biol 1761(11):1391–1399
López-Maury L, García-Domínguez M, Florencio FJ, Reyes JC (2002) A two-component signal transduction system involved in nickel sensing in the cyanobacterium Synechocystis sp. PCC 6803. Mol Microbiol 43(1):247–256
Lim JA, Shin H, Kang DH, Ryu S (2012) Characterization of endolysin from a Salmonella Typhimurium-infecting bacteriophage SPN1S. Res Microbiol 163(3):233–241
Liu H, Yu C, Feng D, Cheng T, Meng X, Liu W, Zou H, Xian M (2012) Production of extracellular fatty acid using engineered Escherichia coli. Microb Cell Fact 11(1):41–54
Liu X, Curtiss R III (2009) Nickel-inducible lysis system in Synechocystis sp. PCC 6803. Proc Natl Acad Sci USA 106(51):21550–21554
Liu X, Curtiss R III (2012) Thermorecovery of cyanobacterial fatty acids at elevated temperatures. J Biotechnol 161(4):445–449
Liu X, Fallon S, Sheng J, Curtiss R III (2011a) CO2-limitation-inducible green recovery of fatty acids from cyanobacterial biomass. Proc Natl Acad Sci USA 108(17):6905–6908
Liu X, Sheng J, Curtiss R III (2011b) Fatty acid production in genetically modified cyanobacteria. Proc Natl Acad Sci USA 108(17):6899–6904
Loessner MJ (2005) Bacteriophage endolysins-current state of research and applications. Curr Opin Microbiol 8(4):480–487
Low LY, Yang C, Perego M, Osterman A, Liddington RC (2005) Structure and lytic activity of a Bacillus anthracis prophage endolysin. J Biol Chem 280(42):35433–35439
Lubitz W, Schmid R, Plapp R (1981) Alterations of the cytoplasmic and outer membranes of Escherichia coli infected with bacteriophage ϕX174. Curr Microbiol 5(1):45–50
Martinez V, Garcia P, Garcia JL, Prieto MA (2011) Controlled autolysis facilitates the polyhydroxyalkanoate recovery in Pseudomonas putida KT2440. Microb Biotechnol 4(4):533–547
McGinn PJ, Price GD, Maleszka R, Badger MR (2003) Inorganic carbon limitation and light control the expression of transcripts related to the CO2-concentrating mechanism in the cyanobacterium Synechocystis sp. strain PCC6803. Plant Physiol 132(1):218–229
McMillan JR, Watson IA, Ali M, Jaafar W (2013) Evaluation and comparison of algal cell disruption methods: microwave, waterbath, blender, ultrasonic and laser treatment. Appl Energy 103:128–134
van Melderen L, Thi MHD, Lecchi P, Gottesman S, Couturier M, Maurizi MR (1996) ATP-dependent degradation of CcdA by Lon protease. J Biol Chem 271(44):27730–27738
Mendes-Pinto M, Raposo M, Bowen J, Young A, Morais R (2001) Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: effects on astaxanthin recovery and implications for bio-availability. J Appl Phycol 13(1):19–24
Millard CS, Chao Y-P, Liao JC, Donnelly MI (1996) Enhanced production of succinic acid by overexpression of phosphoenolpyruvate carboxylase in Escherichia coli. Appl Environ Microbiol 62(5):1808–1810
Molina Grima E, Belarbi EH, Acien Fernandez F, Robles Medina A, Chisti Y (2003) Recovery of microalgal biomass and metabolites: process options and economics. Biotechnol Adv 20(7–8):491–515
Moniruzzaman M, Ingram LO (1998) Ethanol production from dilute acid hydrolysate of rice hulls using genetically engineered Escherichia coli. Biotechnol Lett 20(10):943–947
Numanoğlu Y, Sungur S (2004) β-Galactosidase from Kluyveromyces lactis cell disruption and enzyme immobilization using a cellulose-gelatin carrier system. Process Biochem 39(6):705–711
Peralta-Yahya PP, Keasling JD (2010) Advanced biofuel production in microbes. J Biotechnol 5(2):147–162
Ramsay J, Berger E, Voyer R, Chavarie C, Ramsay B (1994) Extraction of poly-3-hydroxybutyrate using chlorinated solvents. Biotechnol Tech 8(8):589–594
Rapp P (1995) Production, regulation, and some properties of lipase activity from Fusarium oxysporum f. sp. vasinfectum. Enzyme Microb Technol 17(9):832–838
Rathi DN, Amir HG, Abed RM, Kosugi A, Arai T, Sulaiman O, Hashim R, Sudesh K (2013) Polyhydroxyalkanoate biosynthesis and simplified polymer recovery by a novel moderately halophilic bacterium isolated from hypersaline microbial mats. J Appl Microbiol 114(2):384–395
Resch S, Gruber K, Wanner G, Slater S, Dennis D, Lubitz W (1998) Aqueous release and purification of poly(β-hydroxybutyrate) from Escherichia coli. J Biotechnol 65(2):173–182
Roof WD, Young R (1993) ϕX174 E complements λ S and R dysfunction for host cell lysis. J Bacteriol 175(12):3909–3912
Rosenstein R, Gotz F (2000) Staphylococcal lipases: biochemical and molecular characterization. Biochimie 82(11):1005–1014
de Ruyter P, Kuipers OP, Meijer WC, de Vos WM (1997) Food-grade controlled lysis of Lactococcus lactis for accelerated cheese ripening. Nat Biotechnol 15(10):976–979
Rygus T, Scheler A, Allmansberger R, Hillen W (1991) Molecular cloning, structure, promoters and regulatory elements for transcription of the Bacillus megaterium encoded regulon for xylose utilization. Arch Microbiol 155(6):535–542
São-José C, Nascimento J, Parreira R, Santos MA, Macgrath S, van Sinderen D (2007) Release of progeny phages from infected cells. In: McGrath S, van Sinderen D (eds) Bacteriophages: Genetics and Molecular Biology. Caister Academic Press, Norfolk, pp 309–334
Sanders JW, Venema G, Kok J (1997) A chloride-inducible gene expression cassette and its use in induced lysis of Lactococcus lactis. Appl Environ Microbiol 63(12):4877–4882
Sandvig K, van Deurs B (1992) Toxin-induced cell lysis: protection by 3-methyladenine and cycloheximide. Exp Cell Res 200(2):253–262
Savva CG, Dewey JS, Deaton J, White RL, Struck DK, Holzenburg A, Young R (2008) The holin of bacteriophage lambda forms rings with large diameter. Mol Microbiol 69(4):784–793
Sheng J, Vannela R, Rittmann BE (2011) Evaluation of cell-disruption effects of pulsed-electric-field treatment of Synechocystis PCC 6803. Environ Sci Technol 45(8):3795–3802
Short FL, Blower TR, Salmond GPC (2012) A promiscuous antitoxin of bacteriophage T4 ensures successful viral replication. Mol Microbiol 83(4):665–668
Skerra A (1994) Use of the tetracycline promoter for the tightly regulated production of a murine antibody fragment in Escherichia coli. Gene 151(1–2):131–135
Steinbüchel A, Müller M (1986) Glycerol, a metabolic end product of Trichomonas vaginalis and Tritrichomonas foetus. Mol Biochem Parasitol 20(1):45–55
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12(5):835–840
Wang IN, Deaton J, Young R (2003) Sizing the holin lesion with an endolysin-β-galactosidase fusion. J Bacteriol 185(3):779–787
Weaver JC, Chizmadzhev YA (1996) Theory of electroporation: a review. Bioelectrochem Bioenerg 41(2):135–160
Wirtz E, Leal S, Ochatt C, Cross GAM (1999) A tightly regulated inducible expression system for conditional gene knock-outs and dominant-negative genetics in Trypanosoma brucei. Mol Biochem Parasitol 99(1):89–101
Witte A, Blasi U, Halfmann G, Szostak M, Wanner G, Lubitz W (1990) PhiX174 protein E-mediated lysis of Escherichia coli. Biochimie 72(2–3):191–200
Witte A, Lubitz W (1989) Biochemical characterization of PhiX174-protein-E-mediated lysis of Escherichia coli. Eur J Biochem 180(2):393–398
Young R (1992) Bacteriophage lysis: mechanism and regulation. Microbiol Rev 56(3):430–481
Young R (2002) Bacteriophage holins: deadly diversity. J Mol Microbiol Biotechnol 4(1):21–36
Yu H, Shi Y, Sun X, Luo H, Shen Z (2003) Effect of poly(β-hydroxybutyrate) accumulation on the stability of a recombinant plasmid in Escherichia coli. J Biosci Bioeng 96(2):179–183
Yu H, Yin J, Li H, Yang S, Shen Z (2000) Construction and selection of the novel recombinant Escherichia coli strain for poly(β-hydroxybutyrate) production. J Biosci Bioeng 89(4):307–311
Yu S, Yu S, Han W, Wang H, Zheng B, Feng Y (2010) A novel thermophilic lipase from Fervidobacterium nodosum Rt17-B1 representing a new subfamily of bacterial lipases. J Mol Catal B-Enzym 66(1–2):81–89
Zeikus J (1980) Chemical and fuel production by anaerobic bacteria. Annu Rev Microbiol 34(1):423–464
Zhang X, Pan Z, Fang Q, Zheng J, Hu M, Jiao X (2009) An auto-inducible Escherichia coli lysis system controlled by magnesium. J Microbiol Methods 79(2):199–204
Zhang Z, Blewett J, Thomas C (1999) Modelling the effect of osmolality on the bursting strength of yeast cells. J Biotechnol 71(1):17–24
Zheng H, Yin J, Gao Z, Huang H, Ji X, Dou C (2011) Disruption of Chlorella vulgaris cells for the release of biodiesel-producing lipids: a comparison of grinding, ultrasonication, bead milling, enzymatic lysis, and microwaves. Appl Biochem Biotechnol 164(7):1215–1224
Acknowledgment
This research was financially supported by 100-Talent Project of CAS (for GZ), Director Innovation Foundation of QIBEBT, CAS (Y112141105), National Natural Scientific Foundation of China (31200030), National Science and Technology Program (2012BAD32B06), and National 863 Project of China (SS2013AA050703-2).
Author information
Authors and Affiliations
Corresponding author
Additional information
Yongqiang Gao and Xinjun Feng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Gao, Y., Feng, X., Xian, M. et al. Inducible cell lysis systems in microbial production of bio-based chemicals. Appl Microbiol Biotechnol 97, 7121–7129 (2013). https://doi.org/10.1007/s00253-013-5100-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5100-x