Abstract
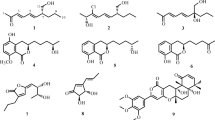
Two strains of endophytic fungi, Penicillium melinii Yuan-25 and Penicillium janthinellum Yuan-27, with strong anti-Pyricularia oryzae activity, were obtained from the roots of Panax ginseng. Based on bioactivity-oriented isolation, a new benzaldehyde derivative, ginsenocin (1), together with six known compounds, methyl 2,4-dihydroxy-3,5,6-trimethylbenzoate (2), 3,4,5-trimethyl-1,2-benzenediol (3), penicillic acid (4), mannitol (5), ergosterol (6), and ergosterol peroxide (7), were separated from the EtOAc extract of Yuan-25 culture, while brefeldin A (8) was isolated as the major constituent from the EtOAc extract of Yuan-27 culture. The chemical structures were determined based on spectroscopic methods. All the isolated compounds 1–8 were evaluated for their cytotoxicity against six human cancer cell lines. Brefeldin A (8) was the most cytotoxic constituent against all the tested cell lines with IC50 values <0.12 μg/ml, while ginsenocin (1) and penicillic acid (4) also exhibited potent cytotoxicity with IC50 values ranging from 0.49 to 7.46 μg/ml. Our results suggest that endophytic fungi isolated from P. ginseng are a promising natural source of potential anticancer agents.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Panax ginseng C.A. Meyer, belonging to the family Araliaceae, is a slow-growing perennial plant with fleshy roots and mainly distributed in the Northern Hemisphere, typically in cooler climates. Its roots, famous as ginseng, have been regarded as one of the most invaluable herbal remedies in Chinese traditional medicine since ancient times (Qi et al. 2011), commonly used as a health tonic with supposed benefits for adaptogenic, antiaging, prophylactic, and restorative remedies (Coon and Ernst 2002; Ernst 2010). As we know, active constituents found in ginseng include ginsenosides, polysaccharides, peptides, polyacetylenic alcohols, and fatty acids, which are responsible for the versatile pharmacological effects of ginseng on the cardiovascular, immune, and central nervous systems (Park et al. 2005). Recently, more and more studies have been conducted to evaluate the excellent anticancer activities of ginseng, revealing ginsenosides and polysaccharides to be the major active constituents (Shin et al. 2000; Park et al. 2005; Saw et al. 2010; Li et al. 2012a, b).
Endophytic fungi are microorganisms living within the tissues of host plants without causing visible disease symptoms at a particular time (Rodriguez et al. 2009). Since the discovery of the anticancer agent taxol from Taxomyces andreanae, an endophytic fungal strain isolated from Taxus brevifolia (Stierle et al. 1993), interest in bioactive compounds from plant-derived endophytes has increased considerably. Endophytes are, therefore, regarded as an emerging resource of biologically active secondary metabolites, attracting increasing attention in recent years (Schulz et al. 2002; Strobel 2003; Yu et al. 2010). They can produce various metabolites of different structure types including alkaloids, lignans, terpenoids, steroids, xanthones, coumarins, benzopyranones, cytochalasins, etc. (Schulz et al. 2002), which exhibited a variety of bioactivity such as anticancer, antifungal, antibacterial, and antiviral activities (Gunatilaka 2006).
In our preliminary research, an oil-producing endophyte characterized as Paecilomyces sp. was isolated from the roots of P. ginseng, which exhibited potent cytotoxic and antifungal properties. Further chemical analysis revealed that both of the ether extracts of Paecilomyces sp. and ginseng contained an interesting compound, falcarinol, a natural pesticide and anticancer agent (Xu et al. 2009). In continuation to our search for novel cytotoxic secondary metabolites from endophyte cultures (Xu et al. 2009; Sun et al. 2011; Zheng et al. 2012; Wu et al. 2012), our present study afforded two strains of endophytic fungi (Penicillium melinii Yuan-25 and Penicillium janthinellum Yuan-27) with high anti-Pyricularia oryzae activity from the roots of P. ginseng collected in Changchun, Jilin Province, People’s Republic of China. Through bioassay-oriented fractionation, a new benzaldehyde derivative, ginsenocin (1), together with six known compounds, methyl 2,4-dihydroxy-3,5,6-trimethylbenzoate (2), 3,4,5-trimethyl-1,2-benzenediol (3), penicillic acid (4), mannitol (5), ergosterol (6), and ergosterol peroxide (7), were separated from the EtOAc extract of Yuan-25 culture, while brefeldin A (8) was isolated as the major constituent from the EtOAc extract of Yuan-27 culture. We report herein the details of the isolation and identification of endophytes and compounds and the cytotoxicity evaluation for those isolated compounds.
Materials and methods
Isolation and identification of the endophytic fungus
Healthy root of P. ginseng was collected in Changchun, Jilin Province, People’s Republic of China. Samples were washed and cut into about 5-mm2 segments with surface sterilized by sequentially dipping into 5 % sodium hypochlorite (5 min) and 75 % ethanol (3 min), rinsed with sterile water, and finally allowed to surface-dry under sterile condition. The material was then deposited on a Petri dish containing potato dextrose agar (PDA) medium with 200 μg/ml ampicillin and 200 μg/ml streptomycin to inhibit bacterial contamination and then incubated at room temperature for 7–14 days. The hyphal tip was removed and placed on PDA media, incubated at 26 °C, and replated until a pure culture was obtained.
Two strains of endophytic fungi, Yuan-25 and Yuan-27, were identified according to its 5.8S gene and internal transcribed spacer (ITS; ITS1 and ITS2 regions) sequence by using the universal primers ITS5 and ITS4 according to the previous reported protocol (Guo et al. 2003). The 5.8S gene and ITS regions sequence was compared by BLAST search with reference sequences in GenBank and aligned with CLUSTAL_X software (Thompson et al. 1997) using 1,000 bootstrap replicates. The phylogenetic trees were performed using the neighbor-joining method (Saitou and Nei 1987). Identification at the species taxonomic level was based on ≥97 % ITS similarity (Higgins et al. 2007). The fungal strains, Yuan-25 and Yuan-27, were deposited in the China General Microbiological Culture Collection Center (CGMCC) as CGMCC 3.15173 and as CGMCC 3.15174, respectively.
Fermentation and compounds isolation
The endophytic fungi were initially grown on a PDA medium in Petri dishes and then transferred into shake flasks culture by punching out 5 mm of the agar plate culture using a self-designed cutter (Wang et al. 2006). The shake flask culture (250 ml) was carried out containing 100 ml potato dextrose broth medium at 26 °C, 180 rpm for 7 days and then transferred into 5-L Erlenmeyer flasks. About 60 L of fermentation liquid was obtained after incubation under the same condition for 10 days. The mycelial pellets were harvested by filtration and then thoroughly crushed in a mortar. The fermentation broths and ground mycelia were subjected to ultrasound-assisted extraction with equal volume of EtOAc at room temperature for 1 h. The extract solutions were then condensed in a rotating evaporator under reduced pressure. As a result, the EtOAc extract from strain Yuan-25 was obtained as EY-25 (2.5 g) and the EtOAc extract from strain Yuan-27 was obtained as EY-27 (1.0 g). EY-25 (2.5 g) was chromatographed on a silica gel column eluting with a step gradient of petroleum ether–EtOAc (20:1, 10:1, 5:1, 1:1, 1:2, each 1.0 L, v/v) to give five fractions (Fr1–Fr5). Fr2 (180 mg) was subjected on Sephadex LH-20 (MeOH/H2O, 4:1, v/v) followed by preparative thin layer chromatography (TLC) to yield compounds 1 (15 mg), 2 (8 mg), and 3 (10 mg). Fr3 (240 mg) was separated over silica gel with petroleum ether–EtOAc (4:1, 2:1, 1:1, each 0.5 L, v/v) to give three subfractions (subFr1, subFr2, and subFr3). SubFr1 (50 mg) was separated using preparative TLC (petroleum ether–EtOAc, 3:1, v/v) to obtain compound 4 (8 mg). SubFr3 (90 mg) was subjected on Sephadex LH-20 (MeOH/H2O, 4:1, v/v) to yield compounds 6 (6 mg) and 7 (4 mg). Fr4 (200 mg) was subjected on Sephadex LH-20 (MeOH/H2O, 1:1, 500 ml, v/v) to give compound 5 (5 mg). EY-27 (1.0 g) was separated by chromatography on Sephadex LH-20 (MeOH/H2O, 4:1, v/v) followed by repeated recrystallization in MeOH to afford compound 9 (180 mg).
Chemical analysis
ESIMS were measured on an Agilent LC/MSD Trap XCT mass spectrometer, whereas HRESIMS were measured using a Q-TOF micro mass spectrometer (Waters, Milford, MA, USA). Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance 400/500 NMR spectrometer with TMS as an internal standard. Infrared (IR) spectra were recorded on a Bruker Vector 22 spectrometer with KBr pellets. Optical rotations were acquired with a Perkin-Elmer 341 polarimeter. Materials for column chromatography were silica gel (100–200 mesh; Huiyou Silical Gel Development Co. Ltd., Yantai, China), silica gel H (10–40 μm; Yantai), Sephadex LH-20 (40–70 μm; Amersham Pharmacia Biotech AB, Uppsala, Sweden), and YMC-GEL ODS-A (50 μm; YMC, Milford, MA, USA). HSGF254 silica gel TLC plates (Yantai) were used for analytical TLC.
Anti-Pyricularia oryzae bioassay
The anti-P. oryzae activity was evaluated following a reported method (Xu et al. 2009). P. oryzae P-2b was obtained from the Department of Biochemistry and Molecular Biology, College of Basic Medical Sciences, Second Military Medical University and deposited in Agricultural Culture Collection of China (ACCC) as ACCC30320. Briefly, after the spore suspension of P. oryzae P-2b (4 × 104/ml) was added in a 96-well microplate, 50 μl of the sample was placed in each well and incubated at 27 °C for 15 h. The spore growth and mycelium shape were observed and compared with ketoconazole to determine the minimum inhibited concentration (MIC). Each test was done in three replicates and interpretation was based on the average value of the results.
Cytotoxic assay
The 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazoliumbromide (MTT) colorimetric assay, against six human cancer cell lines (MKN45, LOVO, A549, MDA-MB-435, HepG2, and HL-60) (available in the Chinese Academy of Sciences), was performed as described in the literature (Wang et al. 2012). In brief, the cells were cultured in 10 % FBS DMEM medium. Test samples were prepared at six concentrations (0.001, 0.01, 0.1, 1.0, 10.0, and 100.0 μg/ml). After these cell lines were seeded (4–6 × 104) in a 96-well microplate for 12 h, 10 μl of the sample was placed in each well and incubated at 37 °C for 72 h, and then 20 μl MTT (5 mg/ml; Sigma, New York, NY, USA) was added for 4 h. After removing the medium and putting 150 μl dimethyl sulfoxide into each well, the plate was shaken for 10 min to dissolve blue formazan crystals and the absorbance was read at a wavelength of 570 nm on a microplate reader (Labsystems, WellscanMR-2). The cytotoxicity of tested compounds against tumor cells was expressed as IC50 values, which were calculated by LOGIT method. Results are representative of three individual experiments.
Results
Identification of the endophytic fungus
The phylogenetic tree (Fig. 1) inferred from the ribosomal DNA ITS sequences indicated that the endophytic fungus Yuan-25 and Yuan-27 were classified into the clade including Penicillium allii AF218787, P. janthinellum AJ608945, Penicillium concentricum EU551202, Penicillium skrjabinii EU427287, Penicillium carneum DQ339566, and P. melinii AY373923. The endophytic fungus Y-25 closely related to P. melinii AY373923, with the ITS sequence similarity of 100 %, was, therefore, identified as P. melinii. Yuan-27 was closely related to P. janthinellum AJ608945, with the ITS sequence similarity of 100 % and, thus, identified as P. janthinellum.
Structural determination of the compounds
Compound 1, trivially named ginsenocin, was obtained as yellowish powder; [α] 20D +119.0° (c 0.2, MeOH); UV (MeOH) λ max 272 nm; IR (KBr) v max 3,384, 2,917, 1,630, 1,603, 1,249, 1,123, 1,054 cm−1. Its molecular formula was determined to be C15H16O4 by negative HRESIMS [M − H]− at m/z 259.0971 (calcd., 259.0970), indicating 8° of unsaturation, which was supported by its NMR data (Table 1). The IR spectrum exhibited the absorption bands for the hydroxyl (3,384 cm−1) and aldehyde (1,630, 1,603 cm−1) groups.
The 1H NMR spectrum of 1 indicated signals due to one aldehyde group (δ H 10.00, s; δ C 194.0), one tertiary methyl group (δ H 2.10, s; δ C 7.1), one secondary methyl group (δ H 1.30, d, J = 6.1 Hz; δ C 21.0), one methylene group (δ H 2.23 and 2.20; δ C 31.7), four olefinic protons at δ H 6.76 (s), 6.15 (d, J = 9.7 Hz), 6.00 (m), 5.59 (s) assigned to H-5, H-3′, H-4′, and H-7′, respectively, and an oxygenated methine multiplet at δ H 4.10 assigned to H-6′. The 13C NMR and distortionless enhancement by polarization transfer (DEPT) (Table 1) analysis revealed 15 carbon signals, including 1 methylene, 2 methyls, 6 methines, and 6 quaternary carbons, of which the typical signals were 1 each of an oxygenated trisubstituted olefinic group (δ C 152.3, C-2′; δ C 101.0, C-7′), disubstituted olefinic group (δ C 124.7, C-3′; δ C 128.0, C-4′), methylene adjacent to an olefinic group (δ C 31.7, C-5′), and oxygenated methine carbon (δ C 71.7, C-6′), which undoubtedly constitute a dihydro-2H-pyran-2-ylidene moiety. The remaining seven carbon signals in low field were suggested to constitute a tetrasubstituted benzaldehyde based on further 2D NMR analysis (Fig. 2). In more detail, heteronuclear multiple-bond correlations (HMBC) observed from the aldehyde proton (δ H 10.00, s) to C-1 (δ C 111.7) and C-2 (δ C 163.4) and from the methyl protons (δ H 2.10, s) to C-2 (δ C 163.4), C-3 (δ C 109.1), and C-4 (δ C 160.6) strongly supported a 2,4-dihydroxy-3-methylbenzaldehyde structure moiety in compound 1. HMBC cross-peaks from two olefinic protons H-3′ (δ H 6.15, d, J = 9.7 Hz) and H-4′ (δ H 6.00, m) to C-2′ (δ C 152.3) indicated that the 2H-pyran ring was 5,6-dihydrogenation. A secondary methyl was established to attach to C-6′ deduced from the HMBC correlations from the methyl protons (δ H 1.30, d, J = 6.1 Hz) to C-6′ (δ C 71.7) and C-5′ (δ C 31.7). The 1H–1H correlation spectroscopy (COSY) spectrum revealed the existence of fragment –CH3–CH–CH2–CH = CH–, from C-8′ through C-6′ to C-5′ and C-4′ (Fig. 3) in the pyran ring. Furthermore, key HMBC correlations from the olefinic proton H-7′ (δ H 5.59, s) to C-3′ (δ C 124.7), C-1 (δ C 111.7), and C-5 (δ C 108.6), as well as HMBC correlations from H-5 (δ H 6.76, s) to C-7′ (δ C 101.0), indicated the suggested linkage of the pyran ring and the benzene ring from C-2′ through C-7′ to C-6 in compound 1. In addition, the geometry of Δ2′(7′) was determined to be E on the basis of nuclear Overhauser effect spectroscopy (NOESY) correlations (Fig. 3) between H-5/H-3′ and H-5/H-7′, while the NOESY correlation between H-3′/H-7′ was absent. Comparison of the 13C NMR chemical shift of the chiral carbon adjacent to a secondary methyl group of 1 (C-6′ at δ C 71.7) and two reported 2-methyl-2H-pyran analogs, (2R,3S,6S)-3,6-dihydro-6-methoxy-2-methyl-2H-pyran-3-ol (C-2 at δ C 67.9) and (2S,3R,6R)-6-(benzyloxy)-3,6-dihydro-2-methyl-2H-pyran-3-ol (C-2 at δ C 70.1) (Guo and O’Doherty 2008), led us to assign the absolute configuration of C-6′ in 1 as S. Accordingly, the structure of compound 1 was, therefore, deduced to be 2,4-dihydroxy-3-methyl-6-[(2E,6S)-(6-methyl-5,6-dihydro-2H-pyran-2-ylidene)methyl]-benzaldehyde, as shown in Fig. 4.
Based on the NMR and MS data, compounds 2–8 were identified as, methyl 2,4-dihydroxy-3,5,6-trimethylbenzoate (Soman et al. 1999), 3,4,5-trimethyl-1,2-benzenediol (Han et al. 2009), penicillic acid (Kimura et al. 1996), mannitol (Kamoldinov et al. 2011), ergosterol (Li et al. 2012a, b), ergosterol peroxide (Wang 2012), and brefeldin A (Wang et al. 2002), respectively.
Anti-Pyricularia oryzae activity
The EtOAc extracts of P. melinii Yuan-25 (EY-25) and P. janthinellum Yuan-27 (EY-27) displayed strong inhibitory activity against P. oryzae with MIC values of 7.8 and 15.6 μg/ml, respectively (Table 2).
Cytotoxic activity
Brefeldin A (8) was the most cytotoxic constituent against the six tested cell lines with IC50 values <0.12 μg/ml, while ginsenocin (1) and penicillic acid (4) also exhibited potent cytotoxicity with IC50 values ranging from 0.49 to 7.46 μg/ml (Table 3). In addition, ergosterol (6) and ergosterol peroxide (7) showed moderate cytotoxic activity, while compounds 2, 3, and 5 exhibited very weak or no cytotoxicity.
Discussion
During the long period of coevolution, influenced by their host plants (Aly et al. 2010), some endophytes have the ability to produce identical or similar metabolites as their host plants, such as paclitaxel, podophyllotoxin, camptothecin, vinblastine, hypericin, and diosgenin (Zhao et al. 2011). In our previous study, an oil-producing Paecilomyces sp. endophyte, with potent cytotoxic and antifungal properties, was isolated from the roots of P. ginseng (Xu et al. 2009). Further chemical analysis revealed that Paecilomyces sp. shared an interesting constituent, falcarinol, a natural pesticide and anticancer agent, with its host ginseng. Therefore, in order to search for similar antitumor compounds as those of P. ginseng, the EtOAc crude extracts from the fermentation broths and ground mycelia of isolated 48 endophytic fungal taxa (data not shown) were first tested through P. oryzae bioassay, which is a good primary model for screening of antifungi and antitumor strains (Gunji et al. 1983). As a result, the crude extracts of P. melinii Yuan-25 and P. janthinellum Yuan-27 displayed strong inhibitory activity against P. oryzae, with MIC values of 7.8 and 15.6 μg/ml, respectively, which were, therefore, selected for the present phytochemical study on their cytotoxic metabolites.
In our study, we found that the endophytic fungus P. melinii Yuan-25 produced different classes of metabolites, while P. janthinellum Yuan-27 was a brefeldin A-producing endophyte. Compound 1 was a new member of benzaldehyde metabolites and it exhibited potent cytotoxicity with IC50 values ranging from 0.49 to 5.03 μg/ml (Table 3). Compound 2 was previously isolated from the fungus Mortierella vinacea (Soman et al. 1999) and it was isolated from Penicillium species for the first time. Though possessing similar benzene ring moiety to ginsenocin (1), compound 2 showed no or very weak toxicity to those cell lines at tested concentrations, indicating that the aldehyde group or the pyran ring enhanced the cytotoxicity of ginsenocin (1). Compound 3 was found as a common metabolite presence in several Penicillium endophyte cultures with antibacterial activity (Han et al. 2008, 2009). It showed very weak cytotoxicity in our assay. Penicillic acid (4) can be synthesized by a large number of Penicillium and Aspergillus fungi (Ciegler et al. 1971), which is a mycotoxin that can induce DNA single-strand breaks. In contrast to its carcinogenic potential, penicillic acid also possessed antitumor activity (Suzuki et al. 1971). Our study also validated the strong cytotoxicity of penicillic acid (IC50 values, 0.80–7.46 μg/ml). However, it has been proven to be too toxic for use in therapy attributed to its low LD50 of 100 mg/kg (subcutaneous) for mice (Ciegler et al. 1971). Mannitol (5) is a sugar alcohol widely used in the food and pharmaceutical industries because of its unique functional properties, which is found in a wide variety of natural products, including almost all plants and also several Penicillium strains and marine fungi (Hendriksen et al. 1988; Shawkat et al. 2012; Cui et al. 2008). It did not show any cytotoxicity towards the tested cancer cell lines. Ergosterol (6) is the principal sterol in fungi cell membrane, known as an important pharmaceutical intermediate for vitamin D2, cortisone, etc. (Jasinghe et al. 2005). Both ergosterol (6) and ergosterol peroxide (7) have been isolated from a variety of fungi, yeast, lichens, and sponges (Kuo et al. 2005, 2011). Previous study has already demonstrated the antitumor activity of ergosterol and ergosterol peroxide in vitro (Kwon et al. 2002). In the present work, they also exhibited moderate cytotoxic activity against all the tested cancer cell lines. Brefeldin A (8) was initially obtained from the culture medium of Penicillium brefeldianis (Kim and Kochevar 1995) and proved to be of great value as an inhibitor of protein trafficking in the endomembrane system of mammalian cells (Klausner et al. 1992). In our study, brefeldin A (8) was the most cytotoxic constituent against all the tested cell lines with IC50 values <0.12 μg/ml, equivalent to the positive control doxorubicin (Table 3). Brefeldin A is now primarily used in biological research to study protein transport and also recognized as a promising anticancer lead compound (Klausner et al. 1992; Sausville et al. 1996) with relatively low toxicity (LD50 >200 mg/kg for mice (intraperitoneal)) (Haerri 1963).
Our results indicate that ginsenocin (1) and brefeldin A (8) could be valuable candidates as potent tumor inhibitors and be beneficial in the therapy of cancer diseases. However, the anticancer properties of these compounds produced by endophytic fungi may be different from that of P. ginseng. The anticancer effect of P. ginseng was generally due to the immune function improvement by ginsenosides and ginseng polysaccharides (Shin et al. 2000; Park et al. 2005), whereas the cytotoxicity mainly contributes to the anticancer effect of these metabolites isolated from endophyte cultures. Our study, therefore, suggests that endophytes from P. ginseng are promising sources of natural bioactive and novel constituents, though without metabolites like ginsenosides.
References
Aly AH, Debbab A, Kjer J, Proksch P (2010) Fungal endophytes from higher plants: a prolific source of phytochemicals and other bioactive natural products. Fungal Diversity 41(1):1–16
Ciegler A, Detroy RW, Lillehoj EB (1971) Microbial toxins (chapter 6): patulin, penicillic acid, and other carcinogenic lactones. Academic, New York, p 417
Coon JT, Ernst E (2002) Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 25(5):323–244
Cui HB, Mei WL, Han Z, Wu J, Lin HP, Hong K, Dai HF (2008) Antibacterial metabolites from the fermentation broth of marine fungus 095407. Chin J Med Chem 18:131–134
Ernst E (2010) Panax ginseng: an overview of the clinical evidence. J Ginseng Res 34(4):259–263
Gunatilaka AAL (2006) Natural products from plant-associated microorganisms: distribution, structural diversity, bioactivity, and implications of their occurrence. J Nat Prod 69(3):509–526
Gunji S, Arima K, Beppu T (1983) Screening of antifungal antibiotics according to activities inducing morphological abnormalities. Agric Biol Chem 47(9):2061–2069
Guo H, O'Doherty GA (2008) De novo asymmetric synthesis of anthrax tetrasaccharide and related tetrasaccharide. J Org Chem 73(14):5211–5220
Guo LD, Huang GR, Wang Y, He WH, Zheng WH, Hyde KD (2003) Molecular identification of white morphotype strains of endophytic fungi from Pinus tabulaeformis. Mycol Res 107(Pt 6):680–688
Haerri E (1963) A fungal metabolite which is a macrocyclic lactone exhibiting a wide range of antibiotic activity produced by Penicillium brefeldianum Dodge. Helv Chim Acta 46:1235
Han Z, Mei WL, Cui HB, Zeng YB, Lin HP, Hong K, Dai HF (2008) Antibacterial constituents from the endophytic fungus Penicillium sp. of mangrove plant Cerbera manghas. Acta Chim Sinica 29:749–752
Han Z, Mei WL, Zhao YX, Deng YY, Dai HF (2009) A new cytotoxic isocoumarin from endophytic fungus Penicillium sp. 091402 of the mangrove plant Bruguiera sexangula. Chem Nat Comp 45:805–807
Hendriksen HV, Mathiasen TE, Adler-Nissen J, Frisvad JC, Emborg C (1988) Production of mannitol by Penicillium strains. J Chem Technol Bitechnol 43(3):223–228
Higgins KL, Arnold AE, Miadlikowska J, Sarvate SD, Lutzoni F (2007) Phylogenetic relationships, host affinity, and geographic structure of boreal and arctic endophytes from three major plant lineages. Mol Phylogenet Evol 42(2):543–555
Jasinghe VJ, Perera CO, Barlow PJ (2005) Bioavailability of vitamin D2 from irradiated mushrooms: an in vivo study. Brit J Nutr 93(6):951–956
Kamoldinov KS, Eshbakova KA, Bobakulov KM, Abdullaev ND (2011) Components of Fraxinus raibocarpa. Chem Nat Comp 47(3):448–449
Kim HL, Kochevar J (1995) Isolation of brefeldin A. Gen Pharmacol 26(2):363–364
Kimura Y, Nakahara S, Fujioka S (1996) Aspyrone, a nematicidal compound isolated from the fungus, Aspergillus melleus. Biosci Biotechnol Biochem 60:1375–1376
Klausner RD, Donaldson JG, Lippincott-Schwartz J (1992) Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol 116(5):1071–1080
Kuo LM, Chen KY, Hwang SY, Chen JL, Liu YY, Liaw CC, Ye PH, Chou CJ, Shen CC, Kuo YH (2005) DNA topoisomerase I inhibitor, ergosterol peroxide from Penicillium oxalicum. Planta Med 71(1):77–79
Kuo CF, Hsieh CH, Lin WY (2011) Proteomic response of LAP-activated RAW 264.7 macrophages to the anti-inflammatory property of fungal ergosterol. Food Chem 126(1):207–212
Kwon HC, Zee SD, Cho SY, Choi SU, Lee KR (2002) Cytotoxic ergosterols from Paecilomyces sp. J300. Arch Pharm Res 25(6):851–855
Li C, Cai J, Geng J, Li Y, Wang Z, Li R (2012a) Purification, characterization and anticancer activity of a polysaccharide from Panax ginseng. Int J Biol Macromol 51(5):968–973
Li JT, Chen QQ, Zeng Y, Wang Q, Zhao PJ (2012b) A new phenol compound from endophytic Phomopsis sp. DC01. Nat Prod Res 26:2008–2012
Park JD, Rhee DK, Lee YH (2005) Biological activities and chemistry of saponins from Panax ginseng. Phytochem Rev 4:159–175
Qi LW, Wang CZ, Yuan CS (2011) Ginsenosides from American ginseng: chemical and pharmacological diversity. Phytochemistry 72(8):689–699
Rodriguez RJ, White JF Jr, Arnold AE, Redman RS (2009) Fungal endophytes: diversity and functional roles. New Phytol 182(2):314–330
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4(4):406–425
Sausville EA, Duncan KL, Senderowicz A, Plowman J, Randazzo PA, Kahn R, Malspeis L, Grever MR (1996) Antiproliferative effect in vitro and antitumor activity in vivo of brefeldin A. Cancer J Sci Am 2(1):52–58
Saw CLL, Wu Q, Kong ANT (2010) Anti-cancer and potential chemopreventive actions of ginseng by activating Nrf2 (NFE2L2) anti-oxidative stress/anti-inflammatory pathways. Chin Med 5:37–43
Schulz B, Boyle C, Draeger S, AK R m, Krohn K (2002) Endophytic fungi: a source of novel biologically active secondary metabolites. Mycol Res 106(9):996–1004
Shawkat H, Westwood MM, Mortimer A (2012) Mannitol: a review of its clinical uses. Contin Educ Anaesth Crit Care Pain 12(2):82–85
Shin HR, Kim JY, Yun TK, Morgan G, Vainio H (2000) The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control 11(6):565–576
Soman AG, Gloer JB, Wicklow DT (1999) Antifungal and antibacterial metabolites from a sclerotium-colonizing isolate of Mortierella vinacea. J Nat Prod 62:386–388
Stierle A, Strobel G, Stierle D (1993) Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 260(5105):214–216
Strobel GA (2003) Endophytes as sources of bioactive products. Microbes Infect 5:535–544
Sun PX, Zheng CJ, Li WC, Jin GL, Huang F, Qin LP (2011) Trichodermanin A, a novel diterpenoid from endophytic fungus culture. J Nat Med 65:381–384
Suzuki S, Kimura T, Saito F, Ando K (1971) Antitumor and antiviral properties of penicillic acid. Agr Biol Chem 35(2):287–290
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25(24):4876–4882
Wang FW (2012) Bioactive metabolites from Guignardia sp., an endophytic fungus residing in Undaria pinnatifida. Chin J Nat Med 10:72–76
Wang JF, Huang YJ, Fang MJ, Zhang YJ, Zheng ZH, Zhao YF, Su WJ (2002) Brefeldin A, a cytotoxin produced by Paecilomyces sp. and Aspergillus clavatus isolated from Taxus mairei and Torreya grandis. FEMS Immunol Med Microbiol 34:51–57
Wang J, Wu J, Huang W, Tan R (2006) Laccase production by Monotospora sp., an endophytic fungus in Cynodon dactylon. Bioresour Technol 97(5):786–789
Wang LW, Xu BG, Wang JY, Su ZZ, Lin FC, Zhang CL, Kubicek CP (2012) Bioactive metabolites from Phoma species, an endophytic fungus from the Chinese medicinal plant Arisaema erubescens. Appl Microbiol Biotechnol 93(3):1231–1239
Wu LS, Hu CL, Han T, Zheng CJ, Ma XQ, Rahmand K, Qin LP (2012) Cytotoxic metabolites from Perenniporia tephropora, an endophytic fungus from Taxus chinensis var. mairei. Appl Microbiol Biotechnol 97:305–315. doi:10.1007/s00253-012-4189-7
Xu LL, Han T, Wu JZ, Zhang QY, Zhang H, Huang BK, Rahman K, Qin LP (2009) Comparative research of chemical constituents, antifungal and antitumor properties of ether extracts of Panax ginseng and its endophytic fungus. Phytomedicine 16(6):609–616
Yu HS, Zhang L, Li L, Zheng CJ, Guo L, Li WC, Sun PX, Qin LP (2010) Recent developments and future prospects of antimicrobial metabolites produced by endophytes. Microbiol Res 165:437–449
Zhao J, Shan T, Mou Y, Zhou L (2011) Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev Med Chem 11(2):159–168
Zheng CJ, Li L, Han T, Qin LP (2012) Identification of a quinazoline alkaloid produced by Penicillium vinaceum, an endophytic fungus from Crocus sativus. Pharm Biol 50(2):129–133
Author information
Authors and Affiliations
Corresponding authors
Additional information
Cheng-Jian Zheng and Li-Li Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zheng, CJ., Xu, LL., Li, YY. et al. Cytotoxic metabolites from the cultures of endophytic fungi from Panax ginseng . Appl Microbiol Biotechnol 97, 7617–7625 (2013). https://doi.org/10.1007/s00253-013-5015-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5015-6