Abstract
Microbially synthesized fatty acids are an attractive platform for producing renewable alternatives to petrochemically derived transportation fuels and oleochemicals. Free fatty acids (FFA) are a direct precursor to many high-value compounds that can be made via biochemical and ex vivo catalytic pathways. To be competitive with current petrochemicals, flux through these pathways must be optimized to approach theoretical yields. Using a plasmid-free, FFA-producing strain of Escherichia coli, a set of chemostat experiments were conducted to gather data for FFA production under phosphate limitation. A prior study focused on carbon-limited conditions strongly implicated non-carbon limitations as a preferred media formulation for maximizing FFA yield. Here, additional data were collected to expand an established kinetic model of FFA production and identify targets for further metabolic engineering. The updated model was able to successfully predict the strain’s behavior and FFA production in a batch culture. The highest yield observed under phosphate-limiting conditions (0.1 g FFA/g glucose) was obtained at a dilution rate of 0.1 h−1, and the highest biomass-specific productivity (0.068 g FFA/gDCW/h) was observed at a dilution rate of 0.25 h−1. Phosphate limitation increased yield (∼45 %) and biomass-specific productivity (∼300 %) relative to carbon-limited cultivations using the same strain. FFA production under phosphate limitation also led to a cellular maintenance energy ∼400 % higher (0.28 g/gDCW/h) than that seen under carbon limitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial production of biofuels from renewable sugars is a promising strategy to supplement and eventually replace fossil fuels currently used in gasoline, diesel, and jet engines (Peralta-Yahya et al. 2012). Free fatty acids (FFA) are attractive precursors to energy dense compounds that can be made via biochemical and catalytic pathways (Lennen and Pfleger 2012). In addition to fuels, FFA can be used to produce oleochemicals that are currently derived from edible plant oils. In vivo, fatty acids can be converted to alkanes (Schirmer et al. 2010), olefins (Mendez-Perez et al. 2011; Rude et al. 2011), esters (Kalscheuer et al. 2006; Nawabi et al. 2011), fatty alcohols (Steen et al. 2010), polyhydroxyalkanoates (Agnew et al. 2012), and methyl ketones (Goh et al. 2012). Alternatively, catalytic processes can be used to make esters and alkanes ex vivo (James et al. 2012; Lennen et al. 2010). While many of these compounds can act as drop in replacements for current fuels and chemicals (Knothe 2010), yields need to approach theoretical levels in order for biologically produced products to become cost competitive with current petrochemical processes.
A central challenge to producing these chemicals is optimizing the flux of carbon through fatty acid metabolism to optimize product yields. Many groups have implemented metabolic engineering strategies to improve the titer and yield of FFA in Escherichia coli (reviewed in Lennen and Pfleger 2012). Most production strategies utilize a chain length-specific thioesterase to cleave desired intermediates in fatty acid biosynthesis. This strategy dramatically increases production of fatty acids over wild type and typically generate yields around 0.06 g fatty acid/g consumed carbon source (Steen et al. 2010; Lu et al. 2008; Zheng et al. 2012). Supplemental metabolic engineering strategies targeting central metabolism have been used to further increase fatty acid yield to 0.14 g fatty acid/g carbon source consumed (Ranganathan et al. 2012). The highest reported yield of fatty acids, 73 % of the theoretical limit for E. coli, was achieved by overexpressing FadR, a regulator of fatty acid metabolism, in a thioesterase expressing strain (Zhang et al. 2012). While these yields are promising, the maximum theoretical yield of fatty acids from glucose is achieved when the flux of carbon to biomass is minimized. The majority of FFA production studies have used carbon as the limiting nutrient in their media formulation, thereby limiting potential yields. To date, no study has examined nutrient limitation as a means to approach the theoretical limit of FFA yield in E. coli.
Advanced cultivation strategies such as fed batch are needed to achieve the optimal balance of cell growth and product synthesis. Design of these strategies is greatly facilitated by kinetic models of the producing organisms. In past work, a kinetic model of FFA production in E. coli under carbon limitation predicted increased yield, and biomass-specific productivity as media formulations moved toward non-carbon limitation (Youngquist et al. 2012). This prediction is consistent with efforts to use nitrogen starvation as a trigger of lipid accumulation in oleaginous microbes (Ratledge and Wynn 2002; Hassan et al. 1996). The effects of different essential elements limiting cellular E. coli growth has been well-studied with significant research on growth effects (Wanner and Egli 1990), differences in stationary phase (Peterson et al. 2005), and transcriptional effects (Ferenci 2008; Hua et al. 2004; Matsuoka and Shimizu 2011). Cultures of E. coli grown under nitrogen-limiting conditions exhibit decreased yield of biomass on glucose (Hua et al. 2004; Kumar and Shimizu 2011) and increased yield of desired products, such as poly-hydroxybutyrate (Johnson et al. 2010). E. coli cultivated under phosphate-limiting conditions also exhibit a decreased biomass yield on various carbon sources (Johansson et al. 2005; Marzan and Shimizu 2011). In contrast to nitrogen-limited cultures, the metabolic activity of these cells remains high in stationary phase, with a low level of protein oxidation (Ballesteros et al. 2001; Gerard et al. 1999). Metabolically active but non-growing cells are attractive for the production of chemicals as no initial substrate is directed to biomass formation and substrate uptake rates remains high. Thus, phosphate limitation has been used in E. coli to increase the production of shikimic acid (Johansson et al. 2005) and recombinant proteins (Huber et al. 2011). Additionally, use of phosphate limitation has led to increased production of vancomycin in Amycolatopsis orientalis (McIntyre et al. 1996) and green fluorescent protein in Hansenula polymorpha (Kottmeier et al. 2010). However, phosphate limitation has not been specifically studied in the production of FFA from E. coli. For these reasons, phosphate limitation is an attractive strategy for controlling E. coli growth while maintaining a high rate of glucose utilization and FFA synthesis.
Here, we studied the impact of cultivation variables (growth rate, nutrient limitation) on FFA production and biomass accumulation in a FFA producing E. coli. In shake flask experiments, cells cultivated in phosphate-limited media generated higher FFA titers and yields. Next, a series of chemostat experiments were performed to quantitatively model the impact of cultivation variables. The concentrations of FFA, biomass, CO2, residual glucose, and fermentation products in chemostat effluents were measured in order to close a carbon mass balance around the bioreactor. The data collected were used to update a kinetic model (Youngquist et al. 2012) to account for FFA production as a function of nutrient limitation and dilution rate and find conditions that maximize FFA yield and biomass-specific productivity. The model was used to accurately predict the biomass and FFA yield in batch cultivation.
Materials and methods
Bacterial strain
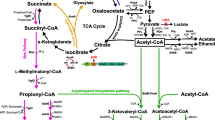
All experiments were performed using E. coli TY05 (Youngquist et al. 2012) which contains three chromosomal copies (replacing fadD, fadE, and fadAB) of an expression cassette for the acyl-ACP thioesterase (BTE) from Umbellularia californica under the control of the Ptrc promoter. When BTE expression is induced with isopropyl β-d-1-thiogalactopyranoside (IPTG), E. coli TY05 produces C12:0, C12:1, C14:0, and C14:1 FFA.
Chemicals, enzymes, and other materials
Enzymes were purchased from New England Biolabs (Ipswich, MA, USA). DNA purification materials were purchased from Qiagen (Venlo, the Netherlands). Chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA) or Fisher Scientific (Hampton, NH, USA) unless otherwise specified.
Batch shake flask studies
FFA production under different nutrient limitation conditions was determined by cultivating E. coli TY05 in 250 mL shake flasks containing 50 mL morpholinepropanesulfonic acid (MOPS) minimal medium (Neidhardt et al. 1974) supplemented with 1.0 % glucose, 1.32 mM dibasic potassium phosphate, 0.276 mM potassium sulfate, and 9.5 mM ammonium chloride with variations depending on nutrient limitation. For nitrogen limitation, the total amount of ammonium chloride in the media was 4.75 mM. To test carbon limitation, the medium was supplemented with 0.4 % glucose. Under phosphate limitation, the medium contained 0.240 mM phosphate. Media conditions for phosphate and nitrogen limitation were determined based on the original formulation of MOPS minimal media (Neidhardt et al. 1974) and are consistent with past phosphate limitation experiments in MOPS minimal media (Baek and Lee 2006; Gerard et al. 1999). Concentrations significantly under the C/P ratio limit of 120 moles of carbon (molC) per mole of phosphate (molP) were used throughout.
For all studies, single colonies from freezer stocks were inoculated into 5 mL of LB and incubated overnight at 37 °C with shaking. Shake flask production cultures were inoculated to an OD600 of 0.04 from the overnight cultures and incubated at 37 °C until each reached OD600 0.2, when IPTG (1 mM final concentration) was added to induce BTE. After 40 h of incubation at 37 °C, samples for fatty acid methyl-ester analysis, glucose consumption, and cell density were taken, processed, and quantified.
Chemostat studies
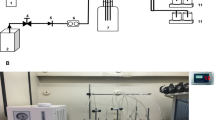
Chemostat experiments were performed in a 3-L stirred bioreactor (Applikon Biotechnology Inc, Schiedam, the Netherlands) using a 1.5-L working volume. Temperature, pH, airflow, dissolved oxygen, off-gas composition, liquid flow, feed and waste masses, and stirrer speed were controlled/recorded as described previously (Youngquist et al. 2012). Chemostat experiments were inoculated using the following propagation schedule. Cultures (5 mL) of TY05 grown in LB at 37 °C for 10 h were used to inoculate 50 mL of MOPS minimal media supplemented with 1.0 % glucose. The 50-mL culture was grown at 37 °C overnight and was used as the inoculum for the chemostat. For dilution rate experiments, modified MOPS minimal media (containing 370 μM phosphate) supplemented with 1.0 % glucose, 0.276 mM potassium sulfate, and 9.5 mM ammonium chloride were inoculated to an OD600 of 0.04. Induction with 1 mM IPTG occurred when the OD600 reached 0.2. The reactor was operated in batch mode for 9 h, at which point flow of fresh media was initiated. Outflow was controlled by maintaining a constant liquid level through the placement of the outlet line at a height corresponding to a 1.5-L working volume. The OD600 of the culture was monitored periodically (18 samples per 24 h) during batch and continuous operation. Steady-state determination and sampling procedures were performed as previously described (Youngquist et al. 2012). During the dilution rate variation experiments, two chemostat experiments were performed, with flow regimes tested of 0.10, 0.05, 0.3, 0.2 and 0.25, 0.075, 0.15, and 0.4 h−1, respectively. As such, growth rates ranging from 7 to 60 % of μ max were covered (μ max was found to be 0.69 h−1 under phosphate limitation).
For C/P ratio experiments, starter cultures followed the same propagation scheme as the dilution rate experiments. Once all samples were taken for a given C/P ratio, the sterile media feed was changed to MOPS minimal media supplemented with a different glucose or phosphate concentration while maintaining a constant dilution rate of 0.1 h−1. Two separate chemostat experiments were performed. The first C/P ratio experiment held the phosphate concentration in the media constant at 370 μM while testing glucose feed concentrations of 7, 4, 20, and 10 g/L, respectively. The second C/P ratio experiment held glucose concentrations constant at 1.0 % while varying feed phosphate concentrations of 370, 185, and 530 μM. These steady states correspond to C/P ratios of 1,800, 900, 630, and 360 molC/molP.
Batch bioreactor studies
Controlled batch cultivation experiments were performed using the same equipment as the chemostat experiments. Starter cultures were prepared using the same propagation scheme as the chemostat experiments. A 1.5-L working volume of MOPS minimal media (containing 370 μM phosphate) supplemented with 0.7 % glucose, 0.276 mM potassium sulfate, and 9.5 mM ammonium chloride was used. Cultures were induced with 1 mM IPTG at OD600 0.2. Fatty acid, cell density (OD600), and supernatant samples were taken periodically over 48 h after induction. pH was maintained at 7.00 ± 0.05 via the addition of 10 % (v/v) NH4OH base.
Product analysis
Culture samples for analyzing fatty acids via GC/MS were taken and derivatized to methyl esters as described previously (Lennen et al. 2010; Youngquist et al. 2012). All C12:0, C12:1, C14:0, and C14:1 were considered FFAs as past results indicate that less than 10 % of these species are found in membrane fractions (Youngquist et al. 2012). Supernatant samples for HPLC quantification of sugars, alcohols, and short chain carboxylic acids were collected and processed as described previously (Youngquist et al. 2012). Supernatant samples were also analyzed for phosphate content using a Malachite Green Phosphate Assay Kit (BioAssay Systems, Hayward, CA, USA) following manufacturer’s instructions. Additionally, protein levels were determined using a Bradford assay (Bradford 1976). Concentrations of bovine serum albumin were used to generate a standard curve. To each well of a microtiter plate, 293 μL Bradford reagent and 7 μL sample or standard were added in technical triplicate. Absorbance at 595 nm was measured using a microtiter plate reader (Tecan, Männedorf, Switzerland). A linear correlation between OD600 and dry cell weight (DCW) was determined as described previously (Youngquist et al. 2012).
Kinetic modeling
In order to determine how growth rate changed with the limiting substrate (phosphate) concentration, a Monod (1949) model was initially used for high concentrations of extracellular phosphate (Eq. 1). The maximum growth rate (μ max) was estimated from the initial batch growth curve (prior to switching to chemostat operation). Levels of residual, extracellular phosphate were below the limit of detection for all but two conditions. Values for K PO4 were determined from steady-state phosphate levels at different dilution rates using Eq. 1. Monod models are not accurate after the incorporation of all extracellular phosphate because growth continues after this point in a batch reactor despite the extracellular concentration of limiting substrate going to zero (Baek and Lee 2006; Lubke et al. 1995). Modifications to the Monod model to address the consumption of intracellular polyphosphate, first described by Nyholm (1976), were added in the form of Eq. 2. Here, C a is the minimum internal concentration of phosphate required for a viable E. coli cell, C in is the internal concentration of phosphate, and \( C_{\mathrm{in}}^{\max } \) is the concentration of internal phosphate when there is sufficient extracellular phosphate in the media. \( C_{\mathrm{in}}^{\max } \) was assumed to be the value at which the Pho regulon (phosphate starvation response) is at full expression, 0.4 μM (Wanner 1993; Hsieh and Wanner 2010). A value of 0.28 μM was used for C a, as literature values of intracellular phosphate and polyphosphate concentrations under phosphate limitation (Fagerbakke et al. 1996; Sharfstein et al. 1996) indicated similar levels. Once C PO4 had dropped below \( C_{\mathrm{in}}^{\max } \), it was assumed that C PO4 equaled C in and μ was determined by Eq. 2.
While the standard Herbert–Pirt equation was used to determine glucose uptake (Eq. 3), a simplified Herbert–Pirt equation (Eq. 4) (Herbert et al. 1956) was used to determine the phosphate uptake rate into the cell. In the Herbert–Pirt equation, q s and q p represent the biomass-specific substrate uptake rate and biomass-specific productivity, respectively. The product-associated (bq p) and maintenance-associated (m s) phosphate consumption terms, which are significant under carbon-limitation, were eliminated because phosphate is not consumed to generate extracellular products or for cellular maintenance (Mallette et al. 1964). The remaining constant (a PO4) is the inverse of the maximum biomass yield on phosphate which was determined by a linear plot of q PO4 versus growth rate, with (a PO4) being the slope. Once the relationships between q s for glucose (Eq. 3) and q p versus growth were determined (Eq. 5), a model was generated to predict FFA production in E. coli under all growth conditions for phosphate limitation.
Equations (1)–(5) were solved by Euler method implemented in MATLAB (MathWorks, Inc., Natick, MA, USA) to predict FFA production under given reactor configuration, media composition, and growth rate.
Results
Initial evaluation of non-carbon-limiting conditions
In a previous study, a kinetic model was developed to predict the performance of a FFA producing strain of E. coli (TY05) under carbon limitation (Youngquist et al. 2012). This study concluded that FFA yields were the highest at low growth rates and suggested that moving away from carbon-limiting conditions would lead to greater FFA yield and specific productivity. To initially determine whether to pursue nitrogen or phosphate as the non-carbon-limiting nutrient, E. coli TY05 was cultivated in batch, shake flask experiments using MOPS minimal medium with glucose as the sole carbon source, ammonium chloride as the sole nitrogen source, and potassium phosphate as the sole phosphate source. Both nitrogen and phosphate limitation led to higher FFA yields on glucose than the same cells cultivated under carbon limitation (Fig. 1). Phosphate limitation resulted in the highest yield. Additionally, the phosphate-limited cultures consumed significantly more glucose than the nitrogen-limited cultures, even though their final OD600 values were equivalent. This result is consistent with the observation that glucose uptake significantly slows after nitrogen starvation (Ballesteros et al. 2001).
Continuous cultivation of a FFA producing E. coli at various growth rates under P-limitation
To expand our model of FFA production in E. coli TY05, data were collected from chemostat experiments under phosphorus-limiting conditions. A culture of E. coli TY05 was fed MOPS minimal glucose medium containing 1 mM IPTG for induction of BTE. The medium was phosphorus limited as it contained 0.37 mM phosphate instead of the typical 1.32 mM. During the first phosphorus limitation growth rate experiment, E. coli TY05 was continuously cultured using different dilution rates for a feed containing 10 g/L (1.0 %) glucose. Under each regime, the biomass concentration (calculated from OD600), residual glucose, FFA titer, CO2 evolution rate (calculated from off-gas composition and air flow rate), and the titer of alternative fermentation products (acetate, formate, ethanol, succinate, pyruvate, and lactate) were measured (Table 1). Similar to carbon limitation, residual glucose levels increased and FFA titer decreased with increasing dilution rate. Of the alternative fermentation products assayed, only acetate was present in a measurable quantity, with concentrations decreasing with increasing dilution rate. In contrast to carbon-limited experiments, extracellular acetate appeared under all tested dilution rates. Using mass balances for biomass, glucose, and FFA, the production rates (q-rates) for each were calculated (Figs. 2 and 3). These rates were used to calculate FFA and biomass yields as a function of dilution rate.
Productivity (a, b) and yield (c, d) values calculated for each dilution rate tested. a Specific glucose uptake rate and b CO2 productivity for both carbon (gray)- and phosphorus (dark)-limiting conditions calculated from mass balances around the vessel. c Yield of biomass and d CO2 on glucose for both conditions. Experimental values from phosphorus-limiting conditions are represented by filled symbols, open symbols represent data from chemostats run under carbon limitation (Youngquist et al. 2012), and the model prediction is represented by the dashed line. Error bars represent standard deviations of four to five samples taken over a residence time at each steady state
FFA productivity (a) and yield (b) values calculated for each dilution rate tested. Shown are values for C12 and C14 species under both nutrient limitation conditions. Experimental values from phosphorus-limiting conditions are represented by filled symbols, closed symbols represent previous data from chemostats run under carbon limitation (Youngquist et al. 2012), and the production model is represented by the dashed line. Error bars represent standard deviations of four to five samples taken over a residence time at each steady state
Unlike carbon-limited conditions, significant residual glucose was found in the bioreactor effluent for each of the phosphate-limited dilution rates. Glucose consumption rates (Fig. 2a) were equal for the lowest three dilutions rates in contrast to the monotonically increasing trend observed for carbon limitation. Above a dilution rate of 0.1 h−1, glucose uptake rates increased with a slope similar to that observed for carbon limitation. Carbon dioxide-specific productivity was static at low (<0.1 h−1) and high (>0.3 h−1) dilution rates (Fig. 2b). Biomass yields under phosphorus limitation (Fig. 2c) increased with increasing dilution rate and followed a similar trend to the yield under carbon limitation except with values around one third less for high dilution rates. Larger differences were observed for low dilution rates. The yield of carbon dioxide from glucose (Fig. 2d) decreased with increasing dilution rate under both carbon and phosphorus-limiting conditions, following nearly the same curve.
Similar to carbon-limited cultivations, maximum FFA biomass-specific productivity occurred between dilution rates of 0.2 and 0.25 h−1 (Fig. 3a). However, FFA-specific productivities were consistently three times larger under phosphate limitation, reaching a maximum value (for all FFA species combined) of 0.068 g FFA/gram dry cell weight (gDCW)/h. Unlike the trends observed under carbon limitation, the yield of FFAs on glucose increased from the lowest dilution rate (0.05 h−1) to a maximum value of 0.1 g FFA/g glucose at a dilution rate of 0.1 h−1 (Fig. 3b). FFA yields decreased beyond this maximum but remained significantly greater than the corresponding value obtained under carbon limitation.
Using the biomass-specific productivities in Figs. 2 and 3, a carbon mass balance was performed for each dilution rate. For most dilution rates, over 90 % of the fed carbon was found in residual glucose, biomass, FFA, CO2, and assayed fermentation products. At the two smallest dilution rates, only 80 % of the carbon was accounted for. For these dilution rates, extracellular protein content was assayed using a Bradford reagent, and elevated protein levels were not detected (data not shown). Flow cytometric analysis of cells stained with SYTOX green (permeability assay) showed only a population of intact cells (data not shown) unlike stationary phase samples of similar E. coli strains expressing BTE (Lennen et al. 2011). The breakdown of the carbon products for selected dilution rates can be seen in Fig. 4. Under all but the highest dilution rate, CO2 represented the largest fraction of carbon followed by biomass and FFA.
Kinetic model of FFA production under phosphate limitation
A kinetic model was updated using the chemostat data collected for E. coli TY05 under phosphorus-limiting conditions. As seen in Fig. 3a, the biomass-specific productivity under phosphorus limitation was non-linear with respect to dilution rate, with a similar relationship to that seen under carbon limitation. While an equation with a maximum in q p at an intermediate dilution rate could be fit to the data, addition of a non-growth associated constant (Eq. 5) to the equation previously determined for FFA production under carbon limitation (Youngquist et al. 2012) provided a better fit over both conditions. The fact that E. coli continues to import carbon and remain metabolically active in stationary phase under phosphorus-limiting conditions (Ballesteros et al. 2001) supports the use of a non-growth associated term for the production of FFA under non-carbon limitation. The fact that none of the other model parameters were altered further supported this hypothesis. The optimal value of δ was 0.036 gC12/gDCW/h, while values for α, β, and γ were found to remain at 0.044 gC12/gDCW/h, 0.17 h−1, and 3.4 h, respectively.
To see if there was a similar effect to the yield of biomass on substrate (Y xs) and maintenance energy (m s) terms, the experimental q p terms were factored out of the equation to generate a modified glucose consumption (q s) term as a linear function of growth (Fig. 5). In generating the modified glucose consumption term, the experimental biomass-specific productivities of each component are divided by its maximum theoretical yield and then subtracted from the experimentally observed specific substrate uptake rates. The resulting modified glucose uptake rate versus growth has a linear relationship where maximum biomass yield (inverse slope) and maintenance energy (y-axis intercept) can be determined by a linear fit. The maximum yield of biomass on glucose (Y xs) was found to be 0.54 g/g, in line with published values (Paalme et al. 1995), while m s was found to be 0.28 g glucose/gDCW/h, significantly higher than the value for FFA producing E. coli under carbon limitation (Youngquist et al. 2012) and for wild-type E. coli K-12 (Heijnen and Roels 1981). The resulting modified q s equation was used in conjunction with the q p equation to generate an updated model for yield of FFA on glucose under limiting phosphate conditions (Fig. 3b). The model matched FFA yields for dilutions rates above 0.1 h−1.
The maximum yield of biomass on glucose (\( Y_{\mathrm{x}/\mathrm{s}}^{\max } \), inverse slope) and maintenance energy (m s, intercept) were calculated from a plot of modified glucose consumption (with product formation removed) versus dilution rate. The observed biomass-specific productivity of each component is divided by its maximum theoretical yield and then subtracted from the observed specific substrate uptake rate giving a modified specific substrate uptake rate affected by only the biomass and maintenance terms
Effect of nutrient ratios on FFA-specific productivity
Next, the effect of the media carbon-to-phosphorus ratio on FFA biomass-specific productivity and yield was tested. A set of chemostat experiments was performed holding a constant dilution rate (0.1 h−1) while varying either the concentration of phosphate or glucose in the feed. Experiments were performed using glucose concentrations of 0.4, 0.7, 1.0, and 2.0 % (w/v) in MOPS minimal media containing 370 μM phosphate and using a glucose concentration of 1.0 % (w/v) in MOPS minimal media containing either 185, 370, or 530 μM phosphate. These concentrations correspond to C/P (mole carbon atom per mole phosphate ion) ratios ranging from 360 to 1,800. A C/P molar ratio below 120 is required to enter limiting carbon conditions (Neidhardt et al. 1974). For each C/P ratio, samples for biomass, glucose, FFA, and alternative fermentation products were taken five times over a period of 16 h (1.5 residence times) after a steady state (as defined above) was established. Residual glucose in the reactor effluent was found for all conditions except the lowest ratio (0.4 % glucose in MOPS minimal media containing 370 μM phosphate). For phosphate-limited conditions, biomass, FFA, and CO2 effluent concentrations increased with increased phosphate content in the media feed while glucose variations had no impact. Acetate was produced under all tested conditions except the lowest C/P ratio. When mass balances were calculated, 82–98 % of carbon fed to the system was accounted under all steady states (Table 2).
Data from Table 2 were used to calculate q-rates and yields for all measured compounds. In general, trends in q-rates were independent of whether glucose (C) or phosphate (P) was varied. Specific FFA and CO2 productivities remained nearly constant under all tested conditions (Fig. 6). The average q p-C12 value was 0.049 ± 0.004 gC12 FFA/gDCW/h with small variations over the range of C/P ratios tested. The average q CO2 value was 0.41 ± 0.03 gCO2/gDCW/h with the only significant deviation occurring at the lowest C/P ratio (Fig. 6b). At this low C/P ratio (360) and a low dilution rate, it is likely the culture is bordering on co-limitation where effects of carbon limitation begin to appear. Therefore, no C/P ratios below 360 were tested.
Unlike the other productivities, glucose uptake rates differed significantly between glucose and phosphate variations. As expected, glucose uptake rates decreased with decreasing phosphate concentrations (i.e., increasing C/P ratio), consistent with decreased biomass concentrations (Table 2). When glucose concentrations were varied, glucose uptake rates averaged 0.58 ± 0.05 g glucose/gDCW/h with the only significant deviation occurring at the lowest C/P ratio (Fig. 6a). Despite the differences in glucose uptake, the biomass-specific productivity of FFA (Fig. 6c) was not affected and can be assumed to remain constant at a constant dilution rate under phosphate limitation.
Model validation for alternative fermentation condition
As a test of the FFA production model, three 48-h batch cultures were performed in a bioreactor using MOPS minimal media containing 370 μM phosphate and 0.7 % glucose (Fig. 7). Off-gas composition was recorded continuously throughout to indirectly monitor metabolic activity in stationary phase. Sugar and optical density samples were taken throughout the experiment. FFA samples were taken at 3, 6, 9, 18, 21, 24, 30, and 48 h after induction. However, after the 9-h sample a significant amount of foam accumulated within the reactor and did not exit into a foam overflow trap bottle. For this reason, an accurate FFA titer could not be taken between 9 and 18 h, when the remaining foam in the reactor was collapsed using the addition of antifoam (Antifoam 204, Sigma Aldrich) and the concentration of FFAs collected in the foam trap bottle was determined.
The kinetic model used both q s and q p equations as a function of μ. For the Monod growth model, a K s of 2 μM was estimated from the non-zero, residual phosphate data points (taken at high dilution rates). The model predicted FFA titer was insensitive to the value of K s, in part because the majority of production came after the phosphate concentration had dropped below \( C_{\mathrm{in}}^{\max } \). Most FFA production occurred once C in had dropped to the value of C a, where q p became a constant until all glucose was consumed.
The consumption of glucose and cell density predicted by the model was consistent with the experimental data from the controlled batch fermentation. The measured FFA concentrations were also consistent with predictions up to 9 h and after 18 h. Unfortunately, the inability to collect accurate FFA titers between 9 and 18 h prevented us from confirming whether the FFA production ceased as abruptly as the model and CO2 data suggested. The model accurately estimated the final titer of FFA. Around 0.5 g/L acetate was observed at the 24-h timepoint (immediately prior to full glucose consumption), almost all of which was consumed by the 48-h timepoint. As cells are still metabolically active during the second 24-h period before the complete consumption of acetate, some may go toward producing FFAs, a feature not captured in the model.
Discussion
The experimental and modeling results suggest several areas where improvements in fatty acid production could be made by additional metabolic engineering. First, elimination of one acetate production pathway that also produces carbon dioxide (via a knockout of poxB) could provide a boost as acetate production currently accounts for 3–6 % of the carbon consumed in E. coli TY05. Adding a poxB deletion to strains grown under phosphate limitation could give a minor boost in FFA yield and specific productivity. Second, the observed maintenance energy of 0.28 g glucose/gDCW/h under phosphate limitation is already four times higher than that seen under carbon limitation and much higher than reported values for wild type E. coli (Button 1985; Heijnen and Roels 1981). Higher maintenance energies have been reported for bacteria under excess carbon conditions (Tempest and Neijssel 1984; Zeng and Deckwer 1995); however, this phenomenon appears to be the result of energy spilling (e.g., futile cycles) to consume the excess carbon (Russell 2007). Spurious reactions involved in energy spilling could be identified in a transcriptomic and proteomic analysis of the strain. Further analysis into the cause of the elevated maintenance energy could provide insight into ways to further increase FFA yields. A similar FFA-producing E. coli strain was found to be subject to elevated membrane stress and was less viable in comparison to a non-producing control strain in stationary phase (Lennen et al. 2011). E. coli could be expending energy in response to these stresses, thereby increasing the maintenance energy at the expense of FFA production. Lastly, under phosphate-limited growth, a large percentage of the fed glucose remains unconsumed. One strategy for increasing glucose consumption is to evolve strains under non-carbon-limiting conditions in a very slow growth or stationary phase to select for cells with higher metabolic activity and increased specific glucose uptake rates. Such a strategy was successfully implemented to increase specific glucose uptake rate under nitrogen-limited conditions (Sonderegger et al. 2005). As phosphate-starved cells have greater metabolic activity in stationary phase than nitrogen-starved cells (Ballesteros et al. 2001), there may not be as significant of an improvement in glucose uptake rate. However, any effort to increase the rate of glucose uptake under non-carbon limitations could increase the rate of FFA production and FFA yield if excess carbon flux can be directed through fatty acid biosynthesis. Future efforts will focus on these and other modifications to engineer a strain that will come closer to producing FFAs at their theoretical limit (0.35 g FFA/g glucose) (Lennen and Pfleger 2012).
In summary, phosphate limitation was shown to increase FFA production from an engineered strain of E. coli. Using data collected from chemostat experiments, a kinetic model of FFA production in E. coli was updated to include production under phosphate-limiting conditions. Maximum yields were obtained at low dilution rates, and maximum productivities were obtained at lower to intermediate dilution rates. FFA yields and productivities were not impacted by the ratio of carbon to phosphate as long as the media remained phosphate-limited. The model was used to successfully predict the production of a controlled batch culture under phosphorus limitation. The optimal conditions identified generated a 40 % increase in yield and 300 % increase in productivity versus the optimal conditions observed under carbon limitation.
References
Agnew DE, Stevermer AK, Youngquist JT, Pfleger BF (2012) Engineering Escherichia coli for production of C(1)(2)-C(1)(4) polyhydroxyalkanoate from glucose. Metab Eng 14(6):705–713. doi:10.1016/j.ymben.2012.08.003
Baek JH, Lee SY (2006) Novel gene members in the Pho regulon of Escherichia coli. FEMS Microbiol Lett 264(1):104–109. doi:10.1111/j.1574-6968.2006.00440.x
Ballesteros M, Fredriksson A, Henriksson J, Nystrom T (2001) Bacterial senescence: protein oxidation in non-proliferating cells is dictated by the accuracy of the ribosomes. EMBO J 20(18):5280–5289. doi:10.1093/emboj/20.18.5280
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72(1–2):248–254
Button DK (1985) Kinetics of nutrient-limited transport and microbial-growth. Microbiol Rev 49(3):270–297
Fagerbakke KM, Heldal M, Norland S (1996) Content of carbon, nitrogen, oxygen, sulfur and phosphorus in native aquatic and cultured bacteria. Aquat Microb Ecol 10(1):15–27. doi:10.3354/ame010015
Ferenci T (2008) Bacterial physiology, regulation and mutational adaptation in a chemostat environment. Adv Microb Physiol 53:169-+. doi:10.1016/s0065-2911(07)53003-1
Gerard F, Dri AM, Moreau PL (1999) Role of Escherichia coli RpoS, LexA and H-NS global regulators in metabolism and survival under aerobic, phosphate-starvation conditions. Microbiol-UK 145:1547–1562
Goh E-B, Baidoo EEK, Keasling JD, Beller HR (2012) Engineering of bacterial methyl ketone synthesis for biofuels. Appl Environ Microbiol 78(1):70–80. doi:10.1128/aem.06785-11
Hassan M, Blanc PJ, Granger LM, Pareilleux A, Goma G (1996) Influence of nitrogen and iron limitations on lipid production by Cryptococcus curvatus grown in batch and fed-batch culture. Process Biochem 31 (4):355–361. doi:10.1016/0032-9592(95)00077-1
Heijnen JJ, Roels JA (1981) A macroscopic model describing yield and maintenance relationships in aerobic fermentation processes. Biotechnol Bioeng 23(4):739–763. doi:10.1002/bit.260230407
Herbert D, Elsworth R, Telling RC (1956) The continuous culture of bacteria; a theoretical and experimental study. J Gen Microbiol 14(3):601–622
Hsieh Y-J, Wanner BL (2010) Global regulation by the seven-component Pi signaling system. Curr Opin Microbiol 13(2):198–203. doi:10.1016/j.mib.2010.01.014
Hua Q, Yang C, Oshima T, Mori H, Shimizu K (2004) Analysis of gene expression in Escherichia coli in response to changes of growth-limiting nutrient in chemostat cultures. Appl Environ Microbiol 70(4):2354–2366. doi:10.1128/aem.70.4.2354-2366.2004
Huber R, Roth S, Rahmen N, Buechs J (2011) Utilizing high-throughput experimentation to enhance specific productivity of an E. coli T7 expression system by phosphate limitation. BMC Biotechnol 11
James OO, Maity S, Mesubi MA, Usman LA, Ajanaku KO, Siyanbola TO, Sahu S, Chaubey R (2012) A review on conversion of triglycerides to on-specification diesel fuels without additional inputs. Int J Energy Res 36(6):691–702. doi:10.1002/er.2894
Johansson L, Lindskog A, Silfversparre G, Cimander C, Nielsen KF, Liden G (2005) Shikimic acid production by a modified strain of E. coli (W3110.shik1) under phosphate-limited and carbon-limited conditions. Biotechnol Bioeng 92(5):541–552. doi:10.1002/bit.20546
Johnson K, Kleerebezem R, van Loosdrecht MCM (2010) Influence of the C/N ratio on the performance of polyhydroxybutyrate (PHB) producing sequencing batch reactors at short SRTs. Water Res 44(7):2141–2152. doi:10.1016/j.watres.2009.12.031
Kalscheuer R, Stolting T, Steinbuchel A (2006) Microdiesel: Escherichia coli engineered for fuel production. Microbiol-SGM 152:2529–2536. doi:10.1099/mic.0.29028-0
Knothe G (2010) Biodiesel and renewable diesel: a comparison. Prog Energy Combust Sci 36(3):364–373. doi:10.1016/j.pecs.2009.11.004
Kottmeier K, Mueller C, Huber R, Buechs J (2010) Increased product formation induced by a directed secondary substrate limitation in a batch Hansenula polymorpha culture. Appl Microbiol Biotechnol 86(1):93–101. doi:10.1007/s00253-009-2285-0
Kumar R, Shimizu K (2011) Transcriptional regulation of main metabolic pathways of cyoA, cydB, fnr, and fur gene knockout Escherichia coli in C-limited and N-limited aerobic continuous cultures. Microb Cell Fact 10:3. doi:10.1186/1475-2859-10-3
Lennen RM, Braden DJ, West RM, Dumesic JA, Pfleger BF (2010) A process for microbial hydrocarbon synthesis: overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnol Bioeng 106(2):193–202. doi:10.1002/bit.22660
Lennen RM, Kruziki MA, Kumar K, Zinkel RA, Burnum KE, Lipton MS, Hoover SW, Ranatunga DR, Wittkopp TM, Marner WD II, Pfleger BF (2011) Membrane stresses induced by overproduction of free fatty acids in Escherichia coli. Appl Environ Microbiol 77(22):8114–8128. doi:10.1128/aem.05421-11
Lennen RM, Pfleger BF (2012) Engineering Escherichia coli to synthesize free fatty acids. Trends Biotechnol 30(12):659–667. doi:10.1016/j.tibtech.2012.09.006
Lu XF, Vora H, Khosla C (2008) Overproduction of free fatty acids in E. coli: implications for biodiesel production. Metab Eng 10(6):333–339. doi:10.1016/j.ymben.2008.08.006
Lubke C, Boidol W, Petri T (1995) Analysis and optimization of recombinant protein-production in Escherichia coli using the inducible phoA promoter of the Escherichia coli alkaline-phosphatase. Enzyme Microb Technol 17(10):923–928. doi:10.1016/0141-0229(94)00130-j
Mallette MF, Cowan CI, Campbell JJ (1964) Growth and survival of Escherichia coli in medium limited in phosphate. J Bacteriol 87:779–785
Marzan LW, Shimizu K (2011) Metabolic regulation of Escherichia coli and its phoB and phoR genes knockout mutants under phosphate and nitrogen limitations as well as at acidic condition. Microb Cell Fact 10:39. doi:10.1186/1475-2859-10-39
Matsuoka Y, Shimizu K (2011) Metabolic regulation in Escherichia coli in response to culture environments via global regulators. Biotechnol J 6(11):1330–1341. doi:10.1002/biot.201000447
McIntyre JJ, Bull AT, Bunch AW (1996) Vancomycin production in batch and continuous culture. Biotechnol Bioeng 49(4):412–420. doi:10.1002/(sici)1097-0290(19960220)49:4<412::aid-bit8>3.0.co;2-s
Mendez-Perez D, Begemann MB, Pfleger BF (2011) Modular synthase-encoding gene involved in alpha-olefin biosynthesis in Synechococcus sp strain PCC 7002. Appl Environ Microbiol 77(12):4264–4267. doi:10.1128/aem.00467-11
Monod J (1949) The growth of bacterial cultures. Annu Rev Microbiol 3(1):371–394. doi:10.1146/annurev.mi.03.100149.002103
Nawabi P, Bauer S, Kyrpides N, Lykidis A (2011) Engineering Escherichia coli for biodiesel production utilizing a bacterial fatty acid methyltransferase. Appl Environ Microbiol 77(22):8052–8061. doi:10.1128/AEM.05046-11
Neidhardt FC, Bloch PL, Smith DF (1974) Culture medium for enterobacteria. J Bacteriol 119(3):736–747
Nyholm N (1976) Mathematical-model for microbial-growth under limitation by conservative substrates. Biotechnol Bioeng 18(8):1043–1056. doi:10.1002/bit.260180803
Paalme T, Kahru A, Elken R, Vanatalu K, Tiisma K, Vilu R (1995) The computer-controlled continuous culture of Escherichia coli with smooth change of dilution rate (A-stat). J Microbiol Methods 24(2):145–153. doi:10.1016/0167-7012(95)00064-x
Peralta-Yahya PP, Zhang F, del Cardayre SB, Keasling JD (2012) Microbial engineering for the production of advanced biofuels. Nature 488(7411):320–328. doi:10.1038/nature11478
Peterson CN, Mandel MJ, Silhavy TJ (2005) Escherichia coli starvation diets: essential nutrients weigh in distinctly. J Bacteriol 187(22):7549–7553. doi:10.1128/jb.187.22.7549-7553.2005
Ranganathan S, Tee TW, Chowdhury A, Zomorrodi AR, Yoon JM, Fu Y, Shanks JV, Maranas CD (2012) An integrated computational and experimental study for overproducing fatty acids in Escherichia coli. Metab Eng 14(6):687–704. doi:10.1016/j.ymben.2012.08.008
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51. doi:10.1016/s0065-2164(02)51000-5
Rude MA, Baron TS, Brubaker S, Alibhai M, Del Cardayre SB, Schirmer A (2011) Terminal olefin (1-alkene) biosynthesis by a novel P450 fatty acid decarboxylase from Jeotgalicoccus species. Appl Environ Microbiol 77(5):1718–1727. doi:10.1128/aem.02580-10
Russell JB (2007) The energy spilling reactions of bacteria and other organisms. J Mol Microbiol Biotechnol 13(1–3):1–11. doi:10.1159/000103591
Schirmer A, Rude MA, Li XZ, Popova E, del Cardayre SB (2010) Microbial biosynthesis of alkanes. Science 329(5991):559–562. doi:10.1126/science.1187936
Sharfstein ST, VanDien SJ, Keasling JD (1996) Modulation of the phosphate-starvation response in Escherichia coli by genetic manipulation of the polyphosphate pathways. Biotechnol Bioeng 51(4):434–438. doi:10.1002/(sici)1097-0290(19960820)51:4<434::aid-bit6>3.3.co;2-5
Sonderegger M, Schumperli M, Sauer U (2005) Selection of quiescent Escherichia coli with high metabolic activity. Metab Eng 7(1):4–9. doi:10.1016/j.ymben.2004.05.005
Steen EJ, Kang YS, Bokinsky G, Hu ZH, Schirmer A, McClure A, del Cardayre SB, Keasling JD (2010) Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature 463(7280):559-U182. doi:10.1038/nature08721
Tempest DW, Neijssel OM (1984) The status of YATP and maintenance energy as biologically interpretable phenomena. Annu Rev Microbiol 38:459–486. doi:10.1146/annurev.micro.38.1.459
Wanner BL (1993) Gene regulation by phosphate in enteric bacteria. J Cell Biochem 51(1):47–54. doi:10.1002/jcb.240510110
Wanner U, Egli T (1990) Dynamics of microbial-growth and cell composition in batch culture. FEMS Microbiol Rev 75(1):19–44. doi:10.1111/j.1574-6968.1990.tb04084.x
Youngquist JT, Lennen RM, Ranatunga DR, Bothfeld WH, Marner WD 2nd, Pfleger BF (2012) Kinetic modeling of free fatty acid production in Escherichia coli based on continuous cultivation of a plasmid free strain. Biotechnol Bioeng 109(6):1518–1527. doi:10.1002/bit.24420
Zeng AP, Deckwer WD (1995) A kinetic-model for substrate and energy-consumption of microbial-growth under substrate-sufficient conditions. Biotechnol Progr 11(1):71–79. doi:10.1021/bp00031a010
Zhang F, Ouellet M, Batth TS, Adams PD, Petzold CJ, Mukhopadhyay A, Keasling J (2012) Enhancing fatty acid production by the expression of the regulatory transcription factor FadR. Metab Eng. doi:10.1016/j.ymben.2012.08.009
Zheng Y, Li L, Liu Q, Qin W, Yang J, Cao Y, Jiang X, Zhao G, Xian M (2012) Boosting the free fatty acid synthesis of Escherichia coli by expression of a cytosolic Acinetobacter baylyi thioesterase. Biotechnol Biofuels 5:76. doi:10.1186/1754-6834-5-76
Acknowledgments
This work was funded by the DOE Great Lakes Bioenergy Research Center (DOE Office of Science BER DE-FC02-07ER64494). The authors are grateful to William Bothfeld, Alan Higbee, Alex Lareau, Haibo Li, Michael Luc, Mick McGee, Daniel Mendez-Perez, and Yaoping Zhang for their contributions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Youngquist, J.T., Rose, J.P. & Pfleger, B.F. Free fatty acid production in Escherichia coli under phosphate-limited conditions. Appl Microbiol Biotechnol 97, 5149–5159 (2013). https://doi.org/10.1007/s00253-013-4911-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4911-0