Abstract
The industrially important species of corynebacteria viz. Corynebacterium acetoacidophilum appear to be alternative hosts for recombinant protein production; despite many efforts, a strong promoter-based system in corynebacteria has not been established so far. Described here is a T7 promoter-based expression system which was functional in both gram-positive C. acetoacidophilum and gram-negative Escherichia coli in an external inducer independent manner. This is the very first report of a T7 expression system for Corynebacterium sp. Also, it is a useful addition in the existing T7 expression systems of E. coli.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last two decades, the use of recombinant proteins has greatly increased both at industrial and laboratory levels. A large number of putative genes are continuously added to the GenBank, and to decipher the function of these unknown genes we need to have novel expression tools for high throughput screening of heterologous protein expression.
To date, many vector systems have been developed for a broad range of organisms, but bacterial systems remain the most attractive due to its low cost, high growth rate, and easy handling. The gram-negative bacterium Escherichia coli is the most commonly used organism for heterologous production of proteins due to the fact that its genetics is well studied and is an established system.
In E. coli, the T7 expression system (Studier and Moffatt 1986) is the most popular and extensively used system for high-level protein production. In this system, a plasmid contains an expression cassette in which a gene of interest is inserted downstream to an extremely strong promoter from the E. coli bacteriophage T7. This promoter is recognized only by its own RNA polymerase, the T7 RNA polymerase, encoded by gene 1 of bacteriophage T7. The T7 RNA polymerase is highly processive which does not recognize promoters of the host; consequently, it leads to a high level expression of the target protein. For expression, the T7 plasmid carrying gene of interest under T7 promoter is transformed into a specific host, e.g., E. coli BL21 (DE3), that contains a single copy of T7 RNA polymerase gene under control of lacUV5 promoter as a lambda DE3 lysogen which is induced by isopropyl-β-d-thiogalactopyranoside (IPTG). Despite the fact that protein expression is dependent on the inducer IPTG, this system has proven as a valuable tool in molecular biology, and the usefulness of this system in E. coli cannot be underestimated. But, IPTG is very expensive and toxic to the host which in many instances greatly reduce the growth rate of the host (Makrides 1996).
Although a high level of heterologous proteins are routinely produced through this system in E. coli both at industry and laboratory levels, one of the major disadvantages of this organism is that it is unable to efficiently secrete target protein in the medium; thus, it cannot be used for extracellular production of heterologous proteins. Moreover, this organism is not a suitable host to express proteins to be used for therapeutic and pharmaceutical purposes due to the accumulation of lipopolysaccharide (LPS), generally referred to as endotoxins, which are pyrogenic in humans and other mammals. Recombinant proteins produced in E. coli must be purified in second step to become endotoxin-free prior to their therapeutic uses (Perola et al. 2007). In this context, GRAS members of the gram-positive coryneform bacteria are able to efficiently secrete proteins in the medium (Krämer 1994). These are facultative aerobic soil bacteria with high GC content. The members of this genus may also target folded proteins into the extracellular environment using their twin-arginine translocation (TAT) pathway (Kikuchi et al. 2006) and are thus best suited for being used for production of proteins of therapeutic uses.
Corynebacterium glutamicum and related species have been widely used for industrial production of amino acids. C. glutamicum itself produces more than 1.5 million tons of glutamate and 0.75 million tons of lysine each year (Kimura 2003). Despite immense industrial importance of C. glutamicum and related species, their usage as expression host for high-level protein production is still limited.
Few recombinant proteins have been expressed in C. glutamicum and related species which includes α-amylase of Bacillus amiloliquefaciens (Smith et al. 1986), cellulase of Cellulomonas fimi (Paradis et al. 1987), nuclease of Staphylococcus aureus (Liebl et al. 1992), lactose permease of E. coli (Brabetz et al. 1993), ovine interferon gamma as fusion protein with glutathione S-transferase (Billman-Jacob et al. 1994), protease (bprV) of Dichelobacter nodosus (Billman-Jacob et al. 1995), subtilisin (aprE) of Bacillus subtilis (Billman-Jacob et al. 1995), streptokinase as fusion with glutathione S-transferase (Srivastava and Deb 2002), transglutaminases of Streptoverticillium mobaraense (Kikuchi et al. 2003; Date et al. 2003) and Streptomyces mobaraensis (Date et al. 2004; Itaya and Kikuchi 2008), and human epidermal growth factor (Date et al. 2006). The promoters used so far for protein expression are native promoters of corynebacteria itself. Heterologous E. coli promoters (lac, trc, and ara) have been found to be functional in corynebacteria, but native as well as E. coli promoters are not strong enough in corynebacteria to serve the purpose for high-level protein expression (Pátek et al. 2003). The use of sugar-inducible promoters (lac and ara) of E. coli in Corynebacterium spp. are of limited use as the latter completely lack the lactose and arabinose metabolizing pathways (except C. glutamicum ATCC 31831 which grows on l-arabinose as sole carbon source; Kawaguchi et al. 2009), and thus they lack transporters of their corresponding inducers. Cloning of transporter for corresponding inducer did not give very positive results.
In the present study, we developed a two-plasmid expression system for corynebacteria based on T7 RNA polymerase-dependent promoter. Its function was shown in both gram-positive C. acetoacidophilum and gram-negative E. coli. It might be also used for high-level production of heterologous proteins in C. glutamicum and other related corynebacteria.
Materials and methods
Bacterial strains, media, and growth conditions
The bacterial strains used in this study are shown in Table 1. E. coli strains were grown aerobically on a rotary shaker (180–200 rpm) at 37 °C in Luria–Bertani (LB) broth or LB supplemented with 1.5 % agar on plates. The corynebacteria strain used in present study was C. acetoacidophilum ATCC 21476 (Deb et al. 1990) which is very closely related with C. glutamicum as evident from sequencing of 16S rRNA gene and species specific islands (SSIs) sequences. C. acetoacidophilum were grown at 30 °C in brain heart infusion (BHI) broth or BHI–agar. The antibiotics kanamycin and chloramphenicol wherever required were used at concentrations 50 μg ml−1 and 30 μg ml−1 for E. coli and 10 μg ml−1 and 6 μg ml−1 for C. acetoacidophilum, respectively.
Chemicals and enzymes
All chemicals for this study were purchased from either Sigma-Aldrich (St. Louis, USA) or Merck (Darmstadt, Germany). Restriction enzymes, DNA polymerases, ligase, and other DNA modifying enzymes were purchased from either New England Biolabs (MA, USA) or Fermentas (MA, USA).
Isolation and modification of plasmid and genomic DNA
All plasmids used in present study are listed in Table 2. Plasmid DNA from E. coli was isolated using QIAprep Spin Miniprep kit (Qiagen, Germany) following the manufacturer’s instructions. Plasmid DNA from C. acetoacidophilum was isolated manually by the method described by Mukherjee et al. (1990). Genomic DNA from C. acetoacidophilum was isolated using QIAamp Tissue Kit (Qiagen). All DNA manipulations were carried out by standard procedures (Sambrook et al. 1989) and the instructions provided by the material suppliers.
Transformation and electroporation of plasmid DNA into host cell
Chemically competent E. coli cells were transformed with plasmid DNA by heat shock method (Cohen et al. 1972). C. acetoacidophilum cells were made electrocompetent, and subsequently electroporation of plasmid DNA were carried out by methods described by van der Rest et al. (1999).
PCR and oligonucleotides
All DNA amplifications by PCR were carried out with Pfu DNA polymerase in a gradient thermocycler (CA, Bio-Rad). PCR products were purified with QIAquick PCR Purification Kit (Qiagen). All oligonucleotide primers used in this study were synthesized from Sigma-Aldrich (Bangalore, India), which are listed in Table 3.
Gene synthesis and DNA sequencing
The gene sequence of superfolder green fluorescence protein (sfGFP) used in this study was synthesized from GenScript (NJ, USA). The synthesized gene was received as a recombinant plasmid in a cloning vector. The DNA sequences of all recombinants constructed in this study were confirmed by DNA sequencing Sigma-Aldrich (Bangalore, India).
Plasmid compatibility and stability test
Stability and compatibility of plasmids were studied as per the method described by Walia et al. 2007. C. acetoacidophilum harboring either single or two plasmid(s) were inoculated from a single colony into BHI medium containing appropriate antibiotics and grown overnight at 30 °C on a rotary shaker (200–250 rpm). Next day, 100 ml fresh BHI media without antibiotic was inoculated with 100 μl of overnight grown C. acetoacidophilum culture and was allowed to grow for 24 h until the cells reached stationary phase. An appropriate diluted culture was plated on BHI plates without antibiotics. Percentage of plasmid-harboring cells in culture was estimated by cross-counting of number of streaked colonies that appeared after transferring approximately 100 colonies on BHI plates with appropriate antibiotics. Roughly, an overnight grown culture were assumed to have cells of 10th generation; thus, the process of sub-culturing followed by plating without antibiotic and cross-counting streaked colonies on antibiotic plates were continued for 6 days to collect data of 60th generation.
Fluorescence measurements in E. coli and C. acetoacidophilum
The sfGFP, a robustly folded variant of GFP which has more intense fluorescence (Pédelacq et al. 2006), was used as a reporter gene to investigate and validate the auto-induced expression in newly constructed vector system(s) in E. coli and C. acetoacidophilum. The excitation and emission maxima of sfGFP are 488 nm and 515 nm, respectively. To measure the fluorescence intensity, 1 ml each of growing culture of E. coli and C. acetoacidophilum (in triplicate) were collected at different cultivation time points until the culture reached stationary phase and harvested down at 4,000 rpm, 5 min. Pellets were resuspended into the same volume of PBS and washed thrice with same buffer. The absorbance of washed samples was measured in a UV–vis spectrophotometer (Eppendorf, Germany) at OD600, and subsequently the cells were adjusted to an OD600 of 0.5. The in vivo green fluorescence was measured with a fluorescence spectrometer set at an excitation wavelength of 480 nm and emission detection from 500 to 550 nm. The excitation slit was set at 7.5 and the emission slit at 10 nm. Documentation of the spectra was performed using fluorescence data manager software (Perkin Elmer, USA).
Fluorescence microscopy
Growing culture of E. coli or C. acetoacidophilum (1–2 ml) was pelleted down at 5,000 rpm. The pellet was subsequently washed four times with HEPES buffer and it was finally resuspended into an appropriate volume of the same buffer. A small volume of cells in HEPES buffer was fixed on a polylysine pre-treated slide. Microscopy was carried out using a fluorescence microscope (Olympus, USA) with a halogen light source attached to a high-definition camera, and image acquisition software (Andor, UK).
SDS–PAGE analysis
After normalization of cells, total proteins of C. acetoacidophilum cell lysate were separated on 12 %–15 % SDS–PAGE following standard method (Laemmli 1970) which was stained with Coomassie Brilliant Blue R-250 dye.
Results
A T7 RNA polymerase/promoter coupled dual plasmid system was constructed for both gram-negative E. coli and gram-positive C. acetoacidophilum, where one plasmid carries a T7 RNA polymerase gene and another one carries a strong T7 promoter. Two well-characterized corynebacterial replicons, pBL1 replicon of Brevibacterium lactofermentum (Santamaria et al. 1984) and pCG1 replicon of C. glutamicum (Ozaki et al. 1984), were selected for the present study to develop two plasmid-based expression systems in C. acetoacidophilum.
Construction of plasmids carrying T7 RNA polymerase gene
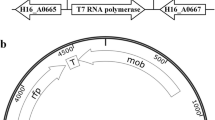
Plasmid pGP1-2 (Tabor and Richardson 1985) was used as source of T7 RNA polymerase gene to construct T7 RNA polymerase-based plasmids. The 2.6-kb PstI–BamHI fragment of pGP1–2 consists of p15A origin of replication and kanamycin resistant gene (Kmr) cassette was PCR amplified using primers JE1-F and JE1-R. Also, a unique restriction site EcoRI was introduced into primer JE1-F just upstream to BamHI restriction site to be used for further cloning site. The Pfu DNA polymerase amplified PCR product was self-ligated with T4 DNA polymerase, and the ligation mixture was subsequently transformed into E. coli Top10 competent cells. The transformed colonies were selected on LA-containing kanamycin plates. Thus, an E. coli replicating small plasmid pJE1 was obtained which contained three adjacent cloning sites BamHI, EcoRI, and PstI (Fig. 1a, b). In the next step, only coding sequence of T7 RNA polymerase gene (gene 1 of T7) was PCR amplified from the same plasmid pGP1–2 using primers RNAP-F and RNAP-R. The PCR product was subsequently cloned into plasmid pJE1 in between BamHI and EcoRI restriction sites and so the obtained recombinant plasmid carrying promoterless T7 RNA polymerase gene was designated as pJE-RNAP (Fig. 1c). The orientation of T7 RNA polymerase and Kmr genes in pJE-RNAP is the same as that of plasmid pGP1–2, i.e., facing each other so that the strong terminator sequence of Kmr falls downstream to promoterless T7 RNA polymerase gene. Later, in order to supply functional promoter(s) to promoterless T7 RNA polymerase gene, promoter(s) of filamentous temperature sensitive mutant Z (ftsZ) of C. acetoacidophilum and promoter of kanamycin resistant gene were separately cloned into pJE-RNAP
Construction of plasmids with T7 RNA polymerase gene for E. coli and Corynebacterium sp. a E. coli replicating plasmid pGP1–2 used as sources of T7 RNA polymerase gene, p15 ori and kanamycin resistant gene cassette, Kmr, as well as promoter of kanamycin resistance gene, P-kan; b plasmid pJE1 carrying E. coli ori, p15A, and kanamycin resistance gene cassette, Kmr; c promoterless T7 RNA polymerase harboring plasmid pJE-RNAP achieved after cloning of only coding sequences of T7 RNA polymerase gene into pJE1; d plasmids pJE-ftsRNAP and pJE-kRNAP achieved after cloning of promoters of ftsZ and Kmr, respectively, into plasmid pJE-RNAP upstream to T7 RNA polymerase gene; e plasmid pEPR1 used as a source of pCG1 replicon; f E. coli–Corynebacterium sp. shuttle plasmids pJEC-ftsRNAP and pJEC-kRNAP achieved after cloning of pCG1 replicon into pJE-ftsRNAP and pJE-kRNAP, respectively
C. glutamicum and related genera viz. Mycobacterium tuberculosis are known to possess multiple promoters for their corresponding ftsZ gene, present as a cluster within 500 bp upstream ftsZ start codon (Letek et al. 2007; Kiran et al. 2009); therefore, about 500 bp upstream promoter region of ftsZ was fished out from C. acetoacidophilum genome by PCR, using sequence-specific primers PFTSZ-F and PFTSZ-R, and the PCR amplified ftsZ promoter regions were cloned in between PstI and EcoRI restriction sites of plasmid pJE-RNAP. This resultant recombinant plasmid was designated as pJE-ftsRNAP (Fig. 1d). In another construct, 400 bp upstream promoter region of Kmr gene was PCR amplified from plasmid pGP1–2 using primers PKAN-F and PKAN-R, and was cloned into pJE-RNAP at PstI–EcoRI, and this recombinant plasmid was designated as pJE-kRNAP (Fig. 1d). Thus, we obtained two E. coli replicating plasmids harboring T7 RNA polymerase gene; in the first one the T7 RNA polymerase gene is under the control of multiple ftsZ promoter of C. acetoacidophilum, whereas in the second one the same is under the control of a constitutive promoter, the Tn9 promoter of Kmr gene.
Both plasmids pJE-kRNAP and pJE-ftsRNAP were further manipulated into shuttle plasmids to be used for C. acetoacidophilum. The 2.8-kb pCG1 replicon of corynebacteria was released from pEPR1 (Knoppova et al. 2007) by NruI digestion and was subsequently cloned at dispensable DraI site of pJE-ftsRNAP and pJE-kRNAP, and so obtained E. coli–Corynebacterium sp. shuttle plasmids were designated as pJEC-ftsRNAP and pJEC-kRNAP, respectively (Fig. 1e, f).
Construction of T7 promoter-based shuttle expression vectors
Plasmids based on pBL1 and pCG1 replicons replicate in Corynebacterium sp. by rolling circle mode but having independent requirements for their replication (Fernandez-Gonzalez et al. 1994; Archer and Sinskey 1993) and, therefore, are supposed to be compatible in corynebacterial cells. Since pCG1 replicon had been used for constructing plasmids carrying the T7 RNA polymerase gene, the pBL1 replicon was used here to construct T7 promoter-based shuttle plasmids.
The intact pBL1 replicon of corynebacteria was released from plasmid pBK2 (Mukherjee et al. 1990) by HincII digestion as a 2.7-kb fragment, and it was subsequently ligated with 4.2 kb PvuII–PvuII fragment of pET28a which consists of ColE1 origin of replication, kanamycin resistant (Kmr) gene cassette, and T7 expression region. The resultant corynebacteria–E. coli shuttle vector was designated as pBJ1 (Fig. 2a–c). Since the plasmids harboring T7 RNA polymerase carried the same Kmr gene cassette, which had to be co-transformed in same host cell, thus another antibiotic resistant gene cassette was needed for T7 promoter carrying plasmid. Therefore, 1 kb intact chloramphenicol resistant (Cmr) gene cassette was excised from plasmid pACYC184 (Rose 1988) by Sau3AI digestion and subsequently cloned into pBJ1 at dispensable BglII site located upstream to T7 promoter region, and so obtained 8-kb shuttle plasmid was designated as pBJ2 (Fig. 2c–e). The Cmr gene is known to be functional in both E. coli and corynebacteria (Rose 1988; Eikmanns et al. 1991). Plasmid pBJ2 carried both Kmr gene cassette and Cmr gene cassette, and its size was too large to be an ideal expression vector; thus, plasmid pBJ2 was trimmed into a 5.7-kb shuttle plasmid, pBJ3, possessing only the Cmr marker. To obtain plasmid pBJ3, plasmid pBJ2 was digested with HaeII, and a 1.5-kb fragment, carrying both T7 expression cassette and Cmr marker, was excised out and subsequently their ends were blunted with T4 DNA polymerase. In a separate restriction digestion, the 4.2-kb fragment was released from pBJ2 by NruI and EcoRV double digestion. This fragment, carrying both pBL1 replicon of corynebacteria and ColE1 replicon of E. coli, was subsequently ligated with 1.5 kb HaeII-blunt fragment, carrying both T7 expression region and Cmr gene cassette, and thus the shuttle plasmid pBJ3 was obtained (Fig. 2e–g). Since the NcoI site of MCS is no longer available for cloning due to another NcoI site that falls into Cmr gene, therefore, the NcoI recognition site of the Cmr gene was modified through PCR-based site-directed mutagenesis using primers mut-F and mut-R, and thus obtained final construct was termed as pBJ4 (Fig. 2g, h). The commercially available E. coli T7 plasmid pET28a (Novagen) has both N- and C-terminal 6× His tags to facilitate Ni–NTA affinity purification of recombinant proteins. The Ni–NTA affinity chromatography-based purification does not depend on the host chosen for recombinant protein expression; rather, its only requirement is that the recombinant protein should have a His tag (Crowe et al. 1994). Because the T7 expression cassettes of E. coli–Corynebacterium sp. T7 shuttle plasmid pBJ4 is derived from pET28a, therefore if desirable, both N- or C-terminal His-tagged recombinant proteins can be expressed through plasmid pBJ4 and can subsequently be purified by Ni–NTA affinity chromatography.
Construction of T7 promoter-based E. coli–Corynebacterium sp. shuttle expression plasmids. a Corynebacterial plasmid pBK2 used as a source of corynebacterial replicon, pBL1; b plasmid pET28a, a T7 promoter-based expression vector for E. coli; c E. coli–Corynebacterium sp. T7 shuttle plasmid pBJ1 derived from pET28a and pBK2, carrying Kmr gene cassette; d plasmid pACYC184, a source of Cmr gene cassette; e E. coli–corynebacteria T7 shuttle plasmid pBJ2 derived from pBJ1 and pACYC184, Kmr and Cmr; f plasmid pBJ2; g E. coli–Corynebacterium sp. T7 shuttle plasmid pBJ3 derived after trimming non-essential regions of plasmid pBJ2; h E. coli–Corynebacterium sp. T7 shuttle plasmid pBJ4 derived after site-directed mutagenesis of plasmid pBJ3 at NcoI site of Cmr gene
Corynebacterial replicons pBL1 and pCG1 are compatible in C. acetoacidophilum and thus suited for dual plasmid system
Compatibility and co-stability of BL1 and pCG1 replicons were tested in C. acetoacidophilum using recombinant plasmids JEC-ftsRNAP (pCG1 replicon) and pBJ4 (pBL1 replicon). Both plasmids were sequentially co-transformed into C. acetoacidophilum through electroporation, and final transformants were selected on both kanamycin- and chloramphenicol-containing BHI plates. Both plasmids were found to be stable in corynebacteria for up to 60 generations without any selection pressure. Stability of both plasmids in C. acetoacidophilum was further confirmed by plasmid isolation at the end of a batch cultivation (Fig. S1).
High-level auto-induced expression of sfGFP through T7 promoter in E. coli
The intact coding sequence of sfGFP was PCR amplified from synthetic construct pUC–sfGFP using primers SFGFP-F and SFGFP-R, and was subsequently cloned under strong T7 promoter of pBJ4 at NcoI–SalI sites to obtain pBJ4-sfGFP. The recombinant plasmid was transformed into routinely used T7 polymerase host E. coli BL21 (DE3) along with Top10 (kRNAP) and Top10 (ftsRNAP) expression hosts.
In Top10 (kRNAP) and Top10 (ftsRNAP) expression hosts, the T7 RNA polymerase gene was supplied through low copy number plasmids pJE-kRNAP and pJE-ftsRNAP, respectively. In Top10 (kRNAP), T7 RNA polymerase gene is under the control of a constitutive promoter (P-kan) of Tn9-derived Kmr resistant gene, whereas in Top10 (ftsRNAP) the same is under control of the ftsZ promoter (P-ftsZ) of C. acetoacidophilum. In BL21 (DE3), chromosomally integrated single copy of T7 RNA polymerase gene is under control of the IPTG-inducible lacUV5 promoter which was made constitutive by adding a saturating concentration of IPTG (1 mM) into medium at the time of inoculation.
Our results show that in Top10 (ftsRNAP) host, sfGFP expression is too low during initial growth phase, but as the growth of culture proceeds towards late-log phase and stationary phase, the expression level of sfGFP dramatically increases. Notably, the expression level of sfGFP at stationary phase of Top10 (ftsRNAP) host was much higher than that of Top10 (kRNAP) and BL21 (DE3) hosts which were constitutively expressing T7 RNA polymerase (Fig. 3a). Transformation of E. coli Top10 (ftsRNAP) cells with plasmid pB4-sfGFP gave intense green colonies on antibiotics-containing LA plate (Fig. 4a). High level of sfGFP into Top10 (ftsRNAP) cells was also evident from fluorescence microscopy (Fig. 4b).
Comparative expression of sfGFP into E. coli and C. acetoacidophilum at different growth phases. a Relative fluorescence intensities (smooth lines) and their corresponding optical densities, OD600nm (dotted lines), for E. coli hosts either lacking T7 RNA polymerase gene (Top10/pBJ4-sfGFP) or harboring T7 RNA polymerase under ftsZ promoter(s) [Top10 (pJE-ftsRNAP)/pBJ4-sfGFP], or constitutive Kmr promoter [Top10 (pJE-kRNAP)/pBJ4-sfGFP], or constitutive lacUV5 promoter [BL21 (DE3)/pBJ4-sfGFP]. LacUV5 promoter of BL21 (DE3) was made constitutive by adding 1 mM IPTG at the time of inoculation. Untransformed E. coli Top10 were taken as negative control. b Relative fluorescence intensities (smooth lines) and their corresponding optical densities, OD600nm (dotted lines), for C. acetoacidophilum hosts either lacking T7 RNA polymerase gene (B-30st) or harboring T7 RNA polymerase under ftsZ promoter(s) [B-30st (pJEC-ftsRNAP)/pBJ4-sfGFP], or constitutive Kmr promoter [B-30st (pJEC-kRNAP)/pBJ4-sfGFP]. Untransformed C. acetoacidophilum B-30st were taken as negative control
T7 promoter-derived expression of sfGFP into E. coli Top10 and corynebacteria co-transformed with plasmids pJE-ftsRNAP and pBJ4-sfGFP. a Green E. coli colonies on Luria-agar plates viewed under trans-UV as a result of sfGFP expression; b fluorescence microscopy image of E. coli cells expressing sfGFP; c fluorescence microscopy images of C. acetoacidophilum (B-30st): expression of sfGFP in cells carrying plasmids pJEC-ftsRNAP and pBJ4-sfGFP
T7 Promoter system is functional in C. acetoacidophilum in a growth-phase-dependent manner
To study the function of T7 RNA polymerase gene in C. acetoacidophilum, recombinant plasmid pBJ4-sfGFP was co-transformed into C. acetoacidophilum along with either pJEC-kRNAP or pJEC-ftsRNAP by electroporation. Expression of sfGFP into C. acetoacidophilum was checked by measuring fluorescence intensity as earlier done for E. coli. Although, as compared to E. coli, in C. acetoacidophilum the expression level of sfGFP was found to be lesser, the two-plasmid-based T7 RNA polymerase-dependent system was also found to be functional in C. acetoacidophilum. Our results reveal that in C. acetoacidophilum host that was constitutively expressing T7 RNA polymerase, i.e., B.30st (pJEC-kRNAP), sfGFP expression level was almost constant throughout the growth phases (∼10 A.U.), whereas the expression of recombinant protein (sfGFP) in C. acetoacidophilum host that was expressing T7 RNA polymerase through ftsZ promoter(s), i.e., B-30st (pJEC-ftsRNAP), was almost shut off up to a certain level of growth phase, and after crossing that level, the expression of recombinant protein through T7 promoter started increasing drastically without the addition of any external inducer. Eventually, the stationary phase expression level of sfGFP in B-30st (pJEC-ftsRNAP) host was many folds higher than that of the constitutive one (Fig. 3b). Fluorescence microscopy and SDS–PAGE data further confirm high-level expression of sfGFP into ftsZ promoter-dependent T7 RNA polymerase expressing C. acetoacidophilum host (Figs. 4c and 5).
SDS–PAGE analysis for expression of sfGFP into C. acetoacidophilum. Lane-1 low molecular weight protein marker, 2–4 cell lysates of B-30st cells co-transformed with plasmids pJEC-ftsRNAP and pBJ4-sfGFP after 2 h, 12 h, and 16 h growth, respectively; 5 cell lysate of B-30st without plasmid (negative control). An intense band of sfGFP (26 kDa) is visible in late-log and stationary phase samples in lane 3 (12 h) and lane 4 (16 h), respectively, which is undetectable in lag or very early log phase sample in lane 2 (4 h)
Discussion
In prokaryotes, ftsZ, a tubulin-like cell cycle protein, is conserved throughout the bacteria and the archaea. It polymerizes to make a contractile ring, the Z-ring, during cell division at the division site. The topology, the frequency, and the timing of division are solely regulated by the varying concentration of ftsZ in host cell (Lutkenhaus and Addinall 1997). For ftsZ transcription, multiple promoters have been elucidated in diverse bacteria (Letek et al. 2007; Kiran et al. 2009), and the overall expression level of ftsZ is known to oscillate during cell cycle due to the cumulative activity of various promoters (Garrido et al. 1993).
The ftsZ promoter activity is almost shut off in non-dividing cells and becomes active in the dividing ones since the ftsZ protein plays a pivotal role in cell division. Moreover, the activity of ftsZ promoter has been observed to be growth dependent. During lag and early log phases, where no significant cell division takes place, activity of ftsZ is hardly detectable, but the promoter is highly active by an unknown mechanism when cells reach late-log and stationary phase (Weart and Levin 2003).
In E. coli, BL21 (DE3) is the most prevalent system for high-level recombinant protein production where chromosomally integrated T7 RNA polymerase is under the IPTG-inducible UV5 promoter (Studier and Moffatt 1986). Tabor and Richardson (1985) developed a dual-plasmid-based T7 system where the plasmid pGP1–2 harbors T7 RNA polymerase which is under the control of heat-inducible promoter PL of lambda phage. Chao et al. (2002) developed E. coli BL21 (BAD) strain for toxic protein expression placing the chromosomally integrated T7 RNA polymerase gene under tightly regulated l-arabinose-inducible araBAD promoter of E. coli. These T7-based systems in E. coli are proven essential protein expression tools, but they all are based on external inducers. While IPTG is expensive and imposes toxicity to the growing culture, heat induction may not be a preferred choice as it increases the protease level which may eventually degrade the target proteins. Also, heat may cause significant death of bacterial host cells. An expression system independent of external inducer is a cost-effective system and may be best suited for easy scale-up.
As far as corynebacteria are concerned, very little success is achieved so far with the attempts made to develop an expression system in corynebacteria, inducible and constitutive, capable of producing a high level of target proteins. Lack of lacY and araE in most of the Corynebacterium strains and impermeability of its cell membrane to common inducers, IPTG and l-arabinose, through diffusion process come as bottlenecks to develop an IPTG/l-arabinose-inducible system in Corynebacterium sp. Also, heterologous cloning of lacY and araE transporters into C. glutamicum did not produce desirable results (Zhang et al. 2012).
Expression systems based on strong T7 promoter are widely used for the industrial production of proteins where high-level expression of heterologous protein is of prime concern. In this context, this is the very first report of a T7 promoter-based system in Corynebacterium sp. Its function was shown in both C. acetoacidophilum and gram-negative E. coli. It might be also used for high-level production of heterologous proteins in C. glutamicum and other related corynebacteria.
References
Archer JA, Sinskey AJ (1993) The DNA sequence and minimal replicon of the Corynebacterium glutamicum plasmid pSR1: evidence of a common ancestry with plasmids from C. diphtheriae. J Gen Microbiol 139(8):1753–1759
Billman-Jacobe H, Hodgson AL, Lightowlers M, Wood PR, Radford AJ (1994) Expression of ovine gamma interferon in Escherichia coli and Corynebacterium glutamicum. Appl Environ Microbiol 60(5):1641–1645
Billman-Jacobe H, Wang L, Kortt A, Stewart D, Radford A (1995) Expression and secretion of heterologous proteases by Corynebacterium glutamicum. Appl Environ Microbiol 61:1610–1613
Brabetz W, Liebl W, Schleifer KH (1993) Lactose permease of Escherichia coli catalyzes active β-galactoside transport in a gram-positive bacterium. J Bacteriol 175(22):7488–7491
Chao YP, Chiang CJ, Hung WB (2002) Stringent regulation and high-level expression of heterologous genes in Escherichia coli using T7 system controllable by the araBAD promoter. Biotechnol Prog 18(2):394–400
Cohen SN, Chang AC, Hsu L (1972) Non-chromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci USA 69(8):2110–2114
Crowe J, Döbeli H, Gentz R, Hochuli E, Stüber D, Henco K (1994) 6xHis–Ni–NTA chromatography as a superior technique in recombinant protein expression/purification. Methods Mol Biol 31:371–387
Date M, Yokoyama K, Umezawa Y, Matsui H, Kikuchi Y (2003) Production of native-type Streptoverticillium mobaraense transglutaminase in Corynebacterium glutamicum. Appl Environ Microbiol 69:3011–3014
Date M, Yokoyama K, Umezawa Y, Matsui H, Kikuchi Y (2004) High level expression of Streptomyces mobaraensis transglutaminase in Corynebacterium glutamicum using a chimeric pro-region from Streptomyces cinnamoneus transglutaminase. J Bacteriol 110(3):219–226
Date M, Itaya H, Matsui H, Kikuchi Y (2006) Secretion of human epidermal growth factor by Corynebacterium glutamicum. Lett Appl Microbiol 42:66–70
Deb JK, Malik S, Ghosh VK, Mathai S, Sethi R (1990) Intergeneric protoplast fusion between xylanase producing Bacillus subtilis LYT and Corynebacterium acetoacidophilum ATCC 21476. FEMS Microbiol Lett 59(3):287–292
Eikmanns BJ, Kleinertz E, Liebl W, Sahm H (1991) A family of Corynebacterium glutamicum/Escherichia coli shuttle vectors for cloning, controlled gene expression, and promoter probing. Gene 102(1):93–98
Fernandez-Gonzalez C, Cadenas RF, Noirot-Gros MF, Martin JF, Gil JA (1994) Characterization of a region of plasmid pBL1 of Brevibacterium lactofermentum involved in replication via the rolling circle model. J Bacteriol 176(11):3154–3161
Garrido T, Sanchez M, Palacios P, Aldea M, Vicente M (1993) Transcription of ftsZ oscillates during the cell cycle of Escherichia coli. EMBO J 12(10):3957–3965
Itaya H, Kikuchi Y (2008) Secretion of Streptomyces mobaraensis pro-transglutaminase by coryneform bacteria. Appl Microbiol Biotechnol 78(4):621–625
Jana S, Karan G, Deb JK (2005) Purification of streptomycin adenylyltransferase from a recombinant Escherichia coli. Protein Expr Purif 40(1):86–90
Karan G (2000) Characterization of streptomycin resistant mutant of Corynebacterium acetoacidophilum ATCC 21476. Ph.D. dissertation, Indian Institute of Technology Delhi, India
Kawaguchi H, Sasaki M, Vertès AA, Inui M, Yukawa H (2009) Identification and functional analysis of the gene cluster for L-arabinose utilization in Corynebacterium glutamicum. Appl Environ Microbiol 75(11):3419–3429
Kikuchi Y, Date M, Yokoyama K, Umezawa Y, Matsui H (2003) Secretion of active-form Streptoverticillium mobaraense transglutaminase by Corynebacterium glutamicum: processing of the co-domain by a co-secreted subtilisin-like protease from Streptomyces albogriseolus. Appl Environ Microbiol 69:358–366
Kikuchi Y, Date M, Itaya H, Matsui K,Wu L (2006) Functional analysis of twin-arginine translocation pathway in Corynebacterium glutamicum ATCC 13869. Appl Environ Microbiol 72:7183–7192
Kimura E (2003) Metabolic engineering of glutamate production. Adv Biochem Eng Biotechnol 79:37–57
Kiran M, Maloney E, Lofton H, Chauhan A, Jensen R, Dziedzic R, Madiraju M, Rajagopalan M (2009) Mycobacterium tuberculosis ftsZ expression and minimal promoter activity. Tuberculosis (Edinb) 89(Suppl 1):S60–S64
Knoppova M, Phensaijai M, Vesely M, Zemanova M, Nesvera J, Patek M (2007) Plasmid vectors for testing in vivo promoter activities in Corynebacterium glutamicum and Rhodococcus erythropolis. Curr Microbiol 55:234–239
Krämer R (1994) Secretion of amino acids by bacteria: physiology and mechanism. FEMS Microbiol Rev 12:75–94
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Letek M, Ordóñez E, Fiuza M, Honrubia-Marcos P, Vaquera J, Gil JA, Castro D, Mateos LM (2007) Characterization of the promoter region of ftsZ from Corynebacterium glutamicum and controlled overexpression of FtsZ. Int Microbiol 10(4):271–282
Liebl W, Sinskey AJ, Schleifer K (1992) Expression, secretion, and processing of staphylococcal nuclease by Corynebacterium glutamicum. J Bacteriol 174:1854–1861
Lutkenhaus J, Addinall SG (1997) Bacterial cell division and the Z ring. Annu Rev Biochem 66:93–116
Makrides SC (1996) Strategies for achieving high-level expression of genes in Escherichia coli. Microbiol Rev 60(3):512–538
Mukherjee KJ, Deb JK, Ramachandran KB (1990) Construction of vector for Brevibacterium lactofermentum and study of its stability in continuous culture. J Biotechnol 16:109–122
Ozaki A, Katsumata R, Oka T, Furuya A (1984) Functional expression of the genes of Escherichia coli in gram-positive Corynebacterium glutamicum. Mol Gen Genet 196:175–178
Paradis FW, Warren RAJ, Kilburn DG, Miller RC (1987) The expression of Cellulomonas fimi cellulase genes in Brevibacterium lactofermentum. Gene 61:199–206
Pátek M, Nesvera J, Guyonvarch A, Reyes O, Leblon G (2003) Promoters of Corynebacterium glutamicum. J Biotechnol 104(1–3):311–323
Pédelacq JD, Cabantous S, Tran T, Terwilliger TC, Waldo GS (2006) Engineering and characterization of a superfolder green fluorescent protein. Nat Biotechnol 24(1):79–88
Pérola OM, André ML, Priscila GM, Carlota RY, Thereza CVP, Adalberto P Jr (2007) Methods of endotoxin removal from biological preparations: a review. J Pharm Pharmaceut Sci 10(3):388–404
Rose RE (1988) The nucleotide sequence of pACYC184. Nucleic Acids Res 16(1):355
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Santamaria RI, Gil JA, Mesas JM, Martin JF (1984) Characterization of an endogenous plasmid and development of cloning vectors and a transformation system in Brevibacterium lactofermentum. J Gen Microbiol 130:2237–2246
Smith MD, Flickinger JL, Lineberger DW, Schmidt B (1986) Protoplast transformation in coryneform bacteria and introduction of an alpha-amylase gene from Bacillus amyloliquefaciens into Brevibacterium lactofermentum. Appl Environ Microbiol 51(3):634–639
Srivastava P, Deb JK (2002) Construction of fusion vectors of corynebacteria: expression of glutathione-S-transferase fusion protein in Corynebacterium acetoacidophilum ATCC 21476. FEMS Microbiol Lett 212(2):209–216
Studier FW, Moffatt BA (1986) Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol 189(1):113–130
Tabor S, Richardson CC (1985) A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci USA 82:1074–1078
van der Rest ME, Lange C, Molenaar D (1999) A heat shock following electroporation induces highly efficient transformation of Corynebacterium glutamicum with xenogenic plasmid DNA. Appl Microbiol Biotechnol 52:541–545
Walia RW, Deb JK, Mukherjee KJ (2007) Development of expression vectors for Escherichia coli based on the pCR2 replicon. Microb Cell Fact 10:6–14
Weart RB, Levin AP (2003) Growth rate-dependent regulation of medial ftsZ ring formation. J Bacteriol 185:2826–2834
Zhang Y, Shang X, Lai S, Zhang G, Liang Y, Wen T (2012) Development and application of an arabinose-inducible expression system by facilitating inducer uptake in Corynebacterium glutamicum. Appl Environ Microbiol 78(16):5831–5838
Acknowledgments
The authors acknowledge financial and other supports given by the Indian Institute of Technology Delhi and Ministry of Drinking Water and Sanitation, GOI. MJE was supported by fellowships from the Department of Biotechnology, India as Junior Research Fellowship (DBT–JRF) and Indian Institute of Technology Delhi as Teaching Assistantship.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jahar Kanti Deb is deceased.
Electronic supplementary material
Below is the link to the electronic supplementary materials.
ESM 1
(PDF 43 kb)
Rights and permissions
About this article
Cite this article
Equbal, M.J., Srivastava, P., Agarwal, G.P. et al. Novel expression system for Corynebacterium acetoacidophilum and Escherichia coli based on the T7 RNA polymerase-dependent promoter. Appl Microbiol Biotechnol 97, 7755–7766 (2013). https://doi.org/10.1007/s00253-013-4900-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4900-3