Abstract
Pyruvate decarboxylases (PDCs) are a class of enzymes which carry out the non-oxidative decarboxylation of pyruvate to acetaldehyde. These enzymes are also capable of carboligation reactions and can generate chiral intermediates of substantial pharmaceutical interest. Typically, the decarboxylation and carboligation processes are carried out using whole cell systems. However, fermentative organisms such as Saccharomyces cerevisiae are known to contain several PDC isozymes; the precise suitability and role of each of these isozymes in these processes is not well understood. S. cerevisiae has three catalytic isozymes of pyruvate decarboxylase (ScPDCs). Of these, ScPDC1 has been investigated in detail by various groups with the other two catalytic isozymes, ScPDC5 and ScPDC6 being less well characterized. Pyruvate decarboxylase activity can also be detected in the cell lysates of Komagataella pastoris, a Crabtree-negative yeast, and consequently it is of interest to investigate whether this enzyme has different kinetic properties. This is also the first report of the expression and functional characterization of pyruvate decarboxylase from K. pastoris (PpPDC). This investigation helps in understanding the roles of the three isozymes at different phases of S. cerevisiae fermentation as well as their relevance for ethanol and carboligation reactions. The kinetic and physical properties of the four isozymes were determined using similar conditions of expression and characterization. ScPDC5 has comparable decarboxylation efficiency to that of ScPDC1; however, the former has the highest rate of reaction, and thus can be used for industrial production of ethanol. ScPDC6 has the least decarboxylation efficiency of all three isozymes of S. cerevisiae. PpPDC in comparison to all isozymes of S. cerevisiae is less efficient at decarboxylation. All the enzymes exhibit allostery, indicating that they are substrate activated.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
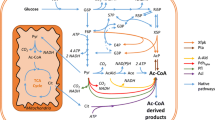
Pyruvate decarboxylases (PDCs) are an important class of enzymes which are at the branch point of the respiratory and fermentative pathways in many organisms. PDC carries out non-oxidative decarboxylation of pyruvate to form acetaldehyde in the presence of its cofactors, Mg2+ (Schellenberger 1967) and thiamine pyrophosphate (TPP) (Lohmann and Schuster 1937). Acetaldehyde is then converted to various metabolites such as ethanol and acetate. Thus, the efficiency of this enzyme is a key factor in diverting the flow of pyruvate away from the tricarboxylic acid cycle (TCA) and towards fermentation. PDCs are reported in various bacteria, yeast, fungal species, and plants (König 1998). The crystal structures of PDCs from a few organisms including Saccharomyces cerevisiae (ScPDC1) (Arjunan et al. 1996), Kluyveromyces lactis (KlPDC) (Kutter et al. 2006), and Zymomonas mobilis (ZmPDC) (Dobritzsch et al. 1998) have been reported. Based on structural information, the catalytic and regulatory mechanisms of the PDCs have been elucidated by various groups (Baburina et al. 1996; Bruhn et al. 1995; Candy and Duggleby 1998; Krieger et al. 2002; Kutter et al. 2006). The level of PDC activity is one of the important differences in the carbon metabolism of Crabtree-positive and Crabtree-negative yeasts. There is on average, sixfold higher PDC activity found in the cell-free extracts of Crabtree-positive yeasts (van Urk et al. 1990).
Another physiological role for ScPDCs has been described: in the absence of pyruvate decarboxylase activity, S. cerevisiae is not able to grow on glucose as acetaldehyde, the substrate for alcohol dehydrogenase (ADH), is not available. The conversion of acetaldehyde to ethanol is a crucial redox step to oxidize the NADH generated in glycolysis. Acetaldehyde is also utilized to generate the cytoplasmic acetyl CoA necessary for lipid biosynthesis. This reaction is the only source of cytosolic acetyl CoA and hence the absence of PDC proves lethal for S. cerevisiae, when grown on glucose (Flikweert et al. 1996) as the only carbon source. In a similar study in K. lactis (a Crabtree-negative yeast), PDC has been found, however, to not be essential for its survival (Breunig et al. 2000).
Six PDC genes have been reported in S. cerevisiae; of these, ScPDC1, ScPDC5, and ScPDC6 have been reported to code for catalytic proteins, where as ScPDC2 and ScPDC3 code for regulatory proteins. ScPDC1 and ScPDC5 are 88 % identical at the protein level. ScPDC1 is responsible for most of the PDC activity in the wild-type yeast, whereas ScPDC5 activity has been detected only in the absence of ScPDC1 (Schaaff et al. 1989). Expression of ScPDC6 was not detected in yeasts cells under normal physiological conditions, not even in a ∆ScPdc1, 5 strain (Hohmann and Cederberg 1990). However, recent reports suggest that it is expressed under sulfur-limiting conditions (Fauchon et al. 2002) and during high sugar stress situations (Erasmus et al. 2003). ScPDC2 has been shown to have a regulatory role on the expression of the catalytic isozymes ScPDC1 and ScPDC5. ΔScPdc2 mutants showed a reduced expression of ScPDC1 and 5 (Hohmann 1993). ScPDC2 also regulates the genes involved in thiamine uptake (Mojzita and Hohmann 2006).
Prior to this study, there has been no systematic comparative analysis of all three purified isozymes of ScPDCs. ScPDC1 containing cell lysates have been characterized, but the other two isozymes have not been kinetically characterized.
PDCs have not only been found in fermentative yeasts but also in respiratory yeasts like K. lactis. Recently, PDCs have been isolated from respiratory yeasts like Ogataea polymorpha (Ishchuk et al. 2008), Scheffersomyces stipitis (Lu et al. 1998), and Wickerhamomyces anomalus (Fredlund et al. 2006). These species are all Crabtree negative.
Komagataella pastoris is methylotrophic yeast and an industrially important organism for recombinant protein production. It is well known for its highly respiratory mode of growth which results in high cell densities. Unlike S. cerevisiae, K. pastoris is respiratory yeast which under normal aerobic conditions does not produce ethanol. Presence of a putative PDC gene has been reported in this yeast (De Schutter et al. 2009; Mattanovich et al. 2009). There are two aspects of the fermentation pathway of K. pastoris which are interesting. Since the organism has a basic fermentative pathway (both PDC and ADH ORFs and activities are present), the first interesting aspect to know is whether this yeast could be developed into a host for production of fermentation products such as ethanol. Second, recombinant protein production has been reported to decrease due to the ethanol production (Inan and Meagher 2001) in the presence of excess glycerol along with partial anaerobic conditions due to high cell densities in batch fermentations. Knowledge of the fermentative activities in K. pastoris, and in particular the role of PDC, would be beneficial towards understanding this phenomenon.
Apart from the decarboxylation reaction, pyruvate decarboxylases are also known for their carboligation capabilities and have been exploited for the synthesis of commercially relevant compounds (Iding et al. 1998). During this reaction, the active aldehyde in the active site is condensed with a second aldehyde as a co-substrate to form hydroxy ketones; when the co-substrate is acetaldehyde, (R)-acetoin is formed (Singer and Pensky 1951). Acetoin is commonly used as a flavoring agent and hence has commercial value. When benzaldehyde is used as co-substrate; (R)-phenyl acetyl carbinol (PAC) is formed (Hildebrandt and Klavehn 1932). (R)-PAC is a chiral intermediate in the formation of (R)-ephedrine and (S)-pseudoephedrine, which have α and β adrenergic activity and are used as nasal decongestants and stimulants (Reynolds 1989). Wild-type S. cerevisiae strains are currently used in whole cell (R)-PAC biotransformation process and the potential of each isozyme for carboligation has not been systematically investigated. In this work, we explore the capabilities of the ScPDC isozymes and PpPDC, for (R)-PAC formation.
We have analyzed and compared the efficiencies of the three ScPDC isozymes and the single isozyme present in K. pastoris towards better understanding the evolutionary significance of each enzyme in terms of its kinetic properties. We have used similar conditions of expression, purification, and characterization for all the isozymes towards maintaining an identical environment for this kinetic comparison.
Materials and methods
Strains
Escherichia coli strain DH5α (Invitrogen Corporation, Carlsbad, CA, USA) was used for routine plasmid manipulation and maintenance. BL21(DE3)plysS (EMD Millipore-Merck, Darmstadt, Germany) was used for recombinant protein expression in E. coli. S. cerevisiae strains BY4741 was obtained from Euroscarf, University of Frankfurt, Germany. YSH pdc null strain 512.17C of S. cerevisiae was kindly provided by S. Hohmann (Department of Cell and Molecular Biology/Microbiology University of Gothenburg, Goeteborg, Sweden). K. pastoris GS115 strain was obtained from Invitrogen Corporation, Carlsbad, CA, USA.
The plasmids used in this study are pET43.1b by Novagen (EMD Millipore-Merck, Darmstadt, Germany), an episomal vector used for expression in E. coli under T7 promoter. It has ampicillin resistance gene which is used as selection marker. pRS426GPD yeast shuttle vector (Mumberg et al. 1995) was used for expression in yeast. Expression is driven by constitutive glyceraldehyde-3-phosphate (GPD) promoter. Selection in E. coli is by ampicillin resistance and in S. cerevisiae by complementation of ura auxotrophy. The vector was kindly provided by P. J. Bhat (Department of Biosciences and Bioengineering, IIT Bombay, Mumbai, India)
Media and other components
Luria–Bertani broth was used for growing E. coli strains and YPD (yeast extract—10 g/l, peptone—20 g/l, and dextrose—20 g/l) was used for growth of S. cerevisiae and K. pastoris. Ethanol and methanol were used at 3.9 g/l when used as carbon source. A defined minimal medium for selection of yeast transformants was composed of 20 g/l dextrose, 6.7 g/l yeast nitrogen base, and 0.5 g/l amino acid mixture without uracil.
Experiments which involved the measurements of PDC activity on different carbon sources had basal media YP (yeast extract—10 g/l, peptone—20 g/l). The concentration of carbon sources used were dextrose—20 g/l (2 % w/v), ethanol—3.9 g/l (0.5 % v/v), methanol—3.9 g/l (0.5 % v/v), glycerol—25.2 g/l (2 % v/v).
Yeast alcohol dehydrogenase (Catalog no G8772) was acquired from Sigma Aldrich (St. Louis, USA). NADH (Catalog no 044018) and sodium pyruvate (Catalog no 194796) was obtained from SRL Ltd (Mumbai, India). Chelating sepharose resins (Catalog no-17-0575-01) from GE Healthcare (Chalfont St Giles, U.K) were used for His-tagged protein purification.
The primers used in the study are listed in Table 1.
Growth conditions
E. coli strains were grown at 37 °C, 250 rpm and S. cerevisiae and K. pastoris strains were grown at 30 °C, 250 rpm in 250 ml or 500 ml flasks for 50 ml and 100 ml culture volumes, respectively.
Cloning of S. cerevisiae PDC genes
ORFs for ScPDC1 (NC_001144.5 coordinates (234081–232390)), ScPDC5 (NC_001144.5 coordinates (410723–412414)) and ScPDC6 (NC_001139.9 coordinates (652981–651290)) were amplified from the genome of S. cerevisae strain BY4741 and cloned in pET43.1.b vector in frame with the sequence for a C-terminal His-tag (encoding 6-histidine residues). They were subsequently subcloned into pRS426GPD by amplification with a gene-specific forward primer and a T7 reverse primer (to include the His-tag at the C-terminus of the resulting gene product). The amplified, tagged, products were cloned into the pRS426GPD vector and were confirmed by sequencing and by restriction digestion.
Cloning of K. pastoris PDC (PpPDC)
PpPDC (NC_012965.1 coordinates (383969–382287)) was amplified from the cDNA of GS115 using gene-specific primers (PpPDCF and PpPDCR), the reverse primer containing the His-tag sequence. The RNA isolation was carried out according to the method described by Schmitt et al. (1990). cDNA was synthesized in accordance with the protocol of 3′RACE by Invitrogen (Catalog no. 18373–019, Carlsbad, USA). The amplified PpPDC gene was confirmed by sequencing. It was then subcloned into pRS426GPD vector and confirmed by restriction analysis
Yeast transformation
Yeast strains were transformed by the LiAc method (Ito et al. 1983). Transformed cells were selected on a defined minimal medium without uracil.
Expression of PDCs
Protein expression in the recombinant S. cerevisiae strain was driven by the constitutive GPD promoter, which expresses PDCs when glucose was used as carbon source. Yeast strains were grown in a defined minimal medium and harvested at an OD600nm of 1.0–1.2. Cells were washed with citrate buffer (pH 6.0) with 1 mM TPP, and 2 mM MgCl2, and were stored at −80 °C until further analysis.
Cell free extract preparation
A yeast cell-free extract was prepared by the glass beads method. Cells were thawed and re-suspended in a buffer containing 20 mM sodium phosphate, 0.5 M NaCl, 10 mM imidazole, 1 mM TPP and 2 mM MgCl2, 1/3 volume of glass beads, and 1 μl of protease inhibitor (Sigma catalogue no P8215). Cell lysates were centrifuged for 30 min at 16,000×g at 4 °C, and supernatants were collected and used immediately or were stored at 4 °C till further use.
Purification of His-tagged PDCs
Chelating sepharose resin was used for purification of His-tagged proteins according to the manufacturer's protocol. Bound PDC proteins were eluted with a series of elution buffers with 0.1, 0.2, and 0.3 M imidazole. The elution fractions were run on denaturing 12 % SDS PAGE and stained with Coomassie brilliant blue stain to observe the purity of eluted PDCs. PDC proteins were eluted in 0.2 M imidazole containing elution buffers. The fractions with >95 % of purity of the expected size (∼60 kDa) were pooled and concentrated using centrifugal filter devices of 10 kDa cut off membranes (EMD Millipore-Merck, Darmstadt, Germany). Glycerol was added to a final concentration of 40 % and the protein was then stored at −20 °C till further use.
Pyruvate decarboxylase activity
PDC activity was determined by a coupled assay using alcohol dehydrogenase (Ullrich et al. 1966). The assay mix contained 33 mM sodium pyruvate, 180 mM citrate buffer pH 6.8, 1 mM TPP and 2 mM MgCl2, 0.11 mM NADH, and 30–40 U of alcohol dehydrogenase. The reaction was started by adding NADH and the decrease in absorbance was measured at 340 nm for 1 min. One unit of PDC activity is defined as the activity required for converting 1 μmol of pyruvate to 1 μmol of acetaldehyde in 1 min.
Determination of kinetic parameters (K 0.5 and V max)
Purified PDC were analyzed for their kinetics properties using ADH coupled assay as described above. A range of substrate pyruvate concentrations from 0.1 to 40 mM was used for determination of steady-state parameters. The specific activity (V 0) was plotted against substrate concentration ([S]), and data were plotted to the Hill equation using the Sigma Plot Software (Systat Software Inc, San Jose, CA), to obtain the values for V max, K 0.5, and n (the Hill coefficient).
pH analysis of the PDCs
pH analysis was done by measuring the PDC activity in a triple buffer (50 mM MES, 100 mM Tris, 50 mM acetic acid) of different pH ranging from pH 4.0 to pH 9.0. This buffer system keeps the ionic strength constant over a wide range of pH. The activity was then plotted against the log pH (Chang et al. 2001).
(R)-PAC measurement for carboligation activity
Equal amount of enzyme solution and carboligase buffer (3 M ethanol, 100 mM pyruvate, and 50 mM benzaldehyde in 250 mM citrate buffer) were incubated for 30 min at 25 °C. The reaction was stopped by adding 10 % trichloro-acetic acid and incubated on ice for 5 min followed by centrifugation at 16,000×g for 5 min. Dilutions were made and added to 900 μl of mobile phase (acetonitrile:water:acetic acid 30:70:0.5) and injected into a HiQ sil C-18 column (supplied by KYA Technologies Corporation, Tokyo, Japan) maintained at 30 °C with a flow rate of 1.5 ml/min for 15 min. Quantities were calculated using standard curve prepared from pure standard ((R)-PAC standards were obtained as a gift from Embio Research Center, Mumbai, India). One unit of carboligation activity is defined as the amount of PDC activity which produces 1.0 μmol of (R)-PAC from benzaldehyde and pyruvate per minute at pH 7.0 and 25 °C.
Results
Cloning, expression, purification of ScPDC isozymes
Initially, the genes for all the ScPDC isozymes were cloned in E. coli expression vector pET43.1b. However, only ScPDC1 was recovered in soluble fraction from transformed E. coli BL21(DE3) cell-free extracts, while ScPDC5 and ScPDC6 were found in inclusion bodies. To express all the isozymes in an active form, these isozymes were subcloned in yeast expression vector pRS426 GPD. PpPDC was also cloned into the same vector. These were then expressed in a pdc null strain of S. cerevisiae. This strain is unable to grow on fermentable carbon sources due to the absence of functional PDCs. The absence of PDCs creates a redox imbalance and a possible shortage of acetyl CoA for lipid synthesis (Flikweert et al. 1996); as in the absence of PDC, there is no alternative pathway to generate acetyl CoA in cytoplasm. The cytoplasmic acetyl CoA is essential for lipid biosynthesis as there is no known mechanism of transport of mitochondrial acetyl CoA to cytoplasm. When a PDC is expressed in this pdc null background, growth on glucose is revived, facilitating a selection strategy for recombinants with the PDC activity. All three isozymes, ScPDC1, ScPDC5, and ScPDC6 as well as PpPDC were able to complement the PDC function and resulted in revival of growth of the pdc null strain on glucose.
PDC isozymes were expressed individually and purified (Fig. 1) to homogeneity from pdc null strain of S. cerevisiae.
Kinetic characterization
Purified PDC isozymes were analyzed for pyruvate-dependent steady-state kinetics (Table 2). All the isozymes of S. cerevisiae and PpPDC demonstrated sigmoidal kinetic behavior consistent with substrate activation (Fig. 2), as has been earlier reported for cell lysates containing ScPDC1 (Hübner et al. 1978). Hill coefficients of more than 1 were observed for ScPDC1 (1.9), ScPDC5 (1.6), ScPDC6 (1.7), and PpPDC (2.1). Similarly, differences in K 0.5 were observed for all the PDCs (Table 2). It was observed that ScPDC5 has the highest V max compared to even ScPDC1, and that ScPDC6 is the slowest among the isozymes of the S. cerevisiae. PpPDC has a lower V max than all the isozymes of the S. cerevisiae. It also has the highest K 0.5 and therefore lowest affinity for pyruvate.
Plots of reaction rates for PDCs versus substrate (pyruvate) concentration. Steady-state kinetics for pyruvate at concentrations of 0.1 to 35 mM (a ScPDC1, c ScPDC5, e ScPDC6, g PpPDC) and subplots demonstrating substrate activation at low pyruvate concentrations (b ScPDC1, d ScPDC5, f ScPDC6, h PpPDC). The experiments were done in triplicates and the error bars represent the standard deviations
pH kinetics
A pH rate profile was generated for all three S. cerevisiae enzymes with varying pH. All PDCs studied here have a pH optimum in the range 6–7 (Table 3), and their pH profiles pH rates) are similar.
Decarboxylation capacities of pdc null strain overexpressing the individual isozymes
Decarboxylation was measured in the cell-free extracts of the pdc null strain overexpressing the various PDCs (Fig. 3). It was found that the decarboxylation activity was highest in the cell-free extracts of the strain expressing ScPDC5, followed by ScPDC1, ScPDC6, and PpPDC. The PDC activities in the cell-free extracts show a similar trend as that of the V max of these enzymes. In the unregulated background (under a different promoter) where there is a saturated level of substrate and enzyme, the only factor which influences the PDC activity in the cell-free extracts is the V max (reaction rate) of the enzymes.
Carboligation capacities of the PDC proteins
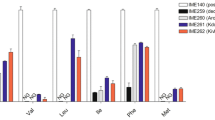
A comparison of the (R)-PAC formation rates of the three isozymes of S. cerevisiae was done by measuring in vitro carboligation of the recombinant strains harboring single PDC isozymes (Fig. 4). It was found that the rate of (R)-PAC was higher for ScPDC5 though unlike their decarboxylation activity patterns, the carboligation rate of ScPDC5 is only marginally higher than that of ScPDC1. ScPDC6 has the lowest carboligation capacity of the S. cerevisiae isozymes but PpPDC was the least efficient of all the PDCs evaluated.
Growth and ethanol production profiles of pdc null strain expressing ScPDC1, 5, 6, and PpPDC
pdc null strains expressing PDCs were analyzed for growth and ethanol production (Fig. 5). Strains containing isozymes of S. cerevisiae showed identical growth patterns, whereas the strain containing PpPDC showed a lower specific growth rate. Stationary phase cell densities were similar however.
The ethanol production profile was similar for ScPDC1- and ScPDC5-expressing strains. There was lower ethanol production with ScPDC6, than with other two isozymes. The PpPDC-expressing strain showed the lowest amount of ethanol production. This corresponds to the relative efficiencies of the enzymes, though the initial lag in the growth of strain expressing PpPDC could be a contributing factor in the lower ethanol production. It was observed that the ethanol levels produced at the onset of stationary phase was the same for strains expressing any of the four isozymes.
PDC activity of K. pastoris in various carbon sources
K. pastoris can use various carbon sources. Hence, PDC activity was assayed for in the crude extracts of cultures grown on methanol, ethanol, glycerol, and glucose. It was observed that the highest PDC activity was in glucose, followed by glycerol. There was negligible activity on ethanol and methanol (Fig. 6).
PDC activity of K. pastoris on various carbon sources. YPD—yeast extract, peptone and dextrose, YPG—yeast extract, peptone, and glycerol, YPE—yeast extract, peptone, and ethanol, YPM—yeast extract, peptone, and methanol, and YP—only yeast extract and peptone. The experiments were done in triplicates and the error bars represent the standard deviations
Discussion
S. cerevisiae contains six genes for PDC. Three of these, ScPDC1, 5, and 6, code for proteins with catalytic activities. All three isozymes have a very high sequence similarity at DNA (90 %) as well as protein (80 %) level. Enzyme kinetic parameters were determined by in vitro experiments with purified enzymes. The proteins were then expressed individually in a pdc null background to study the in vivo behavior of the enzymes.
The kinetic constants of the isozymes ScPDC1, ScPDC5, ScPDC6, and PpPDC differ considerably. ScPDC5 has a higher reaction rate (V max) than ScPDC1, whereas it has a lower affinity for pyruvate as indicated by the higher K 0.5. The efficiency of the enzyme calculated by k cat/K 0.5 indicates that ScPDC1 and ScPDC5 have similar decarboxylation rates. In S. cerevisiae, under fermentative conditions involving very high sugar levels, large amounts of pyruvate are generated. Under such conditions, a high PDC activity is desirable to divert pyruvate to ethanol. The other major enzyme competing for pyruvate, pyruvate dehydrogenase (PDH), has a lower V max for pyruvate (Kresze and Ronft 1981) and is therefore rapidly saturated, limiting the pyruvate flux into the TCA cycle. The ScPDC5 characterized in this study was identified to have a high reaction rate and is thus expected to be expressed in such conditions potentially increasing the total PDC activity of yeast. The high K 0.5 (2.9) of ScPDC5 compared to ScPDC1 (2.0) also seems to be relevant for these conditions, as only excess pyruvate needs to be metabolized via ethanol. The expression of ScPDC6 has been found in only certain conditions such as high glucose stress (Erasmus et al. 2003) and when sulfur is limited (Fauchon et al. 2002; Pereira et al. 2008). ScPDC6 has fewer sulfur-containing amino acids than other isoforms. Thus, even though ScPDC6 is less efficient than the other two enzymes, it has a survival advantage when sulfur is limited.
It was observed that the enzyme kinetics for the ScPDC1, 5, 6, and PpPDC deviated from the typical hyperbolic Michaelis–Menten pattern as could be deduced from the sigmoidal behavior. This was further confirmed by the fact that Hill coefficient for all the PDC isozymes was near 2 which indicates positive cooperativity. PDCs from various yeasts (Baburina et al. 1994; Krieger et al. 2002) and a few Gram-positive bacteria (Lowe and Zeikus 1992) have been found to display sigmoidal kinetics with cooperativity, unlike the Michaelis–Menten kinetics demonstrated by most bacterial PDCs (Dobritzsch et al. 1998). In this respect, all the PDCs characterized here show a similar kinetics to that of other yeast PDCs. The Cys221 amino acid has been indicated in ScPDC1 as responsible for the substrate activation (Baburina et al. 1994). A sequence comparison of the proteins shows that the Cys221 residue is conserved in all the ScPDC catalytic isozymes and PpPDC. Hence, it can be hypothesized that the substrate activation in ScPDC5, 6, and PpPDC has a similar mechanism to the one in ScPDC1.
The catalytic and regulatory mechanisms of ScPDC1 have been extensively studied and various important residues such as His 92, Glu 91 (Baburina et al. 1998), Asp28, His114, Glu 477 (Lu et al. 2000), Glu 51, Gly 413, Ile 415 (Arjunan et al. 1996), Asp 444, Asn471, and Gly473 (Muller et al. 1993) have been identified for catalytic activity and regulatory activity. Sequence comparison shows that all those residues important for substrate activation and catalytic activity are conserved not only in the ScPDCs but also in PpPDC. The similar pH profiles (Table 3) and the conserved amino acids at catalytic and regulatory sites suggest that the basic catalytic mechanism is conserved in the ScPDC isozymes as well as PpPDC.
There are yeast-based industrial processes where the glucose levels are high such as batch fermentation processes in which high concentration of molasses are used. ScPDC1 is easily replaceable by ScPDC5 for any such processes using decarboxylation activity; in such saturated substrate conditions, the lower affinity for pyruvate is inconsequential and the high reaction rate is advantageous. The in vivo experiments with single PDC enzymes expressed in the pdc null background strengthens the point that when there is no native regulation, and when saturated levels of pyruvate are present, there is no difference in the ethanol production capacities of all the PDCs.
The recently published genomes of the GS115 and DSMZ 70382 strains of K. pastoris (De Schutter et al. 2009; Mattanovich et al. 2009) confirmed the existence of a single PDC gene in K. pastoris. The pyruvate decarboxylase activity when determined in the crude extract of K. pastoris was approximately five times less in comparison to the S. cerevisiae PDC activity. When grown on different carbon sources, maximum PDC activity was found during growth on glucose and glycerol, with a relatively higher activity on glucose; no PDC activity was detectable during growth on ethanol and methanol (Fig. 6). Thus, PDC activity in K. pastoris is induced by the presence of glycolytic substrates.
Kinetic parameters of the K. pastoris PDC were determined to establish whether there is a significant difference in the kinetic efficiencies of the PDCs of this Crabtree-negative yeast which might contribute to its lower level of PDC activity. The K 0.5 of PpPDC was determined as 4.2 mM which is significantly higher than that for ScPDC1 (2.0 mM). Thus, PpPDC has lesser affinity for pyruvate in comparison to all three ScPDCs. This ensures that pyruvate in K. pastoris is diverted towards acetaldehyde formation only at a higher pyruvate concentration due to shut down of respiratory metabolism as a result of anaerobic conditions. In anaerobic condition, NADH generated during glycolysis cannot be oxidized through the usual respiratory chain. Hence, an alternate fermentation strategy is required for maintaining the redox balance and survival of the organism. Presence of a PDC in K. pastoris ensures that in such condition it has the alternative route. At the same time, a lower affinity for pyruvate reduces the chances of aerobic fermentation by rapid switching of metabolism to respiration when oxygen becomes available after oxygen limitation. The turnover number of PpPDC (k cat = 8.6 s−1) is also lesser than those for all three catalytic ScPDC isozymes. The efficiency of the enzyme, as determined by k cat/K 0.5, is 2 which is approximately 30 times less than for ScPDC1. The lower decarboxylation efficiency of the PpPDC may be partially responsible for the Crabtree-negative phenotype. There are other factors which could be responsible for this phenotype such as presence of acetaldehyde dehydrogenase (with a possibility of higher affinity for acetaldehyde) and acetyl coA synthetase (the annotated genes are present in the GS115 genomic database), which diverts pyruvate towards acetyl CoA formation, thus channelling pyruvate into the TCA cycle or lipid biosynthesis to increase biomass formation instead of ethanol formation.
As PDCs are commercially used to carry out the biosynthesis of (R)-PAC, the carboligation rates of the PDC isozymes were investigated. Among the isozymes of S. cerevisiae, ScPDC1 and 5 show similar in vitro (R)-PAC yields by the cell-free extracts in the recombinant strain overexpressing single isozymes (Fig. 4). ScPDC6 and PpPDC which have lower carboligation capacities are less suitable for (R)-PAC synthesis. ScPDC1 was also able to convert 4-methoxy-benzaldehyde and 3-chloro-benzaldehyde to corresponding carbinols, but PpPDC was not able to carry out these biotransformations.
ScPDC5 would be the choice of PDC for the industrial fermentation process where overexpression of PDCs without the native regulation is desired. This could be used for decarboxylation processes for ethanol formation and the biotransformations with aldehyde for synthesis of addition products such as acetoin and (R)-PAC.
References
Arjunan P, Umland T, Dyda F, Swaminathan S, Furey W, Sax M, Farrenkopf B, Gao Y, Zhang D, Jordan F (1996) Crystal structure of the thiamin diphosphate-dependent enzyme pyruvate decarboxylase from the yeast Saccharomyces cerevisiae at 2.3 Å resolution. J Mol Bio 256:590–600
Baburina I, Gao YH, Hu ZX, Jordan F, Hohmann S, Furey W (1994) Substrate activation of brewers-yeast pyruvate decarboxylase is abolished by mutation of cysteine-221 to serine. Biochem 33:5630–5635
Baburina I, Moore DJ, Volkov A, Kahyaoglu A, Jordan F, Mendelsohn R (1996) Three of four cysteines, including that responsible for substrate activation, are ionized at pH 6.0 in yeast pyruvate decarboxylase: evidence from Fourier transform infrared and isoelectric focusing studies. Biochem 35:10249–10255
Baburina I, Li HJ, Bennion B, Furey W, Jordan F (1998) Interdomain information transfer during substrate activation of yeast pyruvate decarboxylase: the interaction between cysteine 221 and histidine 92. Biochem 37:1235–1244
Breunig KD, Bolotin-Fukuhara M, Bianchi MM, Bourgarel D, Falcone C, Ferrero II, Frontali L, Goffrini P, Krijger JJ, Mazzoni C, Milkowski C, Steensma HY, Wesolowski-Louvel M, Zeeman AM (2000) Regulation of primary carbon metabolism in Kluyveromyces lactis. Enzyme Microb Technol 26:771–780
Bruhn H, Pohl M, Grotzinger J, Kula MR (1995) The replacement of Trp392 by alanine influences the decarboxylase/carboligase activity and stability of pyruvate decarboxylase from Zymomonas mobilis. Eur J Biochem 234:650–655
Candy JM, Duggleby RG (1998) Structure and properties of pyruvate decarboxylase and site-directed mutagenesis of the Zymomonas mobilis enzyme. Biochim Biophys Acta Protein Struct Mol Enzymol 1385:323–338
Chang YH, Chang AK, Nixon PF, Duggleby RG (2001) Site-directed mutagenesis of the ionizable groups in the active site of Zymomonas mobilis pyruvate decarboxylase. Eur J Biochem 268:3558–3565
De Schutter K, Lin YC, Tiels P, Van HA, Glinka S, Weber-Lehmann J, Rouze P, Van de Peer Y, Callewaert N (2009) Genome sequence of the recombinant protein production host Pichia pastoris. Nat Biotechnol 27:561–566
Dobritzsch D, König S, Schneider G, Lu G (1998) High resolution crystal structure of pyruvate decarboxylase from Zymomonas mobilis. J Biol Chem 273:20196–20204
Erasmus DJ, van der Merwe GK, van Vuuren HJ (2003) Genome-wide expression analyses: metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res 3:375–399
Fauchon M, Lagniel G, Aude JC, Lombardia L, Soularue P, Petat C, Marguerie G, Sentenac A, Werner M, Labarre J (2002) Sulfur sparing in the yeast proteome in response to sulfur demand. Mol Cell 9:713–723
Flikweert MT, van der Zanden L, Janssen WM, Steensma HY, van Dijken JP, Pronk JT (1996) Pyruvate decarboxylase: an indispensable enzyme for growth of Saccharomyces cerevisiae on glucose. Yeast 12:247–257
Fredlund E, Beerlage C, Melin P, Schnurer J, Passoth V (2006) Oxygen and carbon source-regulated expression of PDC and ADH genes in the respiratory yeast Pichia anomala. Yeast 23:1137–1149
Hildebrandt G, Klavehn W (1932) Verfahren zur Herstellung von 1-l-Phenyl-2-methylamino-1-ol. German Patent 548 459
Hohmann S (1993) Characterisation of PDC2, a gene necessary for high level expression of pyruvate decarboxylase structural genes in Saccharomyces cerevisiae. Mol Gen Genet 241:657–666
Hohmann S, Cederberg H (1990) Autoregulation may control the expression of yeast pyruvate decarboxylase structural genes PDC1 and PDC5. Eur J Biochem 188:615–621
Hübner G, Weidhase R, Schellenberger A (1978) Mechanism of substrate activation of pyruvate decarboxylase—1st approach. Eur J Biochem 92:175–181
Iding H, Siegert P, Mesch K, Pohl M (1998) Application of α-keto acid decarboxylases in biotransformations. Biochim Biophys Acta 1385:307–322
Inan M, Meagher M (2001) The effect of ethanol and acetate on protein expression in Pichia pastoris. J Biosci Bioeng 92:337–341
Ishchuk OP, Voronovsky AY, Stasyk OV, Gayda GZ, Gonchar MV, Abbas CA, Sibirny AA (2008) Overexpression of pyruvate decarboxylase in the yeast Hansenula polymorpha results in increased ethanol yield in high-temperature fermentation of xylose. FEMS Yeast Res 8:1164–1174
Ito H, Ukuda Y, Urata K, Imura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
König S (1998) Subunit structure, function and organisation of pyruvate decarboxylases from various organisms. Biochim Biophys Acta Protein Struct Mol Enzymol 1385:271–286
Kresze GB, Ronft H (1981) Pyruvate dehydrogenase complex from baker's yeast. 2. Molecular structure, dissociation, and implications for the origin of mitochondria. Eur J Biochem 119:581–587
Krieger F, Spinka M, Golbik R, Hübner G, König S (2002) Pyruvate decarboxylase from Kluyveromyces lactis. An enzyme with an extraordinary substrate activation behaviour. Eur J Biochem 269:3256–3263
Kutter S, Wille G, Relle S, Weiss MS, Hübner G, König S (2006) The crystal structure of pyruvate decarboxylase from Kluyveromyces lactis. Implications for the substrate activation mechanism of this enzyme. FEBS J 273:4199–4209
Lohmann K, Schuster P (1937) Untersuchungen über die cocarboxylase. Biochem Z 294:188–214
Lowe SE, Zeikus JG (1992) Purification and characterization of pyruvate decarboxylase from Sarcina ventriculi. J Gen Microbiol 138:803–807
Lu P, Davis BP, Jeffries TW (1998) Cloning and characterization of two pyruvate decarboxylase genes from Pichia stipitis CBS 6054. Appl Environ Microbiol 64:94–97
Lu GG, Dobritzsch D, Baumann S, Schneider G, König S (2000) The structural basis of substrate activation in yeast pyruvate decarboxylase—a crystallographic and kinetic study. Eur J Biochem 267:861–868
Mattanovich D, Graf A, Stadlmann J, Dragosits M, Redl A, Maurer M, Kleinheinz M, Sauer M, Altmann F, Gasser B (2009) Genome, secretome and glucose transport highlight unique features of the protein production host Pichia pastoris. Microb Cell Fact 8:53
Mojzita D, Hohmann S (2006) PDC2 coordinates expression of the THI regulon in the yeast Saccharomyces cerevisiae. Mol Genet Genomics 276:147–161
Mumberg D, Müller R, Funk M (1995) Yeast vectors for the controlled expression of heterologous proteins in different genetic backgrounds. Gene 156:119–122
Muller YA, Indqvist Y, Furey W, Schulz GE, Jordan F, Schneider G (1993) A thiamin diphosphate binding fold revealed by comparison of the crystal structures of transketolase, pyruvate oxidase and pyruvate decarboxylase. Structure 1:95–103
Pereira Y, Lagniel G, Godat E, Baudouin-Cornu P, Junot C, Labarre J (2008) Chromate causes sulfur starvation in yeast. Toxicol Sci 106:400–412
Reynolds JEF (1989) Martindale, the extra pharmacopoeia. Pharmaceutical Press, London, UK, pp 1462–1463
Schaaff I, Green JB, Gozalbo D, Hohmann S (1989) A deletion of the PDC1 gene for pyruvate decarboxylase of yeast causes a different phenotype than previously isolated point mutations. Curr Genet 15:75–81
Schellenberger A (1967) Structure and mechanism of action of the active center of yeast pyruvate decarboxylase. Angew Chem Int Ed 6:1024–1035
Schmitt ME, Brown TA, Trumpower BL (1990) A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res 18:3091–3092
Singer TP, Pensky J (1951) Acetoin synthesis by highly purified a-carboxylase. Arch Biochem Biophys 31:457–459
Ullrich J, Wittorf JH, Gubler CJ (1966) Molecular weight and coenzyme content of pyruvate decarboxylase from brewer's yeast. Biochim Biophys Acta 113:595–604
van Urk H, Voll WSL, Scheffers WA, van Dijken JP (1990) Transient-state analysis of metabolic fluxes in Crabtree-positive and Crabtree-negative yeasts. Appl Environ Microbiol 56:281–287
Acknowledgements
PKA was supported by the University Grants Commission (UGC) of India. VU was supported by Council of Scientific and Industrial Research (CSIR), India. The work was partially funded by Department of Science and Technology (DST) and Board of Research in Nuclear Sciences (BRNS), India.
We also thank the anonymous reviewer for useful suggestions and insights.
Author information
Authors and Affiliations
Corresponding author
Additional information
Praveen Kumar Agarwal and Vanita Uppada have equally contributed to this article.
Rights and permissions
About this article
Cite this article
Agarwal, P.K., Uppada, V. & Noronha, S.B. Comparison of pyruvate decarboxylases from Saccharomyces cerevisiae and Komagataella pastoris (Pichia pastoris). Appl Microbiol Biotechnol 97, 9439–9449 (2013). https://doi.org/10.1007/s00253-013-4758-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-4758-4