Abstract
Photosynthetic microorganisms produce relatively large amounts of physiologically active materials which stimulate the physiological activity of other organisms. In this study, mammalian HeLa cells were cultured in different culture media which were Dulbecco's modified Eagle medium (DMEM) with newborn calf serum (NCS), and DMEM including different types of physiologically activating compounds (PACs) extracted from Rhodobacter sphaeroides grown under various culture conditions. R. sphaeroides was grown under the following five different culture conditions: anaerobically in the light, anaerobically in the dark and treated with dimethyl sulfoxide, aerobically in the dark for 48 h, in the light for 48 h, and in the light for 24 h and changed after previous culturing in the dark for 24 h. The growth of HeLa cell was measured by cell counting using a hemocytometer, and the fluorescent intensities of cellular lysosomes were measured to check the level of cellular stress caused by adding PACs. The growth of HeLa cells cultured in DMEM with PACs extracted from R. sphaeroides aerobically grown under dark conditions was enhanced compared to that of cells grown with NCS. We also found that a high concentration of pigments such as bacteriochlorophylls and carotenoids and a high concentration of arginine produced by R. sphaeroides aerobically grown in the dark were implicated in increased growth of the HeLa cells. Therefore, our results suggest that PACs extracted from R. sphaeroides aerobically cultured in dark conditions can enhance the physiological activity of mammalian cells and serve as nontoxic and bioavailable materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rhodobacter sphaeroides is a purple non-sulfur photosynthetic bacterium capable of growing aerobically and anaerobically in the light or anaerobically in the dark in the presence of external electron acceptors such as dimethyl sulfoxide (Rho et al. 2004). When grown anaerobically in the light or dark, R. sphaeroides synthesizes an intracytoplasmic membrane (ICM) system, which constitutes the photosynthetic apparatus and possesses the structural components necessary for light energy capture, subsequent electron transport, and energy transduction (Rho et al. 2004). The ICM is synthesized by R. sphaeroides during growth in dark or light conditions with limited oxygen availability, and it is likely that such conditions are widely encountered in nature (Yeliseev et al. 1996).
CO2 fixation has been intensively investigated in photosynthetic bacteria, and through the Calvin reductive pentose phosphate pathway via ribulose 1,5-bisphosphate carboxylase-oxygenase, these organisms can produce organic compounds, such as sugars from carbon dioxide. In addition, several of these purple, non-sulfur bacteria produce substantial quantities of useful materials such as amino acids, nucleic acid material, vitamins, and bio-active substances (Lee et al. 2010; Sasaki et al. 2005; Katsuda et al. 2004). In addition, for organisms to use light as an energy source, pigments and many specialized proteins are required, and considerable metabolic energy is needed to synthesize and assemble all the components of the photosynthetic apparatus (Gregor and Klug 1999). The photochemically active complexes harvest and convert light energy into chemical energy. The size and structure of the photosynthetic apparatus are also influenced by light (Zhu and Hearst 1986). Bacteriochlorophylls and carotenoids are important structural and functional components of the antenna complex and are involved in the capture of light energy and the subsequent transfer of the excitation energy to the reaction center, where the primary events of photosynthesis take place. Paradoxically, the photosynthetic apparatus is synthesized only in the dark, and synthesis ceases in the presence of light. This seems to restrict the use of light energy, because the photosynthetic complex that remains from a preceding dark period is gradually diluted by growth (Biebl and Wagner-Dobler 2006).
This study focuses on the impact of physiological activation in mammalian cells by mixed organic compounds extracted from R. sphaeroides grown under differential culture conditions. Here, we refer to mixed organic compounds extracted from R. sphaeroides as physiologically activating compounds (PACs). In this study, R. sphaeroides was grown under five different culture conditions: anaerobically in the light, anaerobically in the dark and treated with dimethyl sulfoxide, aerobically in the dark for 48 h, in the light for 48 h, and in the light for 24 h after culturing in the dark for 24 h. We focused on the extracted PACs from R. sphaeroides grown under each culture condition and have analyzed the concentrations of various amino acids, bacteriochlorophylls, and carotenoids in each of the PACs. In addition, HeLa cells were cultivated to evaluate the enhanced growth effect and cellular stresses under PAC-supplemented culture conditions.
Materials and methods
Extraction of PACs from R. sphaeroides
R. sphaeroides KCTC1434 (Korean Collection for Type Culture, Daejeon) was grown in 250 ml Sistrom’s minimal medium (Lee et al. 2010; Sistrom 1962) and incubated anaerobically in the light for 48 h or dark for 48 h in the presence of dimethyl sulfoxide. R. sphaeroides was also cultured aerobically in the dark, light (at 15 W/m2), or dark after culturing under light conditions, at 30 °C using 180 rpm shaking, and each culture was performed for 48 h. The cells were centrifuged at 6,000 rpm for 15 min. The pellet was collected and washed twice with distilled water. The ratio of distilled water to solid, ultrasonic power, and duration were set at 50:1, 40 %, and 30 min, respectively. R. sphaeroides cells were disrupted by sonication (Sonics & Materials, Inc., USA) for 30 min, and the samples were kept in ice. The samples were centrifuged at 13,000 rpm for 10 min to obtain the supernatant-containing PACs, and then PACs were filtered prior to use.
Cell culture condition
HeLa cells were grown as monolayers in DMEM, supplemented with antibiotics, l-glutamine, and 5 % newborn calf serum (NCS). Cells were grown at 37 °C in a humidified atmosphere containing 5 % carbon dioxide (Villanueva et al. 2010). For treatments, an appropriate number of cells were plated into 60-mm culture plates and incubated to allow attachment. After 12-h incubation, NCS and PACs were added to each plate (the final concentration of PACs in the medium was 1, 5, or 10 %) for 24 h at 37 °C. For counting, the cells were washed with phosphate-buffered saline and collected using trypsin-EDTA for 2 min. When counting cells under a microscope, Trypan Blue (10 μL) was mixed with the cells by gentle pipetting. A sample (10 μL) of the mixture was removed and placed on a hemocytometer.
Cytological staining with Lyso Tracker
HeLa cells were grown in a cell culture, rinsed once with phosphate-buffered saline (PBS), and then stained with 100 nM Lyso Tracker Red DND-99 in the medium without serum for 2 min at 37 °C (Yoon et al. 2010a, b). The cells were washed with PBS and fixed with 4 % parachloroform for 15 min at 37 °C. The cells were washed with PBS and observed using confocal laser-scanning microscopy (LSM 510 META) at 543 nm, and images were generated using a Zeiss LSM image browser.
Determination of pigments and amino acid concentrations
Carotenoids were extracted from R. sphaeroides and quantitated as previously described (Gu et al. 2008). Bacteriochlorophyll concentrations were measured after pigment extraction using acetone/methanol (7:2) solvent (Cohen-Bazire et al. 1957). High-performance liquid chromatography (HPLC, Waters Alliance 2690 Analytical HPLC system) was used to determine amino acid content. The system was equipped with a NovaPakTM C14 column and a Waters 747 scanning fluorescence detector (Waters Co., USA). All results represent the data from at least three independent experiments and include a mean value.
Data analysis
All results were obtained from three independent samples conducted simultaneously for error analysis, and the results and correlations for several experimental conditions are shown, along with the standard deviation. The data were analyzed using Sigma Plot (SPS Chicago, IL, USA). A p value <0.05 was considered significant.
Results
Influence of PACs extracted from aerobically and anaerobically cultured R. sphaeroides on the growth of HeLa cells
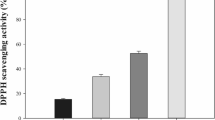
R. sphaeroides grown under aerobic dark conditions, anaerobic light conditions, or anaerobic dark conditions and treated with DMSO showed red, green, and yellow colors, respectively. HeLa cells in DMEM supplemented by PACs extracted from R. sphaeroides grown under each culture condition were cultivated for 24 h. Generally, HeLa cells are supplemented with 5 % NCS for normal cell growth; thus, the total cell numbers of HeLa cells was counted in both NCS- and PAC-supplemented conditions. Compared to the 5 % NCS-supplemented condition, we found that the total number of HeLa cells was increased (1.5-fold of the 5 % NCS-supplemented condition) when 5 % and 10 % PACs were extracted from R. sphaeroides aerobically cultured in the dark (see Fig. 1). On the other hand, PACs from R. sphaeroides grown under anaerobic light conditions and anaerobic dark conditions with DMSO did not significantly impact growth of HeLa cells, even when compared to supplementation with 1 % PACs from cells cultured under aerobic dark conditions. In addition, we have analyzed the extent of cellular stress in HeLa cells cultivated in each condition: without NCS, with 5 % NCS, and PACs extracted from red-, green-, and yellow-colored R. sphaeroides. As shown in Fig. 1c, there were significant increases in fluorescent intensity of lysosomes in HeLa cells treated with 5 % PACs from R. sphaeroides grown under anaerobic light and anaerobic dark conditions with DMSO compared to the 5 % PACs from R. sphaeroides grown under aerobic dark conditions.
The growth of HeLa cells was observed in DMEM with NCS and also DMEM supplemented with PACs rather than NCS. Here, three different types of PACs were prepared: PACs extracted from R. sphaeroides aerobically cultured in the dark (red-colored R. sphaeroides), R. sphaeroides anaerobically cultured in the light (green-colored R. sphaeroides), or R. sphaeroides anaerobically cultured in the dark after treatment with DMSO (yellow-colored R. sphaeroides). The cell number of HeLa cells was compared to conditions without NCS and with 5 % NCS (a), and the HeLa cells were compared to those cultured with 1, 5, and 10 % of the three different types of PACs (b). Fluorescence of lysosomes stained with 100 nM Lyso Tracker Red DND-99 in HeLa cells was analyzed using a confocal microscope (c)
For the analysis of mixed organic compounds, PAC, amino acids were extracted from three different types of PACs and analyzed (Table 1). High concentrations of arginine and leucine were found in PACs from red-colored R. sphaeroides when compared with the other two types.
Influence of PACs extracted from aerobically cultured R. sphaeroides under different light conditions on the growth of HeLa cells
To determine the effective culture conditions of R. sphaeroides for extracting PACs, we analyzed R. sphaeroides aerobically grown under a variety of light conditions. The effectiveness of light conditions was assessed after exposure to the different light conditions, which included only dark condition for 48 h (48:0 h (D/L)), only light condition at 15 W/m2 for 48 h (0:48 h (D/L)), and dark condition for 24 h after light condition at 15 W/m2 for 24 h (24:24 h (D/L)). We did not show significant differences for growth and dry cell weight of R. sphaeroides or total solid weight of PACs under different light conditions for 48 h (data not shown). In addition, to evaluate the growth kinetics of R. sphaeroides under different light conditions, the cell growth was analyzed during 48 h. R. sphaeroides demonstrated slightly increased growth curves under light culturing for 48 h compared with the other two conditions (data not shown).
Using three different types of PACs from R. sphaeroides cultured under each condition, the total numbers and lysosomal intensities of HeLa cells were evaluated under each PAC-supplemented condition. The total number of HeLa cells cultivated in DMEM with 1, 5, and 10 % of each PACs is shown in Fig. 2, and we found that the total number of HeLa cells was similar to that of HeLa cells in 5 % NCS-supplemented DMEM (Fig. 1a), except for that of HeLa cells in DMEM with PACs from R. sphaeroides aerobically cultured in the dark for 48 h. Here, we have also analyzed the effects of 0.5 and 20 % of each PACs, but the highest cell number of HeLa was observed in 10 % PACs from 48:0 h (D/L), 10 % PACs from 0:48 h (D/L), and 1 % PACs from 24:24 h (D/L), respectively (data not shown).
The growth of HeLa cells was observed in DMEM with NCS and also DMEM supplemented with PACs instead of NCS. Here, 1, 5, and 10 % of three different types of PACs were prepared, which were PACs extracted from R. sphaeroides aerobically cultured in the dark for 48 h, R. sphaeroides cultured in the light for 48 h, and R. sphaeroides cultured in the light for 24 h and changed after culturing in the dark for 24 h
As shown in Fig. 3, when compared to Fig. 1c, there were significant increases in the fluorescent intensity of lysosomes in HeLa cells treated with 5 % PACs from R. sphaeroides grown under aerobic light conditions (48 h) and dark (24 h) conditions after light exposure for 24 h. In addition, the extent of cellular stress in HeLa cells as determined by analysis of lysosomal activation due to PACs (0:48 h (D/L) and 24:24 h (D/L)) was similar to that of HeLa cells cultivated without NCS, shown in Fig. 3, in which fluorescent intensity of lysosomes was quantified.
Quantitative analysis for fluorescence of lysosomes in HeLa cells after treatment without NCS, with 5 % NCS, and 5 % PACs extracted from R. sphaeroides aerobically cultured in the dark for 48 h, R. sphaeroides cultured in the light for 48 h, and R. sphaeroides cultured in the light for 24 h and changed after culturing in the dark for 24 h
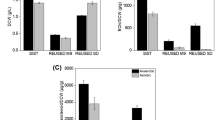
For the analysis of PACs, amino acids were extracted from three different types of PACs and analyzed for concentrations of various amino acids (Table 2). Among the various amino acids, high concentrations of arginine and isoleucine were found in PACs from red-colored R. sphaeroides when compared to the other two types. As shown in Table 1, the high concentration of arginine in PACs from R. sphaeroides grown in 48:0 h (D/L) is considered to be its major mechanism to produce enhanced growth of HeLa cells. R. sphaeroides responded to variations in light conditions by changing its pigment composition. The bacteriochlorophylls and carotenoids in cells grown under different light conditions are shown in Fig. 4. The levels of bacteriochlorophylls and carotenoids dramatically increased when cultured under dark conditions for 48 h compared to culture under light conditions for 48 h and light conditions for 24 h and changed after culturing in dark conditions for 24 h (Fig. 4a, b).
Discussion
In this paper, we present the impact of physiological activation in mammalian cells by mixed organic compounds from R. sphaeroides cultured under differential culture conditions (PACs). The impact of compounds produced according to various conditions was assessed by the cell growth of HeLa. This results show that the growth of HeLa cells with PACs can be maintained at levels similar to those achieved using NCS-supplemented conditions. In addition, the exposure of cells to oxidative stress such hydrogen peroxide and UVB, or nutrient starvation causes early lysosomal rupture, suggesting the enhancement of lysosome activity in adapting to cellular stress (Yoon et al. 2009, 2010a, b; Stoka et al. 2001). The level of lysosomal activation was measured after staining the HeLa cells with Lyso tracker Red, a lysosome-specific fluorescent dye (Yoon et al. 2010a, b). This means that the PACs extracted from R. sphaeroides grown under aerobic dark conditions can increase the growth of HeLa cells, and cellular stress was not induced by supplementing HeLa cells with PACs. We also treated PACs in bovine aortic endothelial cells (BAECs) for checking of BAECs' growth and stress responses using Lyso tracker staining. We found that the effect of PACs in BAEC was consistent to the effect in HeLa cells, meaning that PACs extracted from R. sphaeroides grown under aerobic dark conditions can increase the growth, and also cellular stress was not induced by supplementing PACs to mammalian cells (data not shown).
Here, arginine was first used to enhance the refolding yield of human tissue-type plasminogen activator and later was included in the refolding protocols of various proteins (Cirkovas and Sereikaite 2011). Therefore, the ability of arginine in PACs to increase the solubility of unfolded species as well as folding intermediates is considered to be its major mechanism for enhancing growth of HeLa cells. These data suggest that PACs from R. sphaeroides cultured under aerobic dark conditions would be alternative materials to NCS for mammalian cell growth.
HeLa cells were treated with PACs grown under three different light conditions and concentrations. The cell number increased with increasing PACs concentration under dark condition. These results mean that R. sphaeroides cultured in aerobic dark conditions for 48 h probably produce more useful organic compounds compared to those produced by R. sphaeroides cultured using other conditions. Also, these data showed consistent patterns with increasing levels of lysosomal activity, which can be used to monitor cellular stress. This means that the PACs extracted from R. sphaeroides grown under aerobic dark conditions can increase the growth of HeLa cells, and also cellular stress was not induced by supplementing PACs to HeLa cells, when compared to PACs extracted from R. sphaeroides grown under other conditions.
According to these results, PACs contained pigments, such as bacteriochlorophylls and carotenoids and a lot of amino acids. Also, we have found that the ATP is known as the most important energy-rich compound in cell based on Lee et al. (2012). However, it remains to be confirmed whether another material contains in PACs. A condition for enhancing concentrations of amino acids, bacteriochlorophylls, and carotenoids was suggested and implemented to increase mammalian cell growth. Hence, these results suggest that PACs extracted from R. sphaeroides cultured under aerobic dark conditions can be used as alternative materials to serum for mammalian cell growth.
References
Biebl H, Wagner-Dobler I (2006) Growth and bacteriochlorophyll a formation in taxonomically diverse aerobic anoxygenic phototrophic bacteria in chemostat culture: influence of light regimen and starvation. Process Biochem 41:2143–2159
Cirkovas A, Sereikaite J (2011) Different effects of L-arginine on the heat-induced unfolding and aggregation of proteins. Biologicals 39:181–188
Cohen-Bazire G, Sistrom WR, Stanier RY (1957) Kinetic studies of pigment synthesis by non-sulfur purple bacteria. J Cell Physiol 4:25–68
Gregor J, Klug G (1999) Regulation of bacterial photosynthesis genes by oxygen and light. FEMS Microbiol Letts 179:1–9
Gu Z, Chen D, Han Y, Chen Z, Gu F (2008) Optimization of carotenoids extraction from Rhodobacter sphaeroides. LWT-Food Sci Tech 41:1082–1088
Katsuda T, Lababpour A, Shimahara K, Katoh S (2004) Astaxanthin production by Haematococcus pluvialis under illumination with LEDs. Enzyme Microb Tech 35:81–86
Lee HJ, Jang A, Kim Y-H, Chung H-W, Min J (2012) Improvement of bacterial hydrogen production by ATP in mixed organic compounds extracted from Rhodobacter sphaeroides aerobically cultured under dark conditions. Bioresour Technol 123:678–681
Lee SY, Lee HJ, Park J-M, Lee JH, Park J-S, Shin HS, Kim Y-H, Min J (2010) Bacterial hydrogen production in recombinant Escherichia coli harboring a HupSL hydrogenase isolated from Rhodobacter sphaeroides under anaerobic dark culture. Int J Hydrogen Energ 35:1112–1116
Rho JH, Smith WE, Kaplan S (2004) Effects of oxygen and light intensity on transcriptome expression in Rhodobacter sphaeroides 2.4.1. Redox active gene expression profile. J Biol Chem 279(10):9146–9155
Sasaki K, Watanabe M, Suda Y, Ishizuka A, Noparatnaraporn N (2005) Application of photosynthetic bacteria for medical fields. J Biosc Bioeng 100:481–488
Sistrom WR (1962) The kinetics of the synthesis of photopigments in Rhodopseudomonas sphaeroides. J Gen Microbiol 28:607–616
Stoka V, Turk B, Schendel SL, Kim T-H, Cirman T, Snipas SJ, Ellerby LM, Bredesen D, Freeze H, Abrahamson M, Brömme D, Krajewski S, Reed JC, Yin X-M, Turk V, Salvesen GS (2001) Lysosomal protease pathways to apoptosis. J Biol Chem 276:3149–3157
Villanueva A, de la Presa P, Alonso JM, Rueda T, Martinez A, Crespo P, Morales MP, Gonzalez-Fernandez MA, Valdes J, Rivero G (2010) Hyperthermia HeLa cell treatment with silica-coated manganese oxide nanoparticles. J Phys Chem 114:1976–1981
Yeliseev AA, Eraso JM, Kaplan S (1996) Differential carotenoid composition of the B875 and B800-850 photosynthetic antenna complexes in Rhodobacter sphaeroides 2.4.1: involvement of spheroidene and spheroidenone in adaptation to changes in light intensity and oxygen availability. J Bacteriol 178:5877–5883
Yoon J, Chang S-T, Park J-S, Kim Y-H, Min J (2010a) Functional characterization of starvation-induced lysosomal activity in Saccharomyces cerevisiae. Appl Microbiol Biotechnol 88:283–289
Yoon J, Kim K-J, Choi Y-W, Shin HS, Kim Y-H, Min J (2010b) The dependence of enhanced lysosomal activity on the cellular aging of bovine aortic endothelial cells. Mol Cell Biochem 340:175–178
Yoon J, Park J-M, Jung S-K, Kim K-Y, Kim Y-H, Min J (2009) Characterization of antimicrobial activity of the lysosomes isolated from Saccharomyces cerevisiae. Curr Microbiol 59:48–52
Zhu YS, Hearst JE (1986) Regulation of expression of genes for light-harvesting antenna proteins LH-I and LH-II; reaction center polypeptides RC-L, RC-M, and RC-H; and enzymes of bacteriochlorophyll and carotenoid biosynthesis in Rhodobacter capsulatus by light and oxygen. Proc Natl Acad 83:7613–7617
Acknowledgments
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (MEST) (NRF-2009-0093183). The authors are grateful for their support.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lee, H.J., Park, JY., Yoo, K.S. et al. Activity and characterization of mixed organic compounds extracted from Rhodobacter sphaeroides as alternative materials to serum for mammalian cell growth. Appl Microbiol Biotechnol 97, 9561–9567 (2013). https://doi.org/10.1007/s00253-012-4653-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4653-4