Abstract
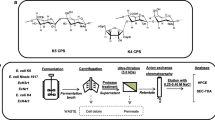
Chondroitin sulfate is a well-known bioactive molecule, widely used as an anti-osteoarthritis drug, that is nowadays mainly produced by animal tissue sources with unsafe extraction procedures. Recent studies have explored an integrated biotechnological–chemical strategy to obtain a chondroitin sulfate precursor from Escherichia coli K4 capsular polysaccharide, demonstrating the influence of environmental and growth conditions on capsule synthesis. In this research work, the flexibility of the strain biosynthetic machinery was investigated to enhance the K4 capsular polysaccharide production by supplementing the growth medium with the monosaccharides (glucuronic acid, galactosamine and fructose) that constitute the chain. Shake flask experiments were performed by adding the sugars singularly or together, by testing monosaccharide different concentrations and times of addition and by observing the bacterial sugar consumption. A K4 capsular polysaccharide production enhancement, compared to the control, was observed in all cases of supplementation and, in particular, significant 68 and 57 % increases were observed when adding 0.385 mM glucuronic acid plus galactosamine or 0.385 mM fructose, respectively. Increased expression levels of the gene kfoC, coding for a K4 polymerase, evaluated in different growth conditions, confirmed the results at the molecular level. Furthermore, batch fermentations, performed in lab-scale reactors (2 L), allowed to double the K4 capsular polysaccharide production values obtained in shake flask conditions, by means of a strict control of the growth parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
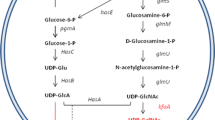
Several Gram-positive and Gram-negative bacterial strains produce a capsular polysaccharide (CPS) that surrounds the surface and shields the cells from environmental, physical and mechanical stresses (Jann and Jann 1992), providing an efficient camouflage in infection and pathogenesis processes (Whitfield 2006; Roberts 1996). CPSs resembling eukaryotic cell glycoconjugates have recently resulted very attractive from scientific and economical points of view: hyaluronic acid (HA) like CPSs, produced by some strains of A and C groups of Streptococci genera (Chong et al. 2005), demonstrated to be a perfect, natural-like replacement of animal source HA and to have potential applications in numerous industrial, medical and surgical fields (Kogan et al. 2007). Among the almost 80 different types of Escherichia coli CPSs, classified in four groups on the basis of serological properties, genetic and biosynthetic criteria (Whitfield 2006), the ones belonging to the group II demonstrated to be very fascinating from a structural point of view because of miming glycoconjugates expressed by the hosts (Jann and Jann 1992). E. coli K5, for example, expresses a CPS whose structure resembles the glycosaminoglycan chain of heparosan (Rodriguez et al. 1988; Vann et al. 1981), while E. coli O5:K4:H4 synthesizes a capsular polysaccharide (K4 CPS) whose repeating disaccharide unit is constituted by glucuronic acid (GlcA) and N-acetylgalactosamine (GalNAc) and that, except for a β-linked terminal fructose (Fruct) residue, is similar to the chondroitin sulphate (CS) backbone (Rodriguez et al. 1988). CS is a glycosaminoglycan that constitutes the extracellular matrix of mammalian connective tissues, but it is also widely used as the active principle of many anti-arthritis and osteoarthritis drugs, having a demonstrated, good and efficient anti-inflammatory action (Ronca et al. 1998; Volpi 2007). CS is currently produced by extraction and purification from animal tissue sources (e.g. bovine trachea or shark fins) using long procedures that are not considered safe because of the risks of virus or prion contaminations. These considerations motivated researchers to find a safer alternative way to produce CS, and the structural similarity between the microbial K4 CPS and the chondroitin chain has opened the prospective to use E. coli K4 as a bacterial tool to set up a new biotechnological productive process by using fermentation strategies (Cimini et al. 2010a; Restaino et al. 2011). As for the other members of group II, also in E. coli K4, a gene cluster, composed of three regions, codes for proteins involved in the biosynthesis, polymerization and transportation of the capsules (Roberts et al. 1988). The chain is built by a cytoplasmatic chondroitin polymerase, a bi-functional glycosyltransferase enzyme with two different active sites (A1 and A2) that extend progressively the nascent chain by adding respectively UDP-glucuronic acid (UDP-GlcA) and UDP-galactosamine (UDP-GalNAc) to the non-reducing end (Zanfardino et al. 2010). When built up, the chain is moved towards the outside of the cell by an ABC transportation system of proteins, mainly responsible of transferring the capsular polysaccharide across the inner and the outer membrane (Smith et al. 1990). The fructose residue might be added sometimes during or after chain biosynthesis, but no specific scientific data have been reported so far. Previous studies have demonstrated that the K4 CPS production is strictly regulated by environmental and growth conditions like pH and, above all, temperature, and that the optimal conditions for the growth (pH of 7.5 and 37 °C) are also the best ones for the chain synthesis (Jann and Jann 1992; Rodriguez et al. 1988). Supporting the increase of biomass formation could facilitate the production of high CPS values, but optimizing metabolic conditions could also increase the production yield (Restaino et al. 2011). On the other hand, the initiation and the elongation of the K4 capsular polysaccharide is a complex synthetic procedure that the cell performs thanks to different metabolic pathways that supply a sufficient number of polysaccharide chain building blocks: a dehydrogenase converts UDP-glucose (UDP-Glc) into UDP-GlcA, while UDP-GalNAc is obtained by epimerization of UDP-glucosamine (UDP-GlcNAc; Fig. 1) (Cimini et al. 2012). Since these two metabolic pathways start from phosporylated forms of glucose (Glc-1P) and fructose (Fruct-6P), respectively, the type of carbon sources, their availability in the growth medium and their uptake rate play a critical role also in the capsular polysaccharide formation. In previous papers, the influence of different types and concentrations of carbon sources on K4 CPS production and yield was demonstrated, and a new semi-defined medium, suitable for industrial applications and capable to support both growth and capsular polysaccharide production, was formulated (Cimini et al. 2010a). So far, new approaches to improve capsular polysaccharide biosynthesis, based on the support of the metabolic pathways by directly supplying the monosaccharides constituting the capsular polysaccharide precursors, have still remained unexplored. Previous studies on polysialic acid formation in E. coli K92 have already demonstrated that the monosaccharide precursors could not be used as the unique carbon sources in the medium in order to obtain high capsular polysaccharide concentrations (Revilla-Nuin et al. 1998; Ezquerro-Sáenz et al. 2006) and that a defined medium is essential to support both growth and associated CPS. In this paper, the flexibility of E. coli K4 capsular polysaccharide biosynthetic mechanisms was tested by supplementing the capsular polysaccharide monosaccharide precursors to the growth medium in order to enhance the K4 CPS production and yield. Shake flask experiments were performed investigating the best supplementation conditions like sugar concentration, time of addition, kinetic of sugar consumption and the influence of these additions on the polymerase gene expression. Batch experiments with monosaccharide supplementation were performed on a 2-L fermentor to test the influence of controlled pH and airflow conditions on the K4 CPS final titer.
Material and methods
Materials
All the growth medium components, the antifoam agent, the ammonium hydroxide and the sulphuric acid as well as salts, acid or alkali components, used to prepare buffers for capillary electrophoresis, for anion exchange chromatography and the monosaccharides supplemented, were purchased by Sigma-Aldrich (USA). Neutralised soya peptone was furnished by Oxoid (UK).
Bacterial strain
The strain E. coli O5:K4:H4 (U1-41, 11307) was purchased from the Culture Collection of the University of Göteborg (Sweden). The strain was stored and maintained in 20 % v/v glycerol stock solutions at −80 °C. A growth medium containing glycerol and soya peptone, already described in a previous paper (Cimini et al. 2010a) [Control medium composition: glycerol (10 g L−1), soya peptone (1 g L−1), KH2PO4 (2 g L−1), K2HPO4 (9.7 g L−1), sodium citrate dihydrate (0.5 g L−1), (NH4)2SO4 (1 g L−1), MgCl2 (0.1 g L-1)] was used for both shake flask and fermentation experiments after being sterilized in autoclave or in situ, respectively. In both types of experiments, the K2HPO4 component was added to the sterilized media as a concentrated stock solution (388 g L−1) after sterilization by microfiltration (cutoff 0.22-μm membranes; Millipore, France).
Shake flask experiments
Different equimolar concentrations (0.128, 0.385, 0.770 and 1.540 mM) of GlcA, GalNAc or fructose were added, singularly or together, to the control medium after sterilization by 0.22-μm membrane filtration, at the beginning of the growth. In few experiments, monosaccharides were also added a second time after 4 or 8 h of the growth. A control medium was inoculated every time for reference. The initial pH and conductivity values of the control and supplemented media were measured on small aliquots (2 mL), by using a coupled pH meter and conductivity meter (Cyberscan 510, Eutech Instruments, Thermo Fisher Scientific Inc.). For each type of experiment, three independent replicates were performed in a rotary air shaker (model Minitron, Infors, Switzerland) with a rotation speed of 200 rpm, at the optimal growth temperature of 37 °C, by using 1-L Erlenmeyer flasks, containing 200 mL of medium and equipped with four baffles. The media were inoculated using 10-mL seed cultures of E. coli K4, coming from glycerol stock preparations and grown overnight on the control medium, with initial cell density corresponding to 0.09 and 0.1 Abs600 nm. Samples of broth were collected at time intervals of 1 h, up to the 24 h, to measure the absorbance at 600 nm (Spectrophotometer DU800, Beckman Coulter, USA), to evaluate the biomass formation and the kinetics of growth. Biomass dry weight was measured during cultivations: variable volumes (2–5 mL) of cultures were filtered on a 0.22-μm propylene membrane (Millipore, France), the pellet deposited on the microfilter was then washed with a volume of physiological saline solution, and successively, the membrane was incubated in an oven at 80 °C (Binder, Germany) for 18 h to achieve constant dry weight. Cell dry weight values were then calculated and correlated in a plot to the absorbance measurements. E. coli K4 growth rates (μ [h-1] ) in the control and supplemented media were reported as average of three values obtained in triplicate experiments; each value was calculated during the exponential phase between 0 and 3 h, according to the formula μ = 1/(T 2 − T 1), × ln (Abs600 nm 2/Abs600 nm 1) where T 2 > T 1. The residual sugar concentration was studied withdrawing samples of supernatant every hour. Growths were stopped after 24 h, when the release of capsular polysaccharide in the medium is known to be maximum (Cimini et al. 2010a). At that time, samples of supernatant were withdrawn to determine the K4 CPS concentration.
Batch experiments
A Biostat CT fermentor, (Braun Biotech International, Sartorius Group, Germany), in situ sterilizable, with a total volume of 2.5 L and a working one of 2 L, was used to perform batch experiments. The system was equipped with pH, temperature and pO2 probes and four peristaltic pumps for the addition of alkali, acid or antifoam solutions. Fermentation data were registered by a Digital Control Unit, during the experiments, while a computer connected to the fermentor, equipped with a MFCS/win software, was used to remotely control all the fermentation parameters and for data storage. The pO2 electrode (Mettler-Toledo, Switzerland) was calibrated using a pure oxygen flow as 100 % value. Batch experiments were performed, as previously described (Cimini et al. 2010a), at 37 °C and pH of 7.5, using as inoculum a shaking flask culture, grown overnight on the control medium. During the process, the pO2 value inside the vessel was kept always higher than 20 %, by modulating the stirring in the range from 400–800 rpm, an airflow value of 1 L min−1 was kept constant, while the pH was maintained stable at 7.5 by addition of 30 % (v/v) NH4OH and/or 30 % (v/v) H2SO4 solutions. Two different types of monosaccharide-supplemented batch experiments were performed in duplicate; with a concurrent addition of 0.385 mM of GlcA and GalNAc, or with a 0.385 mM Fructose supplementation, at the beginning of the growth. The bacterial growth was followed by measuring the absorbance at 600 nm, and samples of supernatant were withdrawn, during the experiments, to analyse glycerol and monosaccharide consumption and K4 CPS production. As already reported (Cimini et al. 2010a), fermentation experiments were stopped after 24 h of growth when the release of capsular polysaccharide in the medium was maximum. Batch fermentations using control medium were performed as reference.
Analytical methods
Monosaccharide and glycerol determination
Samples from shake flask cultures or fermentation broth were collected at different growth times and centrifuged at 1,700×g for 20 min (Avanti J-20 XP, Beckman Coulter, USA) in order to separate the biomass and to recover the supernatants. Two millilitres of supernatants were ultrafiltered/diafiltered on 10-kDa centrifugal filter devices (YM-10 Centricon, Amicon, USA) at 5,000×g and concentrated tenfold. The retentate volumes, containing the K4 CPS, were analysed by capillary electrophoresis (HPCE). Filtrate samples were analysed by high-performance anion exchange (HPAE-PAD; model ICS-3000, Dionex, USA) to determine the concentrations of added monosaccharide and glycerol at different growth times, in both shake flask and batch experiments. Supplemented GlcA and GalNAc analyses were performed by using a Carbopac PA1 column (Dionex, USA), at 25 °C, eluting, in gradient conditions from 10 to 20 % of phase B in 20 min at a flow rate of 1 mL min−1 (150 mM NaOH as mobile phase A and 150 mM NaOH and 1 mM NaAcOH as phase B). Fructose was determined by using a Carbopac PA100 column (Dionex, USA), eluting with 237 mM NaOH at 25 °C, at a flow rate of 1 mL min, while glycerol was analysed by using a Carbopac MA1 column (Dionex), at 25 °C, in isocratic conditions, with a 487-mM NaOH buffer at the flow rate of 0.4 mL min−1. Both monosaccharides and glycerol were detected by a pulsed amperometric detector (reference electrode Ag–AgCl; measuring electrode Au).
Capsular polysaccharide determination
The K4 CPS concentration was determined by HPCE analysis performed on a P/ACE MDQ instrument (Beckman Coulter, USA), according to the previously described method (Restaino et al. 2009). In shake flask experiments, the K4 CPS concentration was analysed after 24 h of growth, while in batch fermentations, the K4 polysaccharide concentration was determined at 8 and 24 h of growth.
Real-time PCR analyses
Quantitative real-time PCR analysis was performed on E. coli K4 cells cultivated in shake flasks. The medium was supplemented with either 0.385 mM GlcA and GalNAc or 0.385 mM Fructose. Biomass samples were collected at three different time points (4, 8 and 24 h) in order to compare mRNA levels of the gene coding for chondroitin polymerase. RNA extracted with a RNeasy Mini Kit (Qiagen, Milano, Italy) was treated with DNase using the DNA-free kit following the supplied protocol (Ambion Inc, Austin, TX) before reverse transcription with the Reverse Transcription System (Promega, Madison, USA). PCR amplification was performed on an iQ5 instrument (Bio-Rad, CA, USA). 16S rRNA was used as housekeeping gene and amplified with primers 16Sq_Up (sequence 5′- GGTGTAGCGGTGAAATGCGTAGAG-3′) and 16Sq_Dw (sequence 5′- CAAGGGCACAACCTCCAAGTC-3′), while kfoC was amplified with primers KfoCq_Up (sequence 5′-TTTTCCCTGCCGCACGATCC -3′) and KfoCq_Dw (sequence 5′-TTCGGTCTGTTGAAGGAGCAATGG -3′). Amplification and analysis were carried out according to the previously described method (Cimini et al. 2010b). Three biological replicates, each one in triplicate, were analysed for every time point.
Results
Shake flask GlcA and GalNAc supplementation
Different amounts of GlcA or GalNAc, final concentrations in the medium ranging from 0.128 to 1.540 mM, were added to the control medium at the beginning of the growth, in shake flask conditions, in order to investigate their influence on E. coli K4 capsular polysaccharide formation. All results were compared with the production data of the control (Cimini et al. 2010a). Monosaccharide additions do not modify medium pH and conductivity significantly (7.43 ± 0.1 and 14.5 ± 0.5 mS/cm, respectively). Supernatant analyses revealed that both monosaccharides were consumed by the bacterial cells, mainly during the exponential growth phase. GlcA was completely used within 3 h, while a small residue (3–5 %) of GalNAc remained in the media after 24 h growth (Fig. 2a). Compared to the growth on control medium, the different sugar consumption did not seem to influence the final biomass formation. On the other hand the growth rate (m) during the exponential phase significantly increased when sugar concentrations higher than 0.385 mM were added (0.01 < p values < 0.05; Table 1). The K4 CPS production and corresponding yield improved when either GlcA or GalNAc concentrations higher than 0.128 and 0.385 mM were used, respectively (Table 1 and Fig. 3). GlcA demonstrated to be more effective than GalNAc: supplementing the media with 0.385 and 0.770 mM of GlcA, the production increased by 26.8 and 37.7 %, while in the case of GalNAc, it resulted to be 12.5 and 26.8 % higher (Fig. 3). Addition of higher concentrations of sugars (1.540 mM) did not generate further K4 CPS production enhancements (Fig. 3).
Shake flasks: GlcA plus GalNAc supplementation
Concurrent supplementations of the two monosaccharides were tested: the data on the residual sugar concentrations showed that the uptake of GlcA was faster than that of GalNAc, as reported for shake flask experiments with singular addition (Fig. 2b). The growth rate (m; p < 0.05 up to 0.77 mM) was influenced, in addition to a great effect noted on both K4 CPS production and yield (Table 1, Fig. 2b and Fig. 3). Significant enhancements (0.168, 0.197, 0.220 g L−1) were obtained when GlcA and GalNAc were simultaneously supplemented at the concentrations of 0.128, 0.385 and 0.770 mM, causing production increases of almost 42, 67 and 87 %, respectively (Fig. 3). The concurrent addition of the two sugars had a clear synergistic effect on K4 CPS synthesis. The increase in production observed was in fact higher than the expected theoretical value calculated as the sum of the experimental data obtained in growths with supplementation of either GlcA or GalNAc (Fig. 4). However, the titer of K4 CPS did not increase linearly with the increase of monosaccharide concentrations. The effect of supplementation seemed to be time- and concentration-dependent; when 0.385 mM GlcA + GalNAc was supplemented at 4 or 8 h of growth, instead of before inoculation, the K4 CPS concentration was similar to the one obtained when cells were grown on the control medium (0.120 ± 0.02 and 0.122 ± 0.02 g L−1, respectively). While when a second addition of 0.385 mM GlcA + GalNAc, for a total of 0.770 mM, was performed, after the initial one, at 4 or 8 h of growth, the capsular polysaccharide production resulted the same as that observed with a single addition at 0 h (Fig. 5), althought the sugars supplied were completely consumed (data not shown). In these cases, the final biomass increased (in the range from 2.70 ± 0.11 to 2.88 ± 0.10 gcdw L−1) lowering the yield and thus supporting the idea that a further addition helped growth but not capsular polysaccharide production. Thus, among all the shake flask experiments, the simultaneous supplementation of 0.385 mM GlcA + GalNAc was considered the most suitable one to enhance the K4 CPS production in terms of both monosaccharide costs and production benefits.
E. coli K4 24-h shake flask growth on supplemented media with addition at 0 h (0.128, 0.385, 0.770, 1.540 mM GlcA, GalNAc or GlcA + GalNAc): comparison of theoretical (as sum of GlcA and GalNAc bars) and experimental data of K4 CPS production increase, in percentage, compared to data on the control medium
Relative mRNA fold expression of chondroitin polymerase in E. coli K4 at 4, 8 and 24 h of growth, in shake flask experiments on media supplemented with 0.385 mM GlcA + GalNAc or with single additions of 0.385 mM GlcA, GalNAc or fructose at 0 h of growth. Results were normalised on gene expression on the medium without supplements. The production of K4 CPS (milligrams per litre) after 24 h of growth in all conditions is also indicated
Shake flasks: fructose supplementation
As a monosaccharide constituting the capsular polysaccharide chain and, mainly, because in its 6-phosphorylated form fructose is considered a key sugar in the metabolic pathway for the formation of UDP-GalNAc (Cimini et al. 2012), shake flask experiments were performed by adding this monosaccharide before inoculation. In all the experiments fructose was completely consumed within 4 h of growth (Fig. 2a), and its additions to the medium, ranging from 0.128 to 0.770 mM, did not seem to significantly modify the bacterial growth in terms of final biomass formation and of growth rate (p > 0.05; Table 1). Production and yield values, instead, were greatly enhanced; by adding 0.385 and 0.770 mM of fructose, the 24-h K4 CPS concentrations reached values of 0.188 and 0.197 g L−1, respectively, with significant increases of production (59 and 67 %; Fig. 3). As previously reported, a further secondary 0.385 mM fructose supplementation at 4 or 8 h did not help to increase the K4 CPS production (Fig. 5). Moreover, also a combined supplementation of 0.385 mM GlcA + GalNAc + fructose did not increase the K4 CPS synthesis that demonstrated to be similar to the one reported for 0.385 mM GlcA + GalNAc experiment (0.205 ± 0.01 g L−1).
Analysis of kfoC expression
In order to correlate the K4 CPS production increase to molecular mechanisms, quantitative real-time PCR experiments were carried out to study the expression pattern of the chondroitin polymerase coding gene following addition to the medium of sugar precursors. Single and combined additions at the time zero of growth were investigated, and all conditions seemed to promote kfoC expression. In particular, when 0.385 mM of GlcA and GalNAc was added to the medium, overexpression was higher after 4 h, slightly lower after 8 h, and it peaked after 24 h of growth (13.9 ± 6) due to the severe drop of expression of the gene in the control medium (Fig. 6). The same expression pattern was obtained when single additions of 0.385 mM fructose or GlucA were performed, although, compared to the previous condition, lower or similar fold increments, respectively, were observed (Fig. 6). Conversely, when only 0.385 mM GalNAc was supplemented to the medium, kfoC mRNA levels were significantly higher also after 8 h of growth, resulting in a growing overexpression trend throughout the experiment (Fig. 6).
Supplemented batch experiments
Batch experiments were performed in a 2.5-L fermentor in order to test the efficiency of monosaccharide supplementation in a bioreactor with controlled pH, temperature and oxygen concentration. Control experiments were performed on control medium, and the results were in agreement with the ones reported in previous papers (Cimini et al. 2010a; Restaino et al. 2011) in terms of final biomass (4.0 ± 0.5 gcdw L−1), K4 CPS production (0.305 ± 0.010 g L−1) and yield (0.073 ± 0.002 g K4 CPS g −1cdw ). Supplemented batch experiments were performed by adding 0.385 mM of GlcA + GalNAc or fructose before inoculation (Fig. 7a, b) producing an increased K4 CPS concentration without changing its structural characteristic (Fig. 8) in terms of capillary electrophoresis profile (Restaino et al. 2009): in both cases, glycerol was consumed in 8 h, as already reported for growth on control medium (Cimini et al. 2010a), while the final biomass reached values of 5.2 ± 0.2 and 4.0 ± 0.2 gcdw L−1, respectively (Fig. 7a, b).
In GlcA + GalNAc-supplemented batches, the monosaccharides were completely used in 6 h of growth. Added sugars contributed to obtain a final K4 CPS production of 0.392 ± 0.010 g L−1 (yield of 0.075 ± 0.001 g K4 CPS g −1cdw ), thus doubling the concentration obtained in supplemented shake flask experiments and resulting almost 28 % higher than the one obtained in control medium batches (Fig. 7a). The addition of fructose produced a final K4 concentration of 0.408 ± 0.010 g L−1, which is similar to that obtained following the addition of GlcA + GalNAc, and a higher yield of 0.098 ± 0.002 g K4 CPS g −1cdw (Fig. 7b). Also in this case, the controlled batch conditions allowed to double the capsular polysaccharide concentration reached in fructose-supplemented shake flask experiments and to have an almost 34 % increased K4 CPS production compared to the batch performed on control medium.
Discussion
E. coli O5:K4:H4 synthesizes a capsular polysaccharide, the K4 CPS, constituted by glucuronic acid, N-acetylgalactosamine and a fructose terminal residue, that is similar to CS, a glycosaminoglycan widely used as the active principle of anti-arthritis and osteoarthritis drugs. Previous papers have already demonstrated that CS could be derived from the K4 CPS, by setting up a novel and safe integrated biotechnological–chemical strategy that uses innovative fermentation technologies (Cimini et al. 2010a; Restaino et al. 2011), membrane-based purification processes (Schiraldi et al. 2011) and a multi-step chemical sulphation synthesis (Bedini et al. 2011). In this paper, a new approach was tested to enhance the E. coli K4 strain capability to produce this commercially valuable polysaccharide by directly supplying the bacteria the monosaccharide precursors of the chain, together with a carbon source like glycerol. E. coli K4 demonstrated to be able to uptake both GlcA and GalNAc; that is not surprising; in fact, genetic analyses have already revealed the existence of a specific phosphotransferase system for the uptake of GalNac in some strains of the E. coli genera, also in the capsulated E. coli K92 one (Brinkkötter et al. 2000; Ezquerro-Sáenz et al. 2006), while the transportation system and the uptake of aldohexuronate in capsulated E. coli K12 were reported by Nemoz et al. (1976). Although both hexuronate and amino sugars are considered good carbon sources for E. coli strains, few papers correlate their use in the media to the production of capsular polysaccharides. Investigating E. coli K92 monosaccharide transport systems, previous researches demonstrated that the use of N-acetylated amino sugars, like GalNac or ManNAc, as the only carbon source in the medium, did not support polysialic acid-like capsular polysaccharide production, even if they were supplied at high concentrations (e.g. >8 g L−1) (Revilla-Nuin et al. 1998; Ezquerro-Sáenz et al. 2006). The use of glucuronic acid in media containing mixed carbon sources, like glucose and xylose, has been reported to study the adaptation of E. coli K12 to grow on different substrates (Cohen 1949). Our data demonstrated that supplying the monosaccharides constituting the E. coli K4 capsular polysaccharide, even at millimolar concentrations, that are at least about 65-fold lower than the major carbon source (glycerol), was successful in improving polysaccharide formation. Shake flask experiments with single monosaccharide additions showed a precise range of sugar concentrations suitable to enhance significantly the K4 CPS production and yield, starting from a minimum threshold that resulted different according to the type of monosaccharide (higher than 0.128 mM for GlcA and higher than 0.385 mM for GalNac). However, the production improvement did not result linearly proportional to the increase of supplemented monosaccharide concentration; when 1.540 mM of either GlcA or GalNAc was added, the K4 CPS concentration did not increase further. GlcA supplementation resulted to be more efficient compared to that of GalNAc in enhancing K4 CPS production. This could be either due to a faster uptake of GlcA or to its influence on the activity of the polymerase. As a matter of fact, UDP-GlcA, bound in a non-productive orientation, was suggested to have a regulation role on the E. coli K4 polymerase A1 site activity and thus on chain elongation (Osawa et al. 2009; Zanfardino et al. 2010). Differences in the CPS production could also depend on the competition with alternative metabolic pathways; in fact, once uptaken, GalNAc could be easily converted into GlcNAc by the cell and used for cell wall or lipopolysaccharide biosynthesis (Wang et al. 2011). The simultaneous supplementation of GlcA and GalNAc, providing both chain precursors, resulted to be more effective in increasing K4 CPS production and yield compared to the single addition of either monosaccharide; a clear synergistic effect was easily noted comparing expected (theorical) and experimental data, thus supporting the idea, already reported for E. coli K5 (Roman et al. 2003; Wang et al. 2011), that the capsular polysaccharide biosynthesis is a process that works better with a balanced co-presence of both building blocks. The 0.385-mM GlcA + GalNAc supplementation resulted the best one in terms of costs versus benefits bringing to an increase of both production and yield (0.197 g L−1 and 0.086 g K4 CPS g −1cdw , respectively); the 0.770-mM addition resulted to be less efficient since it increased both K4 CPS and biomass production thus bringing to yield values similar to that obtained with 0.385-mM supplementation. Fructose is a terminal residue in the K4 CPS chain, and it is probably added once the elongation phase is completed; thus, in this role, its supplementation in the medium should not necessarily affect capsular polysaccharide production. However, in its 6-phosphated form, fructose is a key sugar in the capsular polysaccharide biosynthesis pathway as precursor of UDP-GalNAc. Moreover, it is also a precursor of UDP-GlcA since it is in equilibrium with Glc-6P. Fructose can easily be uptaken by E. coli strains through transporters of the sugar porter family (Saier et al. 2000). By supplementing this sugar to the medium, the K4 CPS concentration significantly increased: in particular, when 0.385 or 0.770 mM fructose concentrations were added, production was enhanced by almost 60 and 74 %, respectively. The yield values obtained in the two trials were however similar due to the biomass increase observed when the 0.770 mM fructose concentration was supplemented. Independently from the type of sugar added, the enhancement of the K4 CPS production resulted to be strictly regulated by the time of addition: increases were noted only when monosaccharides were supplemented before inoculation and not at subsequent times (neither after 4 nor 8 h of growth). Addition during growth resulted to be ineffective even when performed after a first supplementation at 0 h. Molecular analyses were performed, in the best supplementation conditions, in order to verify whether the addition of capsular polysaccharide precursors would also trigger kfoC gene expression. As previously demonstrated (Cimini et al. 2010a; Restaino et al. 2011), the release of K4 polysaccharide in the medium initiates during the late exponential phase, and it is completed after 24 h of growth. In agreement with these data, kfoC expression on the control medium is higher during the exponential phase, when the capsular polysaccharide is synthesized, and it decreases towards the stationary phase. Compared to the control, the mRNA level of the kfoC gene, investigated by real-time PCR, showed that the monosaccharide additions had an effect on its expression; in fact, when both 0.385 mM GlcA + GalNAc were added to the medium, just after cell inoculation, kfoC expression was enhanced, peaking in particular after 24 h of growth when its expression on the control medium is strongly downregulated. In order to understand whether one of the two sugars had a major impact on kfoC mRNA levels, the expression profiles of the gene were also investigated when performing single additions. Results show that the supplementation of GlcA had a stronger effect on increasing kfoC expression compared to GalNAc, and, surprisingly, it was sufficient to reach fold values that are similar to that obtained when both sugars are added. These results clearly indicate that the enrichment of kfoC transcripts does not necessarily imply an enhancement of CPS production, reflecting the complexity of the capsule biosynthesis process. In fact, although fructose supplementation increased the final titer of polysaccharide more than the single additions of GlcA and GalNAc, kfoC overexpression was supported to a lower extent.
The K4 CPS enhancement, obtained in the best supplementation conditions (0.385 mM GlcA + GalNAc or fructose), resulted to be effective also scaling up the process to 2.0-L fermentations, compared to batches performed on the control medium (Cimini et al. 2010a). The strict pH and oxygen control, performed during the batch experiments, allowed to double the capsular polysaccharide concentration obtained in shake flask experiments (0.392 ± 0.010 and 0.408 ± 0.010 g L−1 for GlcA + GalNAc and for fructose, respectively), confirming the strong influence of these two parameters on the K4 CPS biosynthesis, as previously reported (Cimini et al. 2010a). From an economic point of view, both the additions of 0.385 mM GlcA + GalNAc or 0.385 mM fructose charge a contained extra cost on the entire fermentation process, but the fructose supplementation is, indeed, the most promising (less than $20 for GlcA + GalNAc and less than $1 for fructose in case per litre of batch culture). Thus, all our data confirmed the possibility to use the precursors of the capsular polysaccharide chain as tools to enhance the K4 CPS production during the bacterial growth in both shake flask and fermentation conditions. Our results open new perspectives regarding future metabolic investigations that aim at increasing the microbial glycosaminoglycan-like capsular polysaccharide production through the modification of precursor biosynthetic pathways.
References
Bedini E, De Castro C, De Rosa M, Di Nola A, Iadonisi A, Restaino OF, Schiraldi C, Parrilli M (2011) A microbiological–chemical strategy to produce chondroitin sulfate A, C. Angew Chem Int Ed Engl 50(27):6160–6163
Brinkkötter A, Klöb H, Alpert CA, Lengerel JW (2000) Pathways for the utilisation of N-acetyl-galactosamine and galactosamine in Escherichia coli. Mol Microbiol 37:125–135
Cimini D, Restaino OF, Catapano A, De Rosa M, Schiraldi C (2010a) Production of capsular polysaccharide from Escherichia coli K4 for biotechnological applications. Appl Microbiol Biotechnol 85(6):1779–1787
Cimini D, De Rosa M, Viggiani A, Restaino OF, Carlino E, Schiraldi C (2010b) Improved fructosylated chondroitin production by kfoC overexpression in E. coli K4. J Biotechnol 150:324–331
Cimini D, De Rosa M, Schiraldi C (2012) Production of glucuronic acid-based polysaccharides by microbial fermentation for biomedical applications. Biotechnol J 7(2):237–250
Chong BF, Blank LM, Mclaughlin R, Nielsen LK (2005) Microbial hyaluronic acid production. Appl Microbiol Biotechnol 66:341–351
Cohen SS (1949) Adaptive enzyme formation in the study of uronic acid utilization by the K-12 strain of Escherichia coli. J Biol Chem 177(2):607–619
Ezquerro-Sáenz C, Ferrero MA, Revilla-Nuin B, López Velasco FF, Martínez-Blanco H, Rodríguez-Aparicio LB (2006) Transport of N-acetyl-D-galactosamine in Escherichia coli K92: effect on acetyl-amino sugar metabolism and polysialic acid production. Biochimie 88:95–102
Jann K, Jann B (1992) Capsules of Escherichia coli, expression and biological significance. Can J Microbiol 38:705–710
Kogan G, Šoltés L, Stern R, Gemeiner P (2007) Hyaluronic acid: a natural biopolymer with a broad range of biomedical and industrial applications. Biotechnol Lett 29(1):17–25
Nemoz G, Robert-Baudouy J, Stoeberg F (1976) Physiological and genetic regulation of the aldohexauronate transport system in Escherichia coli. J Bacteriol 127(2):706–718
Osawa T, Sugiura N, Shimada H, Hirooka R, Tsuji A, Shirawa T, Fukuyama K, Kimura M, Kimata K, Kakuta Y (2009) Crystal structure of chondroitin polymerase from Escherichia coli K4. Biochem Biophys Res Commun 378:10–14
Roman E, Roberts I, Lidholt K, Kusche-Gullberg M (2003) Overexpression of UDP-glucose dehydrogenase in Escherichia coli results in decreased biosynthesis of K5 polysaccharide. Biochem J 374:767–772
Restaino OF, Cimini D, De Rosa M, De Castro C, Parrilli M, Schiraldi C (2009) High-performance CE of Escherichia coli K4 surface polysaccharides. Electrophoresis 30(22):3877–3883
Restaino OF, Cimini D, De Rosa M, Catapano A, De Rosa M, Schiraldi C (2011) High cell density cultivation of Escherichia coli K4 in a microfiltration bioreactor: a step towards improvement of chondroitin precursor production. Microb Cell Fact 10:10
Revilla-Nuin B, Rodriguez-Aparicio LB, Ferrero MA, Reglero A (1998) Regulation of capsular polysialic acid biosynthesis by N-acetyl-D-mannosamine, an intermediate of sialic acid metabolism. FEBS 426:191–195
Roberts IS (1996) The biochemistry and genetics of capsular polysaccharide production in bacteria. Annu Rev Microbiol 50:285–315
Roberts IS, Mountford R, Hodge R, Jann KB, Boulnois GJ (1988) Common organization of gene clusters for production of different capsular polysaccharides (K antigens) in Escherichia coli. J Bacteriol 170:1305–1310
Rodriguez M-L, Jann B, Jann K (1988) Structure and serological characteristics of the capsular K4 antigen of Escherichia coli O5:K4:H4, a Fructose-containing polysaccharide with chondroitin backbone. Eur J Biochem 177:117–124
Ronca F, Palmieri L, Panicucci P, Ronca G (1998) Anti-inflammatory activity of chondroitin sulfate. Osteoarthr Cartil 6:14–21
Saier MH Jr, Beatty JT, Goffeau A, Harley KT, Huang SC, Jack DL, Jähn PS, Levy K, Liu J, Pao SS, Paulsen IT, Tseng TT, Virk PS (2000) The major facilitator superfamily. J Mol Microbiol Biotechnol 2(2):255
Schiraldi C, Carcarino IL, Alfano A, Restaino OF, Panariello A, De Rosa M (2011) Purification of chondroitin precursor from Escherichia coli K4 fermentation broth using membrane processing. Biotechnol J 6(4):410–419
Smith AN, Boulnois GJ, Roberts IS (1990) Molecular analysis of the Escherichia coli K5 kps locus: identification and characterization of an inner-membrane capsular polysaccharide transport system. Mol Microbiol 4:1863–1869
Vann WF, Schmidt MA, Jann B, Jann K (1981) The structure of the capsular (K5 antigen) of urinary infective Escherichia coli O10:K5:H4. A polymer similar to desulpho-heparin. Eur J Biochem 116:359–364
Volpi N (2007) Analytical aspects of pharmaceutical grade chondroitin sulfate. J Pharm S 96(12):3168–3180
Wang Z, Dordick JS, Linhardt RJ (2011) Escherichia coli K5 heparosan fermentation and improvement by genetic engineering. Bioengineered Bugs 2(1):63–67
Whitfield C (2006) Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75:39–68
Zanfardino A, Restaino OF, Notomista E, Cimini D, Schiraldi C, De Rosa M, De Felice M, Varcamonti M (2010) Isolation of an Escherichia coli K4 kfoC mutant over-producing capsular chondroitin. Microb Cell Fact 9:34
Acknowledgments
This research was supported by Ministero dell’ Istruzione, dell’Università e della Ricerca (MIUR), L297 project: “Produzione biotecnologica di condroitina”.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Restaino, O.F., di Lauro, I., Cimini, D. et al. Monosaccharide precursors for boosting chondroitin-like capsular polysaccharide production. Appl Microbiol Biotechnol 97, 1699–1709 (2013). https://doi.org/10.1007/s00253-012-4343-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4343-2