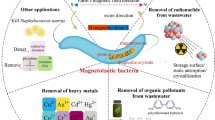
Abstract
Magnetotactic bacteria (MTB), which can orient and migrate along a magnetic line of force due to intracellular nanosized magnetosomes, have been a subject of research in the medical field, in dating environmental changes, and in environmental remediation. This paper reviews the recent development of MTB as biosorbents for heavy metals. Ultrastructures and taxis of MTB are investigated. Adsorptions in systems of unitary and binary ions are highlighted, as well as adsorption conditions (temperature, pH value, biomass concentration, and pretreatments). The separation and desorption of MTB in magnetic separators are also discussed. A green method to produce metal nanoparticles is provided, and an energy-efficient way to recover precious metals is put forward during biosorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution caused by various industries (mining, metallurgy, electroplating, high technology industries, and others) is a serious threat to ecological systems and to the health of its inhabitants. Meanwhile, increasing demand for metal resources results in the scarcity of metal resources in the natural environment. Thus, researchers are addressing the growing need for recovering heavy metals from wastewater to remove the toxic chemical elements and to recycle these metals (Wang and Chen 2009; Zhang et al. 2010). Conventional methods, such as chemical precipitation, ion exchange, electrochemical treatment, and active carbon adsorption, have been tried and improved. However, problems such as low efficiency, high costs, complex processes, and secondary pollution have not been solved thoroughly. In recent years, increasing attention has been paid to biosorbents because of their excellent adsorption properties, low cost, and high availability (Das 2010; Selenska-Pobell and Merroun 2010; Wang and Chen 2009; Xiao et al. 2012, 2010). Nevertheless, separation and recovery of biosorbents from solutions are still challenges in the field (Das 2010). The case of magnetotactic bacteria (MTB) as promising biosorbents was investigated because MTB can be easily separated from wastewater using magnetic fields (Song et al. 2008, 2007; Wang et al. 2011; Xie et al. 2009).
MTB refer to a heterogeneous group of prokaryotes that can orient and migrate along geomagnetic field lines (Schüler and Frankel 1999). They are capable of forming a specific membrane-enveloped intracellular structure called magnetosome (Frankel 2003; Tanaka et al. 2010). Magnetosomes have uniform, species-specific crystal habits with narrow size and shape distributions, and they are generally aligned in chains within bacteria (Tanaka et al. 2010). Magnetite (Fe3O4) or greigite (Fe3S4) are magnetic particles that comprise magnetosomes (Bazylinski et al. 1995; Mann et al. 1990; Schüler and Frankel 1999). MTB widely exist in freshwater, sediments, and oceans, among others (Lin and Pan 2009; Mann et al. 1990; Matsunaga et al. 2005; Sakaguchi et al. 2002). Most of them are microaerophilic and are found in or below the oxic–anoxic transition zone (OATZ) (Flies et al. 2005; Lefevre et al. 2009; Li et al. 2007). Compared with studies on the applications of MTB in the medical and environmental fields, few papers have focused on MTB application in environmental remediation (Bazylinski and Schubbe 2007; Kopp and Kirschvink 2008; Linford et al. 2005; Peng et al. 2000; Xie et al. 2009). Studies focusing on removing metals and trace radionuclides from wastewater using MTB were first presented by Bahaj et al. (1994, 1998a, 1991, 1998b). Subsequently, the adsorption of heavy metals was further investigated (Keim and Farina 2005; Song et al. 2008, 2007; Wang et al. 2011). The mineralization of metal ions occurs in MTB after exposure to metallic elements other than iron (Arakaki et al. 2002; Cai et al. 2011; Song et al. 2008; Tanaka et al. 2010). Moreover, MTB showed selective reinforced competitive biosorption for Au (III) and Ag (I) in Au–Cu and Cu–Ag binary ion systems (Song et al. 2007; Wang et al. 2011).
Confined by the harsh growth conditions of MTB, only a few strains are available in pure culture. Magnetotaxis can be lost in continuous culture or after prolonged storage at 4 °C (Heyen and Schüler 2003; Li et al. 2010, 2007; Lin and Pan 2009; Sun et al. 2008). However, Liu et al. (2010) achieved large-scale production of magnetosomes of Magnetospirillum gryphiswaldense at high cell density by chemostat culture based on pH-stat feeding. New ideas for obtaining magnetosomes can also be inspired by genetic engineering (Matsunaga et al. 2005). The adsorption capacity of MTB for Cd2+ has been improved by cell surface display technology (Tanaka et al. 2008); thus, more attention needs to be paid to this promising biosorbent. The application of MTB in removing and recovering heavy metals is emphasized in this paper. Ultrastructures and taxis of MTB are briefly introduced to promote the application of MTB.
Ultrastructures
Magnetosomes
Features
Magnetosomes consist of magnetite (Fe3O4) or greigite (Fe3S4) coated with membranes (Schüler and Frankel 1999). A combination of greigite and iron pyrite (FeS2), monoclinic pyrrhotite (Fe7S8), mackinawite (tetragonal FeS), and sphalerite-type cubic FeS were also identified in magnetotactic multicellular prokaryotes (MMP) (Bazylinski et al. 1995, 1993; Pósfai et al. 1998). These might be precursor phases of greigite crystals (Pósfai et al. 1998). Iron sulfide magnetosomes in MMP could contain variable concentrations of Cu (up to 10 at.%) (Bazylinski et al. 1993; Pósfai et al. 1998). Cu content may depend on Cu availability and is independent of the mineral phase (Pósfai et al. 1998). Only cobalt and manganese were successfully doped into magnetite particles (Keim et al. 2009; Staniland et al. 2008). Most magnetosomes are ferromagnetic with a size range of 35 nm to 120 nm (Bazylinski and Frankel 2004), whereas smaller particles in the superparamagnetic range (<30 nm) have also been observed (Fischer et al. 2008).
The morphologies and intracellular organization of magnetosomes in different MTB are shown in Fig. 1 (Schüler and Frankel 1999). Magnetosomes scattered throughout the cytoplasm or forming unordered clusters were also reported (Cox et al. 2002; Lin and Pan 2009). The scattering of magnetosomes is attributed to the different stages of magnetosome formation or different environmental conditions. The possibility of genetic mutation could not be ruled out as well. Another possibility is that the clustered magnetosomes are formed by a different biological mechanism and that they serve a different function to magnetosomes in chains.
Crystal morphologies and intracellular organization of magnetosomes in different MTB: a cubo-octahedral, b, d, e, and f elongated hexagonal prismatic, and c bullet-shaped. Magnetosomes are arranged in one (a, b, and c), two (e), or multiple chains (d) or irregularly (f). (Bar equivalent to 100 nm) Copyright © Springer-Verlag. Reproduced with permission
The size and number of magnetosomes can vary considerably in response to culture medium composition and oxygen concentration (Cox et al. 2002; Heyen and Schüler 2003; Kundu et al. 2009; Liu et al. 2008). A correlation between pO2 and magnetosome formation was found in M. gryphiswaldense, Magnetospirillum magnetotacticum and Magnetospirillum sp. AMB-1. Magnetite biomineralization was induced only below a threshold value of 20 mbar O2 in all three strains (Heyen and Schüler 2003). High concentrations of Zn and Ni salts in the growth medium lead to the modification of bacterial and magnetosome properties, especially size, number, and chain alignment of M. magnetotacticum MS-1. The physical properties of MS-1 that are cultured in different media are shown in Table 1, which reveals that the formation of magnetosomes can be influenced and enhanced by chemical manipulation (Kundu et al. 2009).
Flagella
The migration of MTB along geomagnetic field lines depends on the coordinated movement of flagella (Frankel 2003; Van Kampen 1995). Different amounts of flagella are found in various strains of MTB. Desulfovibrio magneticus RS-1 is propelled by a single polar flagellum at its tail end (Sakaguchi et al. 2002). M. gryphiswaldense cells are flagellated in its two poles (Schüler and Frankel 1999). Flagella that originate from a depression on the surface of some uncultured magnetotactic cocci are organized in bundles (Freitas et al. 2003). A mathematical model on the motion of assumptive spherical MTB with a single flagellum was put forward (Nogueira and Lins De Barros 1995).
Granules
Different sizes of granules without any membranes are found in most uncultured MTB. Phosphorus and oxygen are the most common elements in granules. Granules can be classified into two types: phosphorus-rich granules (P granules) and phosphorus–sulfur–iron granules (PSFe granules) (Keim and Farina 2005; Keim et al. 2005; Lins and Farina 1999; Zhu et al. 2010). In rod-shaped bacteria, these granules are positioned symmetrically around the center of the cell. P granules may naturally contain small amounts of manganese. Other elements, such as Na, Al, K, Ca, Zn, Cd, Fe, and Cl, are also detected on some occasions. PSFe granules have similar elemental composition (Keim and Farina 2005; Keim et al. 2001, 2005; Lins and Farina 1999).
Granules are considered as a major site for metal accumulation. Differences in the composition of granules may lead to differences in binding with metal ions. One compelling evidence is the accumulation of larger amounts of gold in PSFe granules than in P granules (Keim and Farina 2005). The capability of granules to assimilate metal ions may indicate a detoxification role. Meanwhile, high solubility of granules allows iron to be further used in metabolism (Byrne et al. 2010; Keim et al. 2005; Lins and Farina 1999). In addition, granules are also identified as lipid storage facilities and may act as storage compounds for energy and carbon needed in maintaining metabolism and synthesis of cellular metabolites during starvation. Another possible function of the large granules is buoyancy regulation (Cox et al. 2002; Keim et al. 2005; Zhu et al. 2010).
Capsules
Capsules are on the cell surface of uncultured MTB (Freitas et al. 2003). They are formed by numerous fibrils that extend to hundreds of nanometers from the cell surface. Capsules are maintained by the combination of electrostatic and hydrophobic–hydrophilic forces. They are known to mineralize different minerals, and most of them contain abundant iron (Freitas et al. 2003; Keim and Farina 2005).
Sulfur globules
Globules are intracellular structures in many strains of MTB (Cox et al. 2002; Zhu et al. 2010). MTB collected from the OATZ of a chemically stratified marine environment characterized by O2/H2S inverse double gradients form S globules (Bazylinski et al. 2004). S globules have no fixed number or specific location (Keim et al. 2005). They may act as electron donor reserves when cells travel from sulfide-rich to sulfide-depleted zones (Cox et al. 2002; Kopp and Kirschvink 2008).
S-layers
The surfaces of most uncultured magnetotactic cocci have S-layers, which are formed by particles organized in hexagonal symmetry (Freitas et al. 2003). S-layers are weakly acidic and completely cover the cell in all stages of growth in both bacteria and archaea. They are supposed to work as protective coats, molecular sieves, and as molecule and ion traps (Sleytr and Beveridge 1999). S-layers in MTB may relate to magnetotaxis. They may inhibit metal precipitation on the cell surface and influence the ability to overcome environmental viscous drags while moving to a suitable place (Freitas et al. 2003).
Membrane vesicles
The cell surfaces of different M. magnetotacticum cells and cocci have numerous spherical or oval-shaped membrane vesicles (Freitas et al. 2003; Lins et al. 2003). The function of vesicles and the chemical nature of these vesicular interiors have not been determined yet.
Taxis
Magnetotaxis
Axial MTB cells can swim in both directions along magnetic field lines, whereas the polar ones swim either parallel to geomagnetic field lines toward the north pole or in an unparallel direction toward the south pole (Frankel 2003; Lefevre et al. 2009). The magnetic polarity of MTB can be changed by applying an artificial magnetic field opposite to the geomagnetic field. Magnetotaxis enable MTB to swim between electron donor-rich and electron acceptor-rich regions (Frankel and Bazylinski 1994). Hence, magnetotaxis turn a three-dimensional problem into a one-dimensional one along the magnetic field, thereby providing a way to short-circuit the diffusion of MTB. The presence of magnetotaxis may also be an evolutionary exaptation; they serve as a metabolic pathway adapted for sensitivity to the geomagnetic field after the original evolution (Kopp and Kirschvink 2008).
Aerotaxis
When a magnetic field and an oxygen gradient are in the opposite direction, MTB hold a position in the OATZ instead of moving down magnetic field lines and accumulating at the area with the lowest oxygen concentration (Bazylinski and Frankel 2004). When a magnetic field toward the cuvette bottom is applied, the aerotaxis of AMB-1 cells occurs if the sample is exposed to atmospheric oxygen. The migration patterns could also be observed without a magnetic field (Smith et al. 2006).
Oxygen is a determinant factor that controls magnetotactic behavior (Lefevre et al. 2009). Modeling the magneto-aerotaxis of AMB-1 indicates that magnetotaxis may enhance the detectability for oxygen but not increase the average speed for moving away from high oxygen concentrations (Smith et al. 2006). Thus, magneto-aerotaxis help MTB move toward a preferred oxygen concentration more efficiently (Bazylinski and Frankel 2004; Frankel et al. 1997; Smith et al. 2006; Xie et al. 2009).
Phototaxis
The relationship between the phototaxis and magnetotaxis of AMB-1 was analyzed by Chen et al. (2011). AMB-1 migrates toward pure white light sources and multiple spectral bands; migration increases with a higher light intensity. The response to light is independent of wavelength and magnetotaxis. Magnetosome synthesis or magnetosome-containing cells increase after exposure to illumination for 20 h. Studies showed that marine magnetic spirilla cells respond to illumination (Chen et al. 2011; Zhu et al. 2010). However, nearly half of them change their swimming direction under the illumination of light with wavelengths from 330 nm to 550 nm, and the other half appear to lose motility and then become agglomerated.
Thermotaxis
When illuminated from the left and heated from the opposite side to create a temperature gradient of 0.1 °C, AMB-1 cells migrate toward both light and heat simultaneously, indicating that AMB-1 cells have a thermotactic response, which is independent from their phototactic response (Chen et al. 2011). Multiple taxis, which may result from a great number of chemoreceptor-like proteins, help MTB search for and move to a more suitable habitat to avoid certain disadvantages more efficiently (Chen et al. 2011; Frankel 2003; Frankel et al. 1997).
Application in removing heavy metals
Adsorption of heavy metals
Adsorption in unitary ion system
The removal of heavy metals and trace radionuclide from wastewater using MTB was first introduced by Bahaj et al. (1994, 1998a, 1991, 1998b). The removal efficiency of heavy metals by MTB was further studied (Song et al. 2008; Wang et al. 2011), as shown in Table 2. The maximum amount of Cd2+ that could bind to 1.0 × 1010 wild-type AMB-1 cells was 60 nmol or more. High accumulation of arsenic and tellurium (Te) ions on the cell surface of AMB-1 cells was also verified. In obtaining better effects and convenience, studies on bioremediation are usually based on the entire MTB group instead of only on a certain strain of MTB. The removal efficiency of Cu (II) in the pH range of 2.0 to 4.5 was 98.07 % to 98.75 %, much higher than that of M. gryphiswaldense MSR-1. The adsorption yield of Au (III) on MTB was 99.53 % to 100 % in the pH range of 1.0 to 5.5 (Song et al. 2007; Wang et al. 2011). Both Langmuir and Freundlich adsorption models were suitable for describing the biosorption equilibrium of Cu (II) ions or Au (III) on MTB, but the Langmuir model matched better with adsorption of Au (III). The maximum adsorption capacity of some biosorbents for Au (III) is summarized in Table 3 (Song et al. 2008, 2007). Furthermore, a genetic modification technique could improve the adsorption ability of MTB. A hexahistidine amino acid was inserted to the membrane-specific protein of AMB-1 and then displayed on the cell surface using cell surface display technology. The quantity of Cd2+ absorbed by the AMB-1 transformant was 40 % higher than that of the wild-type AMB-1, whereas the collection efficiencies of both types of bacteria recovered by magnetic force were almost the same (Tanaka et al. 2008). Details on biosorption are still insufficient because of the limited amount of data, but MTB are obviously viable biosorbents.
The mineralization of metal ions occurs in MTB after exposure to metallic elements other than iron (Arakaki et al. 2002; Cai et al. 2011; Song et al. 2008; Tanaka et al. 2010). Monodisperse nanocrystals composed of Cd, S, and P were observed on the RS-1 cell surface after growing in the presence of Cd2+. More than 95 % of cadmium at an initial concentration of 1.3 mg L−1 was removed by precipitation after cultivation for 240 h. Sulfate-reducing bacteria could precipitate a variety of elements by reduction, including As (V), U (VI), and Tc (VII). Therefore, RS-1 may also accumulate metallic elements other than Cd (Arakaki et al. 2002). After 24 h of exposure to metals, large amounts of gold were trapped by the PSFe granules of magnetotactic cocci, and some were found on the capsules. Crystalline silver sulfide deposits were in the cell envelope (Keim and Farina 2005). The biosynthesis of gold nanoparticles was further studied using MSR-1. Spherical gold nanoparticles accumulated on the cell surfaces through 1 h of biosorption. In the pH range of 1.5 to 4.0, the higher the pH value, the larger the diameter of the gold nanoparticles. Moreover, particle diameter increased with the initial metal concentration. Thus, the size of gold particles can be controlled by the pH value and the initial metal concentration (Cai et al. 2011). Rod-shaped Te nanoparticles were distributed along the magnetosome chain in AMB-1. The number and size of Te crystals increased with the initial Te concentration to the medium, but the formation of magnetite crystals decreased gradually (Tanaka et al. 2010).
Most metallic elements stably existed on the cell surface by binding to the membrane or in the form of inorganic precipitates (Arakaki et al. 2002; Keim and Farina 2005; Keim et al. 2005). Functional proteins on the cell surface, capsules, and granules may play important roles in biomineralization (Cai et al. 2011). Differences in the composition of ultrastructures may lead to the binding of different metal ions. For instance, larger amounts of gold were trapped by a PSFe granule-type of uncultured MTB, whereas silver-containing deposits were in a P granule-type of uncultured MTB (Keim and Farina 2005).
The removal of Te by cell surface adsorption was two orders of magnitude less than the accumulation achieved by crystallization, indicating that chemisorption and biomineralization are important ways of removing heavy metals (Tanaka et al. 2010). Moreover, the minimum inhibitory concentration of heavy metals for MTB is relatively high (Kundu et al. 2009). Biosorption and biomineralization can be controlled by regulating the adsorption conditions (exposure time to metals, pH value, initial metal concentration, and others). Therefore, MTB not only serve as ideal biosorbents, they also provide an organic or natural method for synthesizing metallic nanoparticles.
Adsorption in binary ion system
A variety of metal elements are contained in industrial wastewater. The adsorption of MTB in binary ion systems has been studied. When the initial concentrations of Au (III) and Cu (II) were below 80 mg L−1, the final concentration of both ions in wastewater were less than 1.0 mg L−1 after MTB adsorption. The adsorption of Au (III) was reinforced (95.87 %) and that of Cu (II) was prohibited (8.83 %) with a coexisting concentration of 320 mg L−1 (Song et al. 2007). For the biosorption of MSR-1 in an Ag–Cu system, adsorption for both metallic elements was promoted when the molar ratio of Ag (I) and Cu (II) was below 4:1. Adsorption for Cu (II) decreased, whereas that for Ag (I) was enhanced when the ratio was over 4:1 (Wang et al. 2011). Selective reinforced competitive biosorptions of MTB in binary ion systems occur due to the functional groups and proteins on cell surfaces as well as physical and chemical properties of heavy metals (Song et al. 2007; Wang et al. 2011).
Adsorption conditions
Adsorption conditions include temperature, pH value, biomass concentration, stirring, and pretreatments. The removal efficiencies were almost the same in the range of 10 °C to 35 °C. One possible reason is that the adsorption of metal ions on MTB was mainly chemisorption and ion exchange instead of physical adsorption, which could be easily influenced by temperature (Song et al. 2007; Wang et al. 2011). A higher adsorption effect for heavy metals was seen in highly acidic conditions. The adsorption amounts of Cu (II) and Au (III) increased slightly within the pH range of 1.0 to 4.0 (Wang et al. 2011). As pH increased, more ligands with negative charges on the cell wall would be exposed, and the attraction to positively charged metal ions would increase. In the study of Song et al., however, the adsorption of Cu (II) in the pH range of 2.0 to 4.5 showed no significant difference, as well as that of Au (III) in the pH range of 1.0 to 5.5 (Song et al. 2007).
The adsorption amount rose with biomass concentration within limits. Usually, an appropriate stirring would accelerate adsorption. The removal efficiency increased rapidly in the first 30 min when samples were agitated on a shaker at 130 rpm and adsorption equilibrium was reached in 1 h (Song et al. 2008, 2007; Wang et al. 2011).
Pretreatments, such as drying and treating with sodium hydroxide and hydrochloric acid, would lower adsorption efficiency. This result may be because lipids and proteins in the cell wall involved in biosorption were damaged, thereby reducing the number of binding sites (Das 2010; Keim et al. 2001; Song et al. 2008; Wang et al. 2011).
Separation and desorption of MTB
A low magnetic field separation system for metal-loaded MTB was introduced by Bahaj et al (1998b). A magnetic separator was developed (Fig. 2), by which MTB can be easily separated from aqueous solutions (Song et al. 2008). A pair of NdFeB magnets was placed on two sides of the separator to generate a permanent magnetic field that was vertical to the flowing direction of the feed. The intensity of the magnetic field could be adjusted by changing the distance between the magnets. A high-gradient magnetic field was generated by fixing multilayers of nickel wires inside the separator. Nickel wires were parallel to the flow direction and worked as a trapping matrix to capture MTB. Thus, metal-loaded MTB could be separated and recovered from wastewater. MTB loaded with Au (III) were quickly removed in the first 30 min. The entire biomass could be removed and accumulated on the nickel wires at the magnetic intensity of 1,200 Gs in 180 min. Desorbents (e.g., HNO3, EDTA, NaNO3, and thiourea) were used to elute the Au adsorbed on MTB, among which thiourea proved to be an effective desorbent. About 91.0 % of Au could be recovered from the MTB biomass with the thiourea concentration of 0.8 M. Hence, applications of MTB in the removal and recovery of heavy metals have a promising future.
Prospects
The application of MTB in removing heavy metals has a notable potential. Biosorption and biomineralization are two efficient ways of removing heavy metals. These methods can be controlled by regulating the adsorption conditions. With the help of genetic modification technique, the adsorption ability of MTB will be further enhanced. Moreover, metal-loaded MTB can be easily separated from wastewater by a magnetic separator. The present paper contributes toward an energy-efficient way of recovering precious metals by adsorption and producing metal nanoparticles. More research into selective reinforced competitive biosorption of MTB for heavy metals is needed.
More details on ultrastructures in MTB are required to shed light on the adsorption and reduction law of MTB on heavy metals. In-depth knowledge about the taxis of MTB will also be useful when studying bulk culture samples and their applications. The application of MTB in removing and recovering heavy metals will be further promoted on the basis of a thorough understanding of MTB.
References
Arakaki A, Takeyama H, Tanaka T, Matsunaga T (2002) Cadmium recovery by a sulfate-reducing magnetotactic bacterium, Desulfovibrio magneticus RS-1, using magnetic separation. Appl Biochem Biotechnol 98–100:833–840
Bahaj AS, Ellwood DC, Watson JHP (1991) Extraction of heavy metals using microorganisms and high gradientmagnetic separation. IEEE Trans Magnet 27:5371–5374
Bahaj AS, Croudace IW, James PAB (1994) Treatment of heavymetal contaminants using magnetotactic bacteria. IEEE Trans Magnet 30:4707–4709
Bahaj AS, Croudace IW, James PAB, Moeschler FD, Warwick PE (1998a) Continuous radionuclide recovery from wastwater using magnetotactic bacteria. J Magn Magn Mater 184:241–244
Bahaj AS, James PAB, Moeschler FD (1998b) Low magnetic-field separation system for metal-loaded magnetotactic bacteria. J Magn Magn Mater 177–181:1453–1454
Bazylinski DA, Frankel RB (2004) Magnetosome formation in prokaryotes. Nat Rev Microbiol 2(3):217–230
Bazylinski DA, Schubbe S (2007) Controlled biomineralization by and applications of magnetotactic bacteria. Adv Appl Microbiol 62:22–52
Bazylinski DA, Garratt-Reed AJ, Abedi A, Frankel RB (1993) Copper association with iron sulfide magnetosomes in a magnetotactic bacterium. Arch Microbiol 160:35–42
Bazylinski DA, Frankel RB, Heywood BR, Mann S, King JW, Donaghay PL, Hanson AK (1995) Controlled biomineralization of magnetite (Fe3O4) and greigite (Fe3S4) in a magnetotactic bacterium. Appl Environ Microbiol 61(9):3232–3239
Bazylinski DA, Dean AJ, Williams TJ, Long LK, Middleton SL, Dubbels BL (2004) Chemolithoautotrophy in the marine, magnetotactic bacterial strains MV-1 and MV-2. Arch Microbiol 182(5):373–387
Byrne ME, Ball DA, Guerquin-Kern JL, Rouiller I, Wu TD, Downing KH, Vali H, Komeili A (2010) Desulfovibrio magneticus RS-1 contains an iron- and phosphorus-rich organelle distinct from its bulletshaped magnetosomes. Proc Natil Acad Sci USA 107(27):12263–12268
Cai F, Li J, Sun J, Ji Y (2011) Biosynthesis of gold nanoparticles by biosorption using Magnetospirillum gryphiswaldense MSR-1. Chem Eng J 175:70–75
Chen C, Ma Q, Jiang W, Song T (2011) Phototaxis in the magnetotactic bacterium Magnetospirillum magneticum strain AMB-1 is independent of magnetic fields. Appl Microbiol Biotechnol 90:269–275
Cox BL, Popa R, Bazylinski DA, Lanoil B, Douglas S, Belz A, Engler DL, Nealson KH (2002) Organization and elemental analysis of P-, S-, and Fe-rich inclusions in a population of freshwater magnetococci. Geomicrobiol J 19(4):387–406
Das N (2010) Recovery of precious metals through biosorption—a review. Hydrometallurgy 103:180–189
Fischer H, Mastrogiacomo G, Löffler JF, Warthmann RJ, Weidler PG, Gehring AU (2008) Ferromagnetic resonance and magnetic characteristics of intact magnetosome chains in Magnetospirillum gryphiswaldense. Earth Planet Sci Lett 270(3–4):200–208
Flies CB, Jonkers HM, De Beer D, Bosselmann K, Böttcher ME, Schüler D (2005) Diversity and vertical distribution of magnetotactic bacteria along chemical gradients in freshwater microcosms. FEMS Microbiol Ecol 52(2):185–195
Frankel RB (2003) Biological permanent magnets. Hyperfine Interact 151–152:145–153
Frankel RB, Bazylinski DA (1994) Magnetotaxis and magnetic particles in bacteria. Hyperfine Interact 90:135–142
Frankel RB, Bazylinski DA, Johnson MS, Taylor BL (1997) Magneto-aerotaxis in marine coccoid bacteria. Biophys J 73:994–1000
Freitas F, Keim CN, Kachar B, Farina M, Lins U (2003) Envelope ultrastructure of uncultured naturally occurring magnetotactic cocci. FEMS Microbiol Lett 219(1):33–38
Heyen U, Schüler D (2003) Growth and magnetosome formation by microaerophilic Magnetospirillum strains in an oxygen-controlled fermentor. Appl Microbiol Biotechnol 61:536–544
Keim CN, Farina M (2005) Gold and silver trapping by uncultured magnetotactic cocci. Geomicrobiol J 22(1–2):55–63
Keim CN, Lins U, Farina M (2001) Elemental analysis of uncultured magnetotactic bacteria exposed to heavy metals. Canadian J Microbiol 47:1132–1136
Keim CN, Solórzano G, Farina M, Lins U (2005) Intracellular inclusions of uncultured magnetotactic bacteria. Int Microbiol 8(2):111–117
Keim CN, Lins U, Farina M (2009) Manganese in biogenic magnetite crystals from magnetotactic bacteria. FEMS Microbiol Lett 292:250–253
Kopp RE, Kirschvink JL (2008) The identification and biogeochemical interpretation of fossil magnetotactic bacteria. Earth-Science Rev 86:42–61
Kundu S, Kale AA, Banpurkar AG, Kulkarni GR, Ogale SB (2009) On the change in bacterial size and magnetosome features for Magnetospirillum magnetotacticum (MS-1) under high concentrations of zinc and nickel. Biomater 30:4211–4218
Lefevre CT, Song T, Yonnet JP, Wu L (2009) Characterization of bacterial magnetotactic behaviors by using a magnetospectrophotometry assay. Appl Environ Microbiol 75(12):3835–3841
Li W, Yu L, Zhou P, Zhu M (2007) A Magnetospirillum strain WM-1 from a freshwater sediment with intracellular magnetosomes. World J Microbiol Biotechnol 23:1489–1492
Li J, Ge X, Zhang X, Chen G, Pan Y (2010) Recover vigorous cells of Magnetospirillum magneticum AMB-1 by capillary magnetic separation. Chin J Oceanol Limnol 28(4):826–831
Lin W, Pan Y (2009) Uncultivated magnetotactic cocci from Yuandadu Park in Beijing, China. Appl Environ Microbiol 75(12):4046–4052
Linford N, Linford P, Platzman E (2005) Dating environmental change using magnetic bacteria in archaeological soils from the upper Thames Valley, UK. J Archaeol Sci 32(7):1037–1043
Lins U, Farina M (1999) Phosphorus-rich granules in uncultured magnetotactic bacteria. FEMS Microbiol Lett 172(1):23–28
Lins U, Farina M, Kachar B (2003) Membrane vesicles in magnetotactic bacteria. Microbiol Res 158:317–320
Liu W, Wu H, Liu X, Liu X (2008) Influence of different Fe sources and concentrations on formation of magnetosomes in Acidithiobacillus ferrooxidans. Trans Nonferrous Met Soc China 18:1379–1385
Liu Y, Li GR, Guo FF, Jiang W, Li Y, Li LJ (2010) Large-scale production of magnetosomes by chemostat culture of Magnetospirillum gryphiswaldense at high cell density. Microbial Cell Factories 9
Mann S, Sparks NHC, Board RG (1990) Magnetotactic bacteria:microbiology, biomineralization, palaeomagnetism and biotechnology. Adv Microb Physiol 31:125–181
Matsunaga T, Okamura Y, Fukuda Y, Wahyudi AT, Murase Y, Takeyama H (2005) Complete genome sequence of the facultative anaerobic magnetotactic bacterium Magnetospirillum sp. strain AMB-1. DNA Res 12(3):157–166
Nogueira FS, Lins De Barros HGP (1995) Study of the motion of magnetotactic bacteria. Eur Biophys J 24(1):13–21
Peng X, Jia R, Li R, Dai S, Liu T (2000) Paleo-environmental study on the growth of magnetotactic bacteria and precipitation of magnetosomes in Chinese loess-paleosol sequences. Chin Sci Bull 45(SUPPL):21–25
Pósfai M, Buseck PR, Bazylinski DA, Frankel RB (1998) Iron sulfides from magnetotactic bacteria; structure, composition, and phase transitions. Am Miner 83:1469–1481
Sakaguchi T, Arakaki A, Matsunaga T (2002) Desulfovibrio magneticus sp. nov., a novel sulfate-reducing bacterium that produces intracellular single-domain-sized magnetite particles. Int J Syst Evolutionary Microbiol 52(1):215–221
Schüler D, Frankel RB (1999) Bacterial magnetosomes: microbiology, biomineralization and biotechnological applications. Appl Microbiol Biotechnol 52(4):464–473
Selenska-Pobell S, Merroun M (2010) Accumulation of heavy metals by micro-organisms: biomineralization and nanocluster formation. König H, Claus H, Varma A, Springer NY: 483-500
Sleytr UB, Beveridge TJ (1999) Bacterial S-layers. Trends Microbiol 7(6):253–260
Smith MJ, Sheehan PE, Perry LL, O'Connor K, Csonka LN, Applegate BM, Whitman LJ (2006) Quantifying the magnetic advantage in magnetotaxis. Biophys J 91:1098–1107
Song H, Li X, Sun J, Yin X, Wang Y, Wu Z (2007) Biosorption equilibrium and kinetics of Au(III) and Cu(II) on magnetotactic bacteria. Chin J Chem Eng 15(6):847–854
Song H, Li X, Sun J, Xu S, Hua X (2008) Application of a magntotactic bacterium, Stenotrophomonas sp. to the removal of Au(III) from contaminated wastewater with a magnetic separator. Chemosphere 72:616–621
Staniland S, Williams W, Telling N, Van Der L, Harrison A, Ward B (2008) Controlled cobalt doping of magnetosomes in vivo. Nat Nano 3(3):158–162
Sun J, Zhao F, Tang T, Jiang W, Tian J, Li Y, Li J (2008) High-yield growth and magnetosome formation by Magnetospirillum gryphiswaldense MSR-1 in an oxygen-controlled fermentor supplied solely with air. Appl Microbiol Biotechnol 79:389–397
Tanaka M, Nakata Y, Mori T, Okamura Y, Miyasaka H, Takeyama H, Matsunaga T (2008) Development of a cell surface display system in a magnetotactic bacterium, “Magnetospirillum magneticum” AMB-1. Appl Environ Microbiol 74(11):3342–3348
Tanaka M, Arakaki A, Staniland SS, Matsunaga T (2010) Simultaneously discrete biomineralization of magnetite and tellurium nanocrystals in magnetotactic bacteria. Appl Environ Microbiol 76(16):5526–5532
Van Kampen NG (1995) The turning of magnetotactic bacteria. J Stat Phys 80(1–2):23–33
Wang J, Chen C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Wang Y, Gao H, Sun J, Li J, Su Y, Ji Y, Gong C (2011) Selective reinforced competitive biosorption of Ag(I) and Cu(II) on Magnetospirillum gryphiswaldense. Desalination 270:258–263
Xiao H, Yang P, Peng H, Zhang Y, Deng S, Zhang X (2010) Nitrogen removal from livestock and poultry breeding wastewaters using a novel sequencing batch biofilm reactor. Water Sci Technol 62:2599–2606
Xiao H, Peng H, Deng S, Yang X, Zhang Y, Li Y (2012) Preparation of activated carbon from edible fungi residue by microwave assisted K2CO3 activation—application in reactive black 5 adsorption from aqueous solution. Bioresour Technol 111:127–133
Xie J, Chen K, Chen X (2009) Production, modification and bio-applications of magnetic nanoparticles gestated by magnetotactic bacteria. Nano Res 23(2):261–278
Zhang X, Deng S, Jiang W, Zhang Y, Peng H, Li L, Yang G, Li Y (2010) Energy evaluation of the sustainability of two industrial systems based on wastes exchanges. Resour Conserv Recyl 55:182–195
Zhu K, Pan H, Li J, Kui Y, Zhang S, Zhang W, Zhou K, Yue H, Pan Y, Xiao T, Wu L (2010) Isolation and characterization of a marine magnetotactic spirillum axenic culture QH-2 from an intertidal zone of the China Sea. Res Microbiol 161:276–283
Acknowledgments
This research work was funded by the Science and Technology Department of Sichuan Province (2010JY0040) and the Education Department of Sichuan Province (12ZA124).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, W., Zhang, Y., Ding, X. et al. Magnetotactic bacteria: promising biosorbents for heavy metals. Appl Microbiol Biotechnol 95, 1097–1104 (2012). https://doi.org/10.1007/s00253-012-4245-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4245-3