Abstract
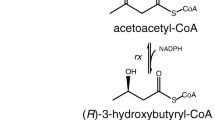
(R)-3-hydroxybutyrate [(R)-3HB] is a useful precursor in the synthesis of value-added chiral compounds such as antibiotics and vitamins. Typically, (R)-3HB has been microbially produced from sugars via modified (R)-3HB-polymer-synthesizing pathways in which acetyl CoA is converted into (R)-3-hydroxybutyryl-coenzyme A [(R)-3HB-CoA] by β-ketothiolase (PhaA) and acetoacetyl CoA reductase (PhaB). (R)-3HB-CoA is hydrolyzed into (R)-3HB by modifying enzymes or undergoes degradation of the polymerized product. In the present study, we constructed a new (R)-3HB-generating pathway from glucose by using propionyl CoA transferase (PCT). This pathway was designed to excrete (R)-3HB by means of a PCT-catalyzed reaction coupled with regeneration of acetyl CoA, the starting substance for synthesizing (R)-3HB-CoA. Considering the equilibrium reaction of PCT, the PCT-catalyzed (R)-3HB production would be expected to be facilitated by the addition of acetate since it acts as an acceptor of CoA. As expected, the engineered Escherichia coli harboring the phaAB and pct genes produced 1.0 g L−1 (R)-3HB from glucose, and with the addition of acetate into the medium, the concentration was increased up to 5.2 g L−1, with a productivity of 0.22 g L−1 h−1. The effectiveness of the extracellularly added acetate was evaluated by monitoring the conversion of 13C carbonyl carbon-labeled acetate into (R)-3HB using gas chromatography/mass spectrometry. The enantiopurity of (R)-3HB was determined to be 99.2% using chiral liquid chromatography. These results demonstrate that the PCT pathway achieved a rapid co-conversion of glucose and acetate into (R)-3HB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microbial cell factories afford a powerful tool for converting renewable biomass into value-added chemicals under mild physicochemical conditions. In particular, the fine synthesis of stereoisomers is an advantage over chemical processes. The microbial production of (R)-3-hydroxybutyrate [(R)-3HB] is a typical example. This chiral compound is known as a monomeric constituent of bacterial polyester P(3HB), and is used as a precursor for synthetic antibiotics (Iimori and Shibasaki 1986), vitamins, pheromones, and also as a building block of biobased polyesters such as P(3HB) (Ren et al. 2010; Tokiwa and Ugwu 2007).
(R)-3HB has been typically obtained by two main approaches, the degradation of P(3HB) and CoA removal from the intermediate compound (R)-3-hydroxybutyryl-coenzyme A [(R)-3HB-CoA] that is generated in the P(3HB) biosynthetic pathway. P(3HB) is synthesized from acetyl CoA via three successive reactions (Fig. 1). β-Ketothiolase (PhaA) catalyzes the condensation of two acetyl CoA molecules into acetoacetyl CoA, which is subsequently converted into (R)-3HB-CoA by NADPH-dependent acetoacetyl CoA reductase (PhaB). Finally, polyhydroxyalkanoate synthase (PhaC) polymerizes (R)-3HB-CoA into a polyester. Coexpression of intracellular P(3HB) depolymerase (PhaZ) together with P(3HB) biosynthetic enzymes (PhaABC) enables the excretion of monomeric (R)-3HB into the medium. The PhaZ pathway has reportedly achieved up to 7.3 g L−1 (R)-3HB production in a shake flask culture (Shiraki et al. 2006). This method has an advantage over (R)-3HB synthesis by the acidic hydrolysis of P(3HB) that generates acid derivatives (de Roo et al. 2002; Shiraki et al. 2006). However, the PhaZ pathway requires a two-step fermentation, accumulation of the P(3HB) polymer and also its degradation. Therefore, the culture time required is relatively long (100 h) and this has significantly added to the cost of production.
To accelerate the production rate, alternative (R)-3HB-producing pathways from (R)-3HB-CoA have been investigated. Gao et al. reported that the coexpression of phosphotransbutyrylase and butyrate kinase together with PhaA and PhaB in Escherichia coli resulted in 2.0 g L−1 (R)-3HB production in a shake flask (Gao et al. 2002). Similarly, (R)-3HB-CoA can be directly hydrolyzed into (R)-3HB by thioesterase (TesB; TesB pathway in Fig. 1), an approach that yielded up to 4.0 g L−1 (R)-3HB production in a shake flask (Liu et al. 2007; Tseng et al. 2009). These processes produced (R)-3HB within a shorter culture time period (24–48 h). However, the (R)-3HB concentrations currently obtained are still lower than those of the PhaZ pathway.
The aim of this study is therefore to design a new (R)-3HB-synthesizing system with higher productivity. To meet this goal, we attempted to generate (R)-3HB from (R)-3HB-CoA using a CoA transferase (referred to as propionyl CoA transferase, PCT). PCT can transfer a CoA moiety to other short-chain organic acids. It should be noted that this reaction allows the release of (R)-3HB from (R)-3HB-CoA and simultaneous coupling with the generation of acetyl CoA from acetate (PCT pathway in Fig. 1). The regeneration of acetyl CoA should facilitate the (R)-3HB synthesis, because acetyl CoA is a precursor of (R)-3HB-CoA, as described above. In this paper, we performed efficient (R)-3HB synthesis from glucose using the PCT pathway, and obtained evidence for the regeneration acetyl CoA from acetate. PCT from Clostridium propionicum was used for the production of (R)-3HB because the enzyme is reported to functionally catalyze the CoA transfer reaction between (R)-3HB and acetate (Jossek et al. 1998). The PCT pathway achieved a higher (R)-3HB concentration and hourly productivity by co-utilizing glucose and acetate than either of these obtained by the TesB pathway.
Materials and methods
Plasmid, strain, and culture condition
pTV118NpctC1AB(STQK) (Taguchi et al. 2008) was digested with XbaI/PstI, blunted with T4 DNA polymerase and self-ligated to eliminate the phaC1 gene, and an EcoRI/SmaI fragment of the pct gene from C. propionicum (accession no. AJ276553), which was synthesized by Operon Biotechnologies (Japan), was inserted into the EcoRI/SmaI sites of the plasmid. The resulting plasmid containing the pct gene from C. propionicum together with the phaA and phaB genes from Ralstonia eutropha was designated pTVpctAB (Fig. 2). pTVpctAB was digested with EcoRI/SmaI, blunted with T4 DNA polymerase and self-ligated to eliminate the pct gene. The resultant plasmid was designated pTVAB.
E. coli BW25113 was purchased from National BioResource Project, Japan. The cells harboring pTVpctAB and pTVAB were aerobically cultured in a test tube for 24 h at 30 °C using 1.5 mL LB medium containing 10 g L−1 glucose, various concentrations of acetate, and 100 μg L−1 ampicillin. Acetate was neutralized to pH 7.0 by sodium hydroxide and passed through a 0.2-μm cellulose acetate filter for sterilization.
Analytical methods
The cell-free supernatant of the culture medium was prepared by centrifugation and passage through a 0.2-μm cellulose acetate filter. The 3HB, acetate, and glucose in the medium were analyzed using HPLC (Jasco, Japan) equipped with an Aminex HPX-87H column (Bio-Rad) and an RI detector. Samples were eluted in the isocratic mode using 0.014 N H2SO4 as the mobile phase. The enantiopurity of 3HB was determined using HPLC equipped with a Sumichiral OA-6100 column (Sumika Chemical Analysis Service, Japan). Samples were eluted in isocratic mode using 1 mM CuSO4 as the mobile phase with detection at λ = 254 nm. (R)- and (S)-3HB were purchased from Kanto Chemical (Japan) and used as the standards.
The conversion of acetate to 3HB was monitored using 13C-labeled acetic acid having 13C-enriched (99 %) carbonyl carbon (purchased from Taiyo Nissan, Japan). The cell-free culture medium was dried in vacuo, and 3HB was derivatized into ethyl-3HB by heating with ethanol and hydrochloric acid in chloroform as described previously (Arai et al. 2002). The ester which was obtained was analyzed using a gas chromatograph/mass spectrometer (GCMS-QP2010Plus, Shimadzu, Japan) to determine the 13C enrichment in 3HB.
Results
PCT-catalyzed 3HB production
The E. coli cells harboring pTVAB and pTVpctAB were grown on glucose (Table 1). The cells harboring pTVpctAB produced 1.0 g L−1 3HB, while no 3HB was detected in the absence of PCT, indicating that PCT essentially contributed to the 3HB production. The 3HB synthesis increased glucose consumption, indicating the link between the flux in the glycolysis pathway and 3HB production. Additionally, the production of 3HB was associated with a decrease in acetate concentration in the medium (2.5 to 1.8 g L−1). This result indicates that the designed PCT pathway functioned as expected (Fig. 1, PCT pathway).
If acetate acts as an acceptor for the CoA-transferring reaction, the presence of a high concentration of acetate would increase the production of 3HB. To examine this possibility, acetate was added to the medium used for the production of 3HB. In the absence of PCT, 3HB was not produced (Table 1). In contrast, in the presence of PCT, the 3HB production was increased depending on the concentration of acetate, reaching 5.2 g L−1 when 6.6 g L−1 acetate was supplemented (Table 1). Since the cultivation time was 24 h, the productivity of this condition was calculated to be 0.22 g L−1 h−1. With a higher concentration of acetate, 3HB production was somewhat decreased. The glucose consumption was potently increased under the 3HB-producing conditions. The pH of the medium was shifted to alkaline, probably because of the conversion of two acetic acid molecules into one 3HB molecule catalyzed by PCT (Fig. 1).
Flux analysis from acetate toward 3HB
The results in Table 1 suggest the functionality of the PCT-assisted metabolic route converting acetate into 3HB (Fig. 1). In order to clearly demonstrate this, the flux from the extracellular acetate toward 3HB was estimated by adding 13C carbonyl carbon-labeled acetic acid into the medium, and then the 13C enrichment in the synthesized 3HB was monitored. With the addition of 3.3 g L−1 13C carbonyl carbon-labeled acetate, 4.7 ± 0.4 g L−1 3HB and 1.6 ± 0.1 g L−1 acetate were detected in the medium at a level that was similar to the result obtained with non-labeled acetate (Table 1). The glucose consumption at this condition was 9.3 ± 0.1 g L−1. Then 3HB was derivatized into an ethyl ester and analyzed using GC/MS. Ethyl 3HB generates an ion fragment in which m/z = 117. The ratio of 13C in 3HB was determined by measuring the relative intensity of the fragments m/z = 117, 118, and 119. As shown in Fig. 3, 3HB molecules synthesized under the presence of 13C carbonyl carbon-labeled acetate contained larger amounts of the heavier fragments (m/z = 118 and 119), clearly indicating that the 3HB molecule was partly derived from exogenous acetic acid. This result indicates that the extracellular acetate was converted into 3HB by the PCT-assisted acetyl CoA-regenerating pathway. According to the relative peak areas of the fragments with m/z = 118 and 119, the ratio of the acetate-derived carbon in 3HB was estimated to be 30 ± 4 %.
GC/MS analysis of 3HB produced by recombinant E. coli harboring pTVpctAB under the presence of 3.3 g L−1 acetic acid (a) and 13C carbonyl carbon-labeled acetic acid (b). Three ion fragments from ethyl 3HB (m/z = 117, 118, or 119) were monitored. Asterisk indicates carbon atoms, which could be 13C enriched, when 13C-labeled acetic acid was converted into 3HB via the proposed PCT pathway (Fig. 1)
Enantiomer analysis of 3HB
Because of the enantioselectivity of PhaB, the 3HB produced by E. coli was expected to be composed of the R-form. To confirm this, the supernatant of the culture medium of E. coli harboring pTVpctAB under the presence of 3.3 g L−1 acetate was subjected to chiral HPLC. As a result, 3HB was eluted as a major peak (Fig. 4c) at the same retention time of (R)-3HB standard (Fig. 4a), and the combination with the standard was eluted as a single peak (Fig. 4d), indicating that the 3HB produced by engineered E. coli was composed of almost entirely the R-form. In addition, there was a minor peak at 9 min, which was close to the retention time of the (S)-3HB standard (Fig. 4b). This small peak was eluted together with the externally added (S)-3HB standard (Fig. 4e), indicating that the sample contained a small amount of (S)-3HB. According to the peak areas, the enantiopurity of (R)-3HB was estimated to be as high as 99.2 mol%.
Discussion
In this study, a new pathway for (R)-3HB production by co-conversion of glucose and acetate was constructed using CoA transferase (Fig. 1). The productivity of (R)-3HB was enhanced by addition of acetate in the medium, achieving the maximum concentration of 5.2 gL−1 (R)-3HB with a productivity of 0.22 g L−1 h−1. These values were higher than those obtained by the TesB pathway (4.0 g L−1 and 0.17 g L−1 h−1) (Liu et al. 2007; Shiraki et al. 2006). Although this concentration was lower than that obtained by the PhaZ pathway (7.2 g L−1 and 0.07 g L−1 h−1), the PCT pathway had an advantage over the PhaZ pathway in terms of productivity. In addition, the (R)-3HB productions using UV mutagenized native P(3HB)-producing strains have been reported. For examples, the mutagenized R. eutropha produced 3.4 g L−1 (R)-3HB with a productivity of 0.07 g L−1 h−1 from gluconate (Vollbrecht and Schlegel 1979), and the mutagenized Azohydromonas lata produced 6.5 g L−1 (R)-3HB with a productivity of 0.07 g L−1 h−1 from glucose (Ugwu et al. 2011). These results also indicate that the PCT pathway in E. coli is particularly effective to achieve the higher production rate of (R)-3HB. In terms of (R)-3HB production via P(3HB) hydrolysis using recombinant E. coli, Lee et al. reported the production of 9.6 g L−1 3HB (Lee and Lee 2003). This is of great interest, but also requires careful confirmation, because there is a counterclaim that the high (R)-3HB production was not reproducible, based on both theoretical grounds and experimental evidence (Uchino et al. 2008).
The key mechanism in enhancing (R)-3HB production is the regeneration of acetyl CoA by the CoA transfer reaction (Fig. 1). The 13C carbonyl carbon-labeling analysis demonstrated that extracellular acetate has been converted into (R)-3HB, strongly supporting the functionality of the proposed pathway. Under this condition, 52 mM glucose was consumed and 45 mM (R)-3HB was produced. Because the ratio of the glucose-derived (R)-3HB was approximately 70 %, the carbon yield from glucose to (R)-3HB was estimated to be 61 %. To further increase the concentration of (R)-3HB in the medium, a strategy of feeding glucose and acetate using a jar fermentor would be effective, based on the previous studies (Liu et al. 2007; Shiraki et al. 2006).
Highly stereoselective synthesis of stereoisomers such as 3HB is a great advantage biological processes have over chemoprocesses. It was reported that nearly 100 % of (R)-3HB was obtained using PhaZ pathway. A high degree of enantiopurity was achieved by the very strict stereoselectivity toward (R)-3HB-CoA exhibited by PhaC (Rehm 2003). Using the PCT pathway, however, a small amount of S-isomer was detected (Fig. 4). The (S)-3HB may be synthesized by β-oxidation enzymes such as enoyl CoA hydratase/3-hydroxyacyl-CoA oxidase (FadB) and its homologue FadJ (formerly YfcX). To further increase the enantiopurity, the use of a dual knockout strain of these genes would be effective (Tappel et al. 2012). On the other hand, because of the non-stereoselective substrate specificity of PCT (Tung and Wood 1975), the PCT pathway is applicable to (S)-3HB production using (S)-3HB-CoA dehydrogenase reported by Tseng et al. (2009).
References
Arai Y, Nakashita H, Suzuki Y, Kobayashi Y, Shimizu T, Yasuda M, Doi Y, Yamaguchi I (2002) Synthesis of a novel class of polyhydroxyalkanoates in Arabidopsis peroxisomes, and their use in monitoring short-chain-length intermediates of β-oxidation. Plant Cell Physiol 43:555–562
de Roo G, Kellerhals MB, Ren Q, Witholt B, Kessler B (2002) Production of chiral R-3-hydroxyalkanoic acids and R-3-hydroxyalkanoic acid methylesters via hydrolytic degradation of polyhydroxyalkanoate synthesized by pseudomonads. Biotechnol Bioeng 77:717–722
Gao HJ, Wu Q, Chen GQ (2002) Enhanced production of D-(−)-3-hydroxybutyric acid by recombinant Escherichia coli. FEMS Microbiol Lett 213:59–65
Iimori T, Shibasaki M (1986) Simple, stereocontrolled synthesis of 1β-methylcarbapenem antibiotics from 3(R)-hydroxybutyric acid. Tetrahedron Lett 27:2149–2152
Jossek R, Reichelt R, Steinbüchel A (1998) In vitro biosynthesis of poly(3-hydroxybutyric acid) by using purified poly(hydroxyalkanoic acid) synthase of Chromatium vinosum. Appl Microbiol Biotechnol 49:258–266
Lee SY, Lee Y (2003) Metabolic engineering of Escherichia coli for production of enantiomerically pure (R)-(−)-hydroxycarboxylic acids. Appl Environ Microbiol 69:3421–3426
Liu Q, Ouyang SP, Chung A, Wu Q, Chen GQ (2007) Microbial production of R-3-hydroxybutyric acid by recombinant E. coli harboring genes of phbA, phbB, and tesB. Appl Microbiol Biotechnol 76:811–818
Rehm BHA (2003) Polyester synthases: natural catalysts for plastics. Biochem J 376:15–33
Ren Q, Ruth K, Thony-Meyer L, Zinn M (2010) Enatiomerically pure hydroxycarboxylic acids: current approaches and future perspectives. Appl Microbiol Biotechnol 87:41–52
Shiraki M, Endo T, Saito T (2006) Fermentative production of (R)-(−)-3-hydroxybutyrate using 3-hydroxybutyrate dehydrogenase null mutant of Ralstonia eutropha and recombinant Escherichia coli. J Biosci Bioeng 102:529–534
Taguchi S, Yamada M, Matsumoto K, Tajima K, Satoh Y, Munekata M, Ohno K, Kohda K, Shimamura T, Kambe H, Obata S (2008) A microbial factory for lactate-based polyesters using a lactate-polymerizing enzyme. Proc Natl Acad Sci U S A 105:17323–17327
Tappel RC, Wang Q, Nomura CT (2012) Precise control of repeating unit composition in biodegradable poly(3-hydroxyalkanoate) polymers synthesized by Escherichia coli. J Biosci Bioeng 113:480–486
Tokiwa Y, Ugwu CU (2007) Biotechnological production of (R)-3-hydroxybutyric acid monomer. J Biotechnol 132:264–272
Tseng HC, Martin CH, Nielsen DR, Prather KL (2009) Metabolic engineering of Escherichia coli for enhanced production of (R)- and (S)-3-hydroxybutyrate. Appl Environ Microbiol 75:3137–3145
Tung KK, Wood WA (1975) Purification, new assay, and properties of coenzyme A transferase from Peptostreptococcus elsdenii. J Bacteriol 124:1462–1474
Uchino K, Saito T, Jendrossek D (2008) Poly(3-hydroxybutyrate) (PHB) depolymerase PhaZa1 is involved in mobilization of accumulated PHB in Ralstonia eutropha H16. Appl Environ Microbiol 74:1058–1063
Ugwu CU, Tokiwa Y, Ichiba T (2011) Production of (R)-3-hydroxybutyric acid by fermentation and bioconversion processes with Azohydromonas lata. Bioresour Technol 102:6766–6768
Vollbrecht D, Schlegel HG (1979) Excretion of metabolites of hydrogen bacteria III. D(−)-3-hydroxybutanoate. Eur J Appl Microbiol 7:259–266
Acknowledgements
We thank J.M. Nduko for the technical assistance of HPLC analysis. E. coli strain was provided by National BioResource Project, Japan. This work was financially supported by Showa Denko K. K. (Japan). Pacific Edit reviewed the manuscript prior to submission.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Matsumoto, K., Okei, T., Honma, I. et al. Efficient (R)-3-hydroxybutyrate production using acetyl CoA-regenerating pathway catalyzed by coenzyme A transferase. Appl Microbiol Biotechnol 97, 205–210 (2013). https://doi.org/10.1007/s00253-012-4104-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4104-2