Abstract
In Escherichia coli, the phosphoenolpyruvate–carbohydrate phosphotransferase system (PTS) is responsible for the transport and phosphorylation of sugars, such as glucose. PTS activity has a crucial role in the global signaling system that controls the preferential consumption of glucose over other carbon sources. When the cell is exposed to carbohydrate mixtures, the PTS prevents the expression of catabolic genes and activity of non-PTS sugars transport systems by carbon catabolite repression (CCR). This process defines some metabolic and physiological constraints that must be considered during the development of production strains. In this review, we summarize the importance of the PTS in controlling and influencing both PTS and non-PTS sugar transport processes as well as the mechanisms of transcriptional control involved in the expression of catabolic genes of non-PTS sugars in E. coli. We discuss three main approaches applied efficiently to avoid these constraints resulting in obtaining PTS− glc+ mutants useful for production purposes: (1) adaptive selection in chemostat culture system of PTS− mutants, resulting in the selection of strains that recovered the ability to grow in glucose, along with the simultaneous consumption of two carbon sources and reduced acetate production; (2) replacement in PTS− strains of the native GalP promoter by strong promoters or the substitution of this permease by recombinant glucose transport system; and (3) enhancement of Crp (crp+) in mgsA, pgi, and ptsG mutants, resulting in derivative strains that abolished CCR, allowing the simultaneous consumption of mixtures of sugars with low acetate production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In Escherichia coli, the presence of a double cellular membrane system necessitates the diffusion of carbohydrates used as a carbon and energy source from the extracellular environment to the periplasmic space through a series of abundant and non-specific porins located in the outer membrane. OmpC, OmpF, and LamB porins constitute the main periplasm entry system for carbohydrates, such as glucose (Gosset 2005). As the cytoplasmatic membrane is a hydrophobic barrier, active carbohydrate transport systems, such as the phosphoenolpyruvate–carbohydrate phosphotransferase system (PTS) and several proteins involved normally in the transport of galactose, such as those of the galactose permease system (GalP) and the active primary MglBAC system, are required to internalize the glucose molecules present in the periplasmic space under different nutritional conditions (Ferenci 2001; Gosset 2005, 2009). PTS is the main system involved in the transport and phosphorylation of several sugars. It has a significant impact on carbon flux and distribution in central carbon metabolism and also has a crucial role in the global signaling system that controls the preferential consumption of glucose over other carbon sources. When the cell is exposed to different carbohydrate mixtures, the PTS prevents the expression of several catabolic genes and activity of non-PTS sugars transport systems by two main processes: carbon catabolite repression (CCR) and inducer exclusion. These processes define some metabolic and physiological constraints that must be considered during the development of industrial production strains. In this review, we discuss various strategies that were developed to avoid metabolic and physiological constraints imposed by PTS. We summarize the importance of the system in controlling the transport process of both PTS and non-PTS sugars. In addition, we summarize transcriptional control mechanisms involved in the expression of catabolic genes of non-PTS sugars in E. coli. The genetic, metabolic, and physiological implications of PTS inactivation and the disruption of those regulatory mechanisms in engineered strains developed for metabolite production are also discussed.
The PTS in E. coli and its impact on growth and product formation
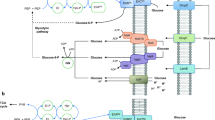
PTS is composed of soluble components: the phosphohistidine carrier protein (HPr), the Enzyme I (EI) component, and the enzyme EII (coded by the ptsHIcrr operon). When glucose is present in the growth medium, the HPr and EI components transfer a phosphoryl group from the glycolytic intermediate phosphoenolpyruvate (PEP) to the sugar-specific enzymes EIIAGlc and EIIBGlc. EIIA and the membrane bound glucose-specific enzyme IICB (EIICBGlc) comprise the glucose-specific PTS, which possess two domains, with an IIC-IIB order, in a single polypeptide chain coded by the ptsG gene. EIIC is an integral membrane protein permease that recognizes and transports the sugar molecules, which are then phosphorylated by EIIB (Fig. 1). There are 21 different identified EII complexes coded in the E. coli chromosome involved in the transport of approximately 20 different carbohydrates. Among these complexes, EIIGlc and EIIMan are involved in glucose transport (Saier et al. 1996; Tcheiu 2001; Gosset 2005). The PTS also plays a crucial role as part of a global signaling system that controls the preferential consumption of glucose over other carbon sources when bacteria are exposed to more than one carbohydrate in a culture medium. The phosphorylation state of the PTS proteins is modulated in response to the available carbon source. The modulated state can regulate other proteins, either through protein binding or by transference of phosphoryl groups. These proteins include the phosphorylated forms of the EIIAGlc (EIIAGlc∼P) and HPr (HPr∼P) proteins, which control CCR and inducer exclusion mechanisms in Enterobacteriaceae (Brückner and Titgemeyer 2002; Deutscher et al. 2006; Deutscher 2008; Bahr et al. 2011).
The PTS and major mechanisms controlling carbohydrate uptake in E. coli. PTS is composed by the cytoplasmatic components EI, HPr, and EIIAGlc and the membrane-bound EIICGlc and EIIBGlc components conforming the EIICBGlc. Phosphorylated forms of PTS components are denoted by ∼P. Bold arrows represent activation mechanisms. Bold doted arrows represent inhibition/repression mechanisms. PEP phosphoenolpyruvate, Pyr pyruvate, UCF unidentified cellular factor, EMP Embden–Meyerhof pathway, TCA tricarboxylic acid cycle, AC adenylate cyclase, LacY specific transporter for lactose, GlpF glycerol permease, GlpK glycerol kinase
Metabolically engineered E. coli strains are used for the production of a diverse number of high-value metabolites, including fermentation products, biofuels, and recombinant proteins in industrial fermentor processes. Media containing a wide range of glucose concentrations as the main carbon source are usually employed, as the sugar is relatively inexpensive and is the preferred carbon and energy source for E. coli (Gosset 2005, 2009; Keasling 2010). Glucose transport and phosphorylation by the PTS consumes one PEP molecule during the catabolism of one glucose molecule by the Embden–Meyerhof pathway (EMP). In addition to its role as a phosphate donor for the PTS, PEP is a precursor in several biosynthetic pathways and also participates directly in energy-generating reactions, such as substrate-level phosphorylation of ADP. PEP also participates indirectly to energy generation as an acetyl coenzyme-A precursor. When E. coli is grown in minimal medium containing glucose as the carbon source, the PTS consumes 50 % of the available PEP, whereas the reactions catalyzed by enzymes, such as PEP carboxylase, pyruvate kinases, UDP-N-acetylglucosamine enolpyruvyl transferase, and 3-deoxy-d-arabinoheptulosonate 7-phosphate (DAHP) synthase, consume approximately 16, 15, 16, and 3 % of remaining PEP, respectively (Flores et al. 2002; Gosset 2005). Several research groups have developed strategies to increase the availability of PEP and redirect it to biosynthetic pathways to achieve an increase in the production and yield of desired products. These strategies involve inactivation of some PTS components (EI, HPr, and EIIAGlc coded by the ptsHIcrr operon). The resulting PTS− strains are unable to use the PTS for the concomitant translocation and phosphorylation of glucose or any other PTS sugar, inducing the cell to enter an extreme nutritional stress condition that disrupts the CCR inhibition circuits (LaDucca et al. 1999; Flores et al. 1996, 2007; Gosset et al. 1996; Gosset 2005; Yao et al. 2011). PTS− strains of E. coli can restore, through evolution, their capability to grow on glucose as a carbon source by selecting alternative transporters, such as GalP or MglBAC, to import glucose and subsequently phosphorylate the sugar using the enzyme glucokinase. The characterization of some of these PTS− mutants has shown the development of genetic changes that allow not only the ability to restore their capability to grow on glucose but also the simultaneous consumption of different carbon sources. This finding is interesting because of the potential to use mixtures of carbohydrates, such as those generated by the hydrolysis of lignocellulosic materials, which could allow an avoidance of the sequential and diauxic consumption patterns determined by CCR (Flores S et al. 2002, 2005a, b; Flores N et al. 2005a, b; Martínez et al. 2008; Sigala et al. 2009; Yao et al. 2011).
Major mechanisms of E. coli autoregulatory carbohydrate uptake control
Carbon catabolite repression
Carbon catabolite repression (CCR) is considered the most important regulatory mechanism in many bacteria. Using CCR, bacteria can select a rapidly metabolizable carbon source, such as glucose, from other carbohydrates present in the growth medium, resulting in a diauxic growth behavior. CCR inhibits the synthesis of enzymes involved in the catabolism of secondary carbon sources by maintaining a low concentration of specific inducers for alternative routes of catabolism. The inhibition is accomplished via the altered activities of specific regulators or the activation of global control proteins, such as the dimeric transcription activator Crp (cyclic AMP (cAMP) receptor protein or catabolite gene-activator protein). In E. coli, CCR involves the cytoplasmatic signal metabolite of carbon and energy sufficiency cAMP, the membrane-bound protein adenylate cyclase (AC), CAP and the EIIAGlc component of the PTS (Postma et al. 1996; Saier et al. 1996; Brückner and Titgemeyer 2002; Gosset 2005; Görke and Stülke 2008; Bahr et al. 2011) (Fig. 1).
CCR regulation is modulated by the phosphorylation state of EIIAGlc. During bacterial growth in the presence of glucose or other PTS substrates, the concentration of intracellular PEP is high and EIIAGlc is preferentially dephosphorylated. The preferential process occurs, as phosphate is required to phosphorylate incoming sugars and activate them for catabolism. EIIAGlc∼P binds to the C-terminal domain of AC and stimulates activity. In membrane fusion experiments involving fusing AC to the Ser chemo-receptor Tsr, the stimulation of AC in the presence of bonded EIIAGlc∼P was only observed in the presence of an additional unidentified factor present in the cellular extracts of E. coli K12 (UCF) (Park et al. 2006; Görke and Stülke 2008). As cAMP levels increase with AC activity, cAMP binds to CRP, and the cAMP–CRP complex activates the promoters of many catabolic genes and operons, such as lactose, melibiose, glycerol, and maltose (Fig. 1). It is thought that such promoters are weak and required to be activated to allow the binding of the RNA polymerase prior to the formation of the gene transcription complex.
Interestingly, the cAMP–CRP complex, in addition to mediating CCR of sugar catabolic genes, plays an important role in other cellular processes. There are 131 described “simple” and “complex” transcriptional units activated, repressed, or with otherwise dual-regulated by cAMP–CRP in the RegulonDB and EcoCyc databases (http://regulondb.ccg.unam.mx/ and http://biocyc.org/ecocyc/index.shtml, respectively). Tricarboxylic acid cycle (TCA), respiratory genes (Gamma-Castro et al. 2010; Keseler et al. 2011), osmoregulation (Landis et al. 1999; Balsalobre et al. 2006), stringent response (Johansson et al. 2000), biofilm formation (Jackson et al. 2002), virulence (Balsalobre et al. 2006), nitrogen assimilation (Mao et al. 2007), iron uptake (Zhang et al. 2005), competence (Sinha et al. 2009), multidrug resistance (Nishino et al. 2008), and also of small non-coding regulatory RNAs, such as the Spot42 and CyaR RNAs (De Lay and Gottesman 2009) are among the regulated transcriptional units.
Other CCR interactions have been described for other PTS components. Dephosphorylated EI interacts with the chemotaxis protein CheA, stimulating its autophosphorylation (Adler and Epstein 1974; Deutscher et al. 2006). HPr binds to glycogen phosphorylase enzyme (GlgP), stimulating glycogen phosphorylation, but P∼HPr prevents this interaction. Dephosphorylated EIIAGlc interacts with FrsA protein, a proposed fermentation/respiration switch, suggesting that EIIAGlc regulates the flux between the respiration and fermentation pathways by sensing the available sugar species via a phosphorylation state-dependent interaction with FrsA (Koo et al. 2004; Deutscher et al. 2006).
Inducer exclusion
Inducer exclusion is considered as one of the major CCR mechanisms in Enterobacteriaceae. The consumption of rapidly metabolizable PTS sugars increases the level of dephosphorylated EIIAGlc, which has been demonstrated to bind under this condition directly with the proteins of several non-PTS sugar transport systems, such as the lactose permease LacY, the melibiose permease MelB, the MalK component of the maltose ABC transport system MalFGK2, and the cytoplasmatic glycerol kinase GlpK, subsequently limiting enzyme activity during glycerol utilization (Plumbridge 2002; Amster-Choder 2005; Deutscher et al. 2006; Deutscher 2008) (Fig. 1).
The efficient binding of dephosphorylated EIIAGlc to its target proteins occurs only when the specific target transport system substrate is present. The E. coli lac operon has been the classic model used to explain the inducer exclusion mechanism (Deutscher et al. 2006; Deutscher 2008; Görke and Stülke 2008). In a diauxic growth period where there is the simultaneous presence of glucose and lactose, as glucose is preferentially consumed, the PEP and EIIAGlc∼P concentrations are low, and abundant dephosphorylated EIIAGlc binds and inactivates LacY, a lactose/proton symporter, responsible for the uptake of lactose and other galactosides. When the glucose has been consumed and lactose remains as the unique available sugar, EIIAGlc is phosphorylated and does not interact with LacY, allowing lactose transport. It has been determined that a conformational change in LacY following periplasmic lactose binding to its specific union residue allows EIIAGlc interaction (Deutscher et al. 2006; Deutscher 2008). The conditional binding of EIIAGlc to LacY in the presence of lactose avoids waste of this PTS component (present at a relatively constant level in E. coli and the Salmonella enterica serovar Typhimurium) if the non-PTS substrate is not present in the environment (Deutscher et al. 2006).
During diauxic growth in the presence of glucose and other non-PTS sugars, the preferential uptake of glucose during the first part of the growth is proposed to be ensured by the inducer exclusion mechanism, whereas the induction of catabolic genes during the adaptive (lag) phase is proposed to be mediated by an increase in the formation of the cAMP–CRP complex, together with the entry/formation of the respective inducer (Fig. 1) (Deutcher et al. 2006; Deutscher 2008; Görke and Stülke 2008).
Carbon catabolic control of transcription factors
The bgl sensory system
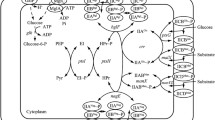
In E. coli, several PTS substrate catabolism operons are controlled by transcription regulators that contain duplicated PTS-regulatory domains. Among them, the bgl sensory system represents a novel family of sensory systems composed of a membrane-bound sugar-sensors/permease and a transcriptional antiterminator that regulates the expression of genes involved in sugar utilization (Amster-Choder 2005; Görke and Stülke 2008).
The bgl operon contains three genes (bglG, bglF, and bglB) required for the uptake and utilization of aromatic β-glucosides. It is proposed that the operon serves as a protective system against toxic β-glucosides found in nature (Schnetz 1987, 1995; Amster-Choder 2005). BglG and BglF comprise a sensory system that controls the termination/antitermination of transcription. BglG is a transcriptional antiterminator, and its activity is modulated by BglF, a PTS permease that catalyzes the concomitant transport and phosphorylation of β-glucosides. As described above, the phosphate flux in the PTS begins with the transfer of a phosphate from PEP to the common cytoplasmatic components EI and HPr. Next, a phosphate is transferred to the different sugar-specific permeases (EII complexes). BglF, similar to many other PTS permeases, is a membrane protein comprised of two hydrophilic domains, EIIABgl and EIIBBgl, that contain a conserved phosphorylation site (H547) and are connected to a hydrophobic IICBgl domain that functions as the sugar translocation channel and the sugar-binding site (Amster-Choder 2005). In addition to its activity as permease, BglF controls the activity of BglG by reversible phosphorylation depending on substrate availability. BglG prevents premature termination of transcription within the bgl transcript by binding to the emerging bgl mRNA at sites that partially overlap putative terminators and stabilizing an alternative conformation of RNA chain. BglG exists either as a phosphorylated monomer or a dephosphorylated dimer that binds to the RNA target. The activity of BglF and BglG depends on phosphorylation state and the presence/absence of β-glucosides. In the absence of substrate (non-stimulated state), BglF phosphorylates BglG, inhibiting its activity as transcriptional antiterminator and leading to the premature termination of the bgl operon at one of two rho-independent sites flanking the first gene. In the presence of β-glucosides (stimulated state), BglF dephosphorylates BglG, allowing its dimerization and binding to the emergent bgl transcript at sites that partially overlap putative terminators and stabilizes an alternative conformation of the mRNA, leading to subsequent expression as β-glucosides are transported and phosphorylated by BglF (Amster-Choder 2005) (Fig. 2). Additionally, it was demonstrated that in the presence of β-glucosides, but in the absence of other PTS sugars, EIIBgl dephosphorylates BglG, which is necessary but not sufficient for its activation. Under this condition, BglG is phosphorylated by HPr at different sites within BglG allowing its dimerization to the active form and alleviate transcription termination within the bgl operon, suggesting that this route of phosphorylation is used to monitor the availability of the various PTS sugars in order to hierarchically tune the expression of the bgl operon (Görke and Rak 1999). In E. coli, there are other reported classes of transcription activators that include regulator proteins, which, depending on the location of their binding site, function both as transcription activators (binding upstream from the promoter) and/or repressors (the binding site overlaps or is located downstream of the promoter). These regulators are proposed to interact with the major σ70-containing RNA polymerase holoenzyme and include the DeoR family of transcription regulators. The family contains repressors such as GlpR, the glycerol-3-P repressor that acts as the repressor of the glycerol-3-phosphate regulon (Lin 1976; Larson et al. 1987; Weissenborn et al. 1992), and SrlR (GutR). GutR is a DNA-binding transcription factor that represses an operon (gut) involved in the transport and utilization of glucitol (Yamada and Sier 1988; Deutcher et al. 2006).
Regulatory functions of PTS. Several PTS components have regulatory functions depending on their phosphorylation/dephosphorylation state and presence/absence of PTS sugars by interacting directly with several target proteins such as Mlc and AC. Soluble Mlc represses mlc and ptsG expression by direct union to the respective promoter, whereas membrane sequestering by EIIBGlc in absence of glucose avoids its repressing action. AC–EIIAGlc∼P–UCF complex in absence of glucose lead the formation of the cAMP–Crp complex repressing transcription of certain genes such as cya and promoting transcription of genes such as crp, mlc, and ptsG. Indirect regulatory effect is driven by the phosphate transference from HPr∼P to EIIBgl (BglF) in absence of β-glucosides, phosphorylating it (BglF∼P) with the subsequent phosphorylation of cytoplasmatic BlgG. Monomeric BglG∼P is unable to prevent premature transcription termination of bgl operon. In presence of β-glucosides, BglF∼P transports and phosphorylates this sugar and cytoplasmatic BglG forms an active dimer which binds to the antiterminator region of bgl operon allowing its transcription. Bold arrows represent activation mechanisms. Bold doted arrows represent inhibition/repression mechanisms. Bold
 denotes transcription of specified genes. PEP phosphoenolpyruvate, Pyr pyruvate, UCF unidentified cellular factor, EMP Embden–Meyerhof pathway, TCA trycarboxilic acid cycle
denotes transcription of specified genes. PEP phosphoenolpyruvate, Pyr pyruvate, UCF unidentified cellular factor, EMP Embden–Meyerhof pathway, TCA trycarboxilic acid cycle
The DgsA (Mlc) regulation system
DgsA, better known as Mlc (“makes large colonies” phenotype) (Hosono et al. 1995), is a transcriptional dual regulator that controls the expression of its own gene (mlc), the PTS genes ptsHI, ptsG, manXYZ, which encodes the mannose PTS and transports several hexoses including glucose and mannose (Kimata et al. 1998; Plumbridge 2001, 2002) along with sgrS (involved in response of glucose-P stress) (Vanderpool and Gottesman 2004; Vanderpool 2007), and malt, the positive regulator of the maltose regulon (Decker et al. 1998). As all the Mlc targets are connected with glucose and Mlc, it is considered as glucose-specific repressor (Plumbridge 2002). Transcription from these genes/operons is repressed by the binding of Mlc to a site overlapping the downstream promoters (except for the ptsHI operon, where Mlc binds close to P0) and is stimulated by cAMP–CRP, which binds upstream from Mlc (Plumbridge 2002) (Fig. 2).
Repression by Mlc and activation by cAMP–CRP are directly related to the phosphorylation state of the PTS components. As it has been previously explained, PTS activity in the presence of a substrate leads to EIICBGlc and EIIAGlc being predominantly dephosphorylated. Under these conditions, EIICBGlc sequesters Mlc away from its DNA-binding sites, leading to the expression of Mlc-repressed genes. The EIIBGlc domain of the membrane-attached PTS component EIICBGlc contains a Cys421 phosphorylation responsible of the interaction with Mlc and sequestering it from the DNA to form a membrane-attached complex EIIBGlc–Mlc. The membrane-bound part of phosphorylated form of EIICBGlc plays a crucial role for Mlc inactivation as it forms a tetrameric Mlc/EIIB complex responsible for of Mlc inactivation by membrane sequestration, in which Mlc loss its DNA binding ability in vivo due to the conformational obstruction by EIIB molecules (Nam et al. 2001, 2008).
Gene repression by Mlc is averted by growth on glucose and other rapidly metabolizable PTS substrates, such as N-acetyglucosamine, mannitol, and, to a lesser extent, fructose and mannose. Non-PTS sugars, such as lactose, melibiose, and sucrose, have no effect; however, maltose (a non-PTS sugar) degradation reduces Mlc repression, probably as a consequence of the intracellular yield of glucose and glucose-1-P (Plumbridge 2002; Deutscher et al. 2006; Deutscher 2008).
The mlc gene is weakly autoregulated, but it is also repressed and activated by CRP. The gene is expressed from two promoters with a Mlc site overlapping the downstream site. A CRP site serves to regulate both promoters by repressing the upstream site and inactivating the downstream site. The downstream site is recognized by RNA polymerases containing either σ70 or σ32 (heat shock) factors. The expression of mlc increases during heat shock but decays when glucose is present, implying that mlc is also subject of post-transcriptional regulation (Decker et al. 1998; Plumbridge 2002).
Energetics of glucose transport in E. coli
The energetic cost of glucose transport in E. coli by PTS and non-PTS systems differs significantly. With the PTS system, periplasmic glucose is concomitantly transported and phosphorylated by the transference of a phosphate group transferred from PEP and carried by PTS components, yielding intracellular glucose-6-P to enter the EMP. Periplasmic glucose transport through non-PTS proteins, such as GalP and MglBAC systems, has been demonstrated under very low glucose concentrations (lower than 1 mM) (Death and Ferenci 1994; Ferenci 2001). Such transport yields intracellular glucose that requires activation (phosphorylation) to enter the glycolytic pathway. Glucose phosphorylation is performed by cytoplasmatic glucokinase (Glk) enzyme employing ATP. The energy equivalent of one mole of PEP required to transport and phosphorylate one mole of incoming glucose by PTS is one mole of ATP. As a consequence, the energy cost of glucose internalization by the EIIGlc or EIIMan transporters is 1 mol of PEP. In contrast, glucose transported by GalP involves 1 H+ and transport by the MglBAC system requires 1 mol of ATP plus 1 mol of ATP for intracellular glucose phosphorylation of the last two transporters. Therefore, PTS is the most energetically efficient system for glucose import in E. coli (Gosset 2005).
Laboratory and industrial fermentor cultures with E. coli employ media containing glucose that is mainly transported and phosphorylated by PTS. In these strains, 50 % of the intermediate PEP resulting from the catabolism of one molecule of transported glucose is used as a phosphate donor for the phosphorylation of translocated glucose by EIICBGlc (Postma et al. 1996; LaDucca et al. 1999), and the remaining PEP is channeled to both the TCA and the shikimic acid pathway (SAP). This characteristic of the PTS system limits the production and yield of industrial metabolites that have PEP as a precursor. In the following sections, we discuss several strategies developed to overcome these potential limiting effects in production strains.
Increasing PEP availability for the production of aromatic metabolites by inactivation of the PTS system
The inactivation of the ptsHIcrr operon results in the elimination of PEP consumption by PTS. The resulting mutants lack the general PTS proteins involved in the phosphotransfer relay to any of the sugar-specific PTS complexes and exhibit a very limited capability to transport glucose supported mainly by GalP, MglBAC, and phosphorylation by Glk from ATP, respectively (PTS− glc− phenotype). Stoichiometric analyses of the metabolic network involved in the synthesis of aromatic metabolites indicate that the maximum theoretical molar yield from glucose could increase twofold in an E. coli PTS− mutant strain if 100 % of the PEP could be redirected to the SAP (LaDucca et al. 1999; Flores et al. 1996; 2007; Gosset et al. 1996; Gosset 2005).
For the purposes of aromatic metabolite production, PTS− mutants derived from wild-type E. coli W3110 strain are used in both batch and fed-batch fermentation systems employing glucose as a carbon source. In these systems, the cloning and expression of alternative glucose permeases systems, such as the glf (Glf glucose facilitator system) from Zymomonas mobilis or the E. coli galP (GalP permease) under control of a strong promoter (Ptrc), have respectively been employed to efficiently restore glucose transport in these strains. At the same time, phosphorylation is performed by native or recombinant Glk. Resultant strains, such as SP1.1pts derivatives, were used as the genetic background for further genetic modifications and shown to be very efficient in the production of shikimic acid (SA) compared with the parent PTS strains (71 g/L, 0.27 mol SA/mol glc vs. 52 g/L 0.18 mol SA/mol glc, respectively) (Knop et al. 2001; Chandran et al. 2003; Krämer et al. 2003). In addition, a W3110 PTS− derivative, designated as VH32, was successfully employed for the production of recombinant protein (TrpLE− pro-insulin and green fluorescent protein) (De Anda et al. 2006), anthranilate (14 g/L in rich medium) (Balderas-Hernández et al. 2009), and l-DOPA (1.51 g/L in rich medium) (Muñoz et al. 2011).
E. coli PTS− mutants are unable to use this system for the import of glucose or any other PTS sugar, resulting in extreme nutritional stress conditions for the cell. In the absence of regulatory components, such as EIIAGlc, HPr partially abolishes the CCR and inducer exclusion inhibition mechanisms, producing global modification of the regulatory and metabolic capabilities that allow the cell to utilize glucose for growth.
In minimal media culture, a PTS− mutant strain designated as PB11 presented a low specific growth rate (μ) (=0.1 h−1) compared with a wild-type JM101strain (μ = 0.7 h−1). An adaptive selection process involving the PB11 strain in a chemostat culture fed glucose at progressively higher rates resulted in the selection of mutants that developed the capacity to grow quickly in glucose. One of these mutant strains, designated PB12, used GalP as the main glucose transporter system and accomplished phosphorylation with Glk. The strain presented a μ = 0.4 h−1 (Flores et al. 1996, 2007). Characterization of this strain indicated a capacity to direct more carbon flux into the aromatic pathway than the wild-type JM101 parental strain as a result of higher PEP availability (Flores et al. 1996; Baez et al. 2001; Flores et al. 2002). Further genetic modifications in this strain allowed production of l-phenylalanine (30–60 % of theoretical maximum) (Báez-Víveros et al. 2004; 2007), l-tyrosine (3 g/L with a yield of 66 mg/g glc) (Chávez-Béjar et al. 2008), and SA (0.29 mol SA/mol glc) (Escalante et al. 2010).
Transcriptomic analysis by RT-qPCR determined that, in fermentor cultures grown in minimal medium with glucose as the carbon source (2 g/L), both the PTS− strains (PB11 and PB12) significantly increased the expression level of several genes coding for sugar transporters, including the gal regulon responsible for glucose transport. Both PTS− mutant strains upregulated the transcription of mglB (coding for the MglB component of MglBAC system) (13- and 9-fold, respectively). Other upregulated gal regulon genes included galP (12.4- and 13.1-fold, respectively) and the regulon repressor galS (4.9- and 3.2-fold, respectively). It was proposed based on transcriptomic evidence from the minimal media/glucose carbon-source growth condition results that both PTS− strains induce the synthesis of galactose as an auto-inducer of the gal regulon, inactivating the repressors GalS and GalR and resulting in the induction of all the gal regulon genes, along with the subsequent internalization of glucose through GalP and MglBAC transporters (Flores et al. 2005a, b). However, the slow growth on glucose of the PB11 strain indicated that phosphorylation of Glk was possibly a limiting step in the transport/activation of incoming glucose. The PB12 strain is a PB11-derived mutant that recovered the capability to exhibit rapid growth in glucose. This behavior could be explained probably as a consequence of the increased transcription of the glk gene (twofold increase) in PB12 compared with the JM101 and PB11 strains. The increase allowed the efficient transport of glucose by GalP and phosphorylation by Glk.
In addition, the differences in growth capacities between these slow/fast glucose-growing strains may also be related to the availability of regulatory molecules, such as cAMP. As previously described, cAMP–CRP plays an important role in transcription activation of many catabolic genes in PTS strains. These genes include most of the gal regulon; however, interestingly, a slight increase in the transcription level of cya and a relatively similar level of transcription of crp genes compared with the wild-type strain in both PTS− mutants suggested that both strains synthesize sufficient cAMP–CRP to activate genes, such as galETK and galS.
Although the main goal in the inactivation of ptsHIcrr operon was to increase the availability of PEP for aromatic metabolite production, further characterization of the PTS− strains PB11 and PB12 indicated that they modified their metabolic capabilities to contend with the resultant extreme nutritional stress condition and developed a permanent scavenging capability as a result of CCR and inducer exclusion inhibition abolition. This adaptation allows the simultaneous utilization of glucose and acetate as carbon sources (Flores S et al. 2002; Flores et al. 2004; 2005a, b). Based on transcriptomic evidence, it was proposed that the upregulation of the gal operon, aceBAK, poxB, acs, glk, pgi, TCA cycle genes, certain respiratory genes, and the gluconeogenic genes maeB, sfcA, pckA, pps, fbaB, fbp, and pfkB has significant importance in the context of the biosynthesis of aromatic compounds, as the protein product of the pps gene converts Pyr into PEP and, thus, contributes to the increase in the metabolic availability of this aromatic precursor (Flores et al. 2004; Sigala et al. 2009).
The gluconeogenic capacity coexisting with glycolytic activity, as detected in the PB12 strain, is a physiological trait that allowed this strain to simultaneously utilize glucose and other carbon sources. This trait could potentially increase the yield of aromatic compounds in this strain, as demonstrated for the simultaneous utilization of mixtures of glucose–arabinose, glucose–gluconate, and glucose–glycerol. This capacity increased the μ in the PB12 strain (transformed with plasmids pRW300aroG fbr and pCLtktA, coding a feedback-resistant DAHP synthase AroG and transketolase I enzymes, respectively), as it metabolizes more moles of carbon source per unit time enhancing the productivity and yield of aromatic compounds, especially in the glucose–glycerol mixture, compared with sole glucose or glycerol cultures. Interestingly, no acetate was detected in the glycerol and the glucose–glycerol batch fermentations (Martínez et al. 2008).
Reduction of acetate production in PTS− strains
In E. coli wild-type strains growing under aerobic growth conditions, as a result of PTS high-glucose uptake rate and its catabolism through the EMP, the acetyl coenzyme-A synthesis rate surpasses the TCA cycle consuming capacity, and the excess is then converted into acetate by the AcK–Pta pathway. Acetate accumulation under aerobic conditions is a common problem in E. coli cultures, as it causes a reduction in both growth rate and recombinant protein productivity (Gosset 2005; de Anda et al. 2006). Several approaches have been developed to obtain productive strains with low acetate accumulation.
A reduction in acetate overflow in E. coli JM103 was achieved by increasing the availability of the PTS regulatory protein Mlc by cloning the mlc gene in a multicopy plasmid. The addition of the plasmid resulted in a 50 % reduction in acetate accumulation. It was proposed that a higher increase in cytoplasmatic Mlc level also increases the repression of ptsG and ptsHI genes. This, in turn, results in a reduction of glucose uptake and flux to the EMP, resulting in both low growth and acetate concentration (Hosono et al. 1995). The inactivation of the PTS and the replacement of the native promoter of GalP by the strong trc promoter in the W3110 wild-type strain resulted in the VH32PTS−GalP+ derivative strain. Batch and fed-batch cultures of this engineered strain maintained similar production and growth rate capabilities compared with the wild-type strain with a reduction in acetate accumulation (de Anda et al. 2006). This strain was also grown in batch fermentor cultures with high glucose concentration (100 g/L) resulting in significant reduction of acetate secretion (less than 80 %) with respect to the W3110 strain as well as more than an 80 % increase in recombinant green fluorescent protein production (Lara et al. 2008).
Abolition of CCR as an alternative to induce simultaneous consumption of sugar mixtures in E. coli cultures
The starting raw materials, such as starch, sucrose, or cellulosic biomass derived from agricultural residues, are potential low-cost feedstock for the production of different types of bioproducts, such as ethanol and l-lactic acid. Hydrolysis of lignocellulose yields a mixture of sugar containing mainly glucose, arabinose, and xylose. Due to CCR and metabolite exclusion inhibition mechanisms, E. coli presents a diauxic consumption of carbohydrates derived from lignocellulosic hydrolysates present in the culture media. The elimination of diauxic behavior and simultaneous consumption of sugar in these mixtures would be advantageous in a fermentative process, as it would be accompanied with a reduction in operating time and increase in productivity (Gosset 2005, 2009; Keasling 2010; Yao et al. 2011).
The inactivation of ptsG in E. coli resulted in a IT1168 PTS− derivative that abolished CCR and allowed simultaneous fermentation mixtures of glucose, arabinose, and xylose to ethanol (yielding 87–94 % of theoretical) (Nichols et al. 2001). In addition, the E. coli ptsG − mutant strain FBR19 was used to ferment a 100 g/L total equal mixture of glucose and xylose to produce lactic acid (yield of 0.77 g lactic acid/g sugar) (Dien et al. 2002). An E. coli W3110 PTS− mutant carrying the ethanologenic pLOI1594 plasmid is an adapted strain able to grow under anaerobic conditions that evolved to obtain glucose+ strain derivatives, some of which were able to restore its μ to 100 % of that observed in the PTS+ parental strain. In experiments performed using a glucose–xylose mixture, xylose was completely consumed, and sugar consumption occurred in a simultaneous manner, indicating that CCR was abolished in these strains, resulting in improved ethanol production (Balderas-Hernández et al. 2011)
The inactivation of the PTS components caused growth reduction as a consequence of diminished glucose uptake capability. While this issue may be resolved as described above, other strategies have been explored to abolish CCR and allow E. coli to consume simultaneously mixtures of sugars, such as those present in lignocellulosic hydrolysates. These efforts have been focused on modifying intracellular cAMP levels as a strategy to alter the role of the cAMP–CRP complex on the expression of several genes and operons responsible for the catabolism of secondary non-PTS sugars. The knockout inactivation of crp (Δcrp) and crp enhancement (crp +) on metabolic regulation was evaluated with ptsG, mlc, and pgi mutants of the E. coli BW25113 strain in continuous and batch cultures. In Δcrp and Δmlc mutants, the TCA cycle and glyoxylate shunt genes were repressed, resulting in acetate accumulation, while in crp + mutants, glycolysis, TCA cycle, and gluconeogenesis genes were upregulated allowing the simultaneous consumption of a mixture of glucose and xylose with low acetate production. This phenomenon was associated with increases in crp transcript levels and deficient ptsG gene expression, resulting in reduced EIICBGlc activity (Yao et al. 2011). Although it was not determined, glucose was probably transported, as in the case of the JM101 PTS− mutants PB11 and PB12, through GalP and MglBAC transport systems, and phosphorylation was facilitated by Glk (Flores et al. 1996, 2007).
Concluding remarks and perspectives
PTS represents the most efficient system for the transport and phosphorylation of sugars, such as glucose, in bacteria such as E. coli. The metabolic traits and transcriptional regulatory control systems associated with PTS represent an important disadvantage in the use of this bacteria for large-scale fermentation processes for the production of metabolites that use PEP as precursor or in the production of bioproducts, such as ethanol, due to its inability to simultaneously consume mixtures of sugars (derived from lignocellulosic hydrolysates, low-cost raw materials). This is a consequence of the CCR and inducer exclusion inhibition systems defining a diauxic behavior growth pattern that forces the sequential consumption of carbon sources.
The inactivation of specific PTS components has been the main approach employed to avoid some of these disadvantages; however, the resultant PTS− strains are exposed to severe nutritional stress conditions because of their inability to consume glucose. Functional replacement in the PTS− strains by glucose transporter systems, such as GalP, MglBAC, or the Z. mobilis Glf, and incoming glucose phosphorylation by Glk ATP-dependent processes resulted in significant strain improvements in the production efficiency of diverse fermentation products.
Among those examples described above, three main approaches have been applied efficiently to obtain PTS− gcl+ mutants that are useful and interesting strains for diverse metabolite production purposes: (1) adaptive selection in chemostat culture system of PTS− mutants, resulting in the selection of strains that naturally recovered the ability to grow in glucose at higher rates, along with the simultaneous consumption of two carbon sources, including gluconeogenic substrates and reduced acetate production; (2) replacement in PTS− strains of the native GalP promoter by strong promoters or the substitution of this permease by recombinant glucose transport system, such as the Z. mobilis Glf system and its efficient phosphorylation by recombinant Glk; and (3) enhancement of Crp (crp +) in mgsA, pgi, and ptsG mutants, resulting in derivative strains that abolished CCR and inducer exclusion mechanisms, allowing the simultaneous consumption of mixtures of sugars with low acetate production. The transcriptomic characterization of specific genes encoding enzymes involved in carbon catabolism and specific biosynthetic pathways have allowed for the analysis of the genetic and regulatory changes developed by some of these strains. This finding has increased our understanding of the physiological implications of the interruption of CCR circuits associated with PTS; however, the further characterization of these strains using global transcriptome, proteome, metabolome, and fluxome analyses will extend overall our understanding of these systems in cellular processes and help to identify the changes responsible for the improved co-assimilation and product formation of sugar mixtures.
References
Adler J, Epstein W (1974) Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci USA 71:2895–2899
Amster-Choder O (2005) The bgl sensory system: a transmembrane signaling pathway controlling transcriptional antitermination. Curr Opin Microbiol 8:127–134
Báez JL, Bolívar F, Gosset G (2001) Determination of 3-deoxy-d-arabino-heptulosonate 7-phosphate productivity and yield from glucose in Escherichia coli devoid of the glucose phosphotransferase transport system. Biotechnol Bioeng 73:530–535
Báez-Viveros JL, Osuna J, Hernández-Chávez G, Soberón X, Bolívar F, Gosset G (2004) Metabolic engineering and protein directed evolution increase the yield of l-phenylalanine synthesized from glucose in Escherichia coli. Biotechnol Bioeng 87:516–524
Báez-Viveros JL, Flores N, Suarez K, Castillo-España P, Bolivar F, Gosset G (2007) Metabolic transcription analysis of engineered Escherichia coli strains that overproduce l-phenylalanine. Microb Cell Fact 6:30
Bahr T, Lüttmann D, März RB, Görke B (2011) Insight into bacterial phosphotransferase system-mediated signaling by interspecies transplantation of a transcriptional regulator. J Bacteriol 193:2013–2026
Balderas-Hernandez VE, Sabido-Ramos A, Silva P, Cabrera-Valladares N, Hernandez-Chavez G, Baez-Viveros JL, Martinez A, Bolivar F, Gosset G (2009) Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb Cell Fact 8:19
Balderas-Hernandez VE, Hernandez-Montalvo V, Bolivar F, Gosset G, Martinez A (2011) Adaptive evolution of Escherichia coli inactivated in the phosphotransferase system operon improves co-utilization of xylose and glucose under anaerobic conditions. Appl Biochem Biotechnol 163:485–496
Balsalobre C, Johansson J, Uhlin BE (2006) Cyclic AMP-dependent osmoregulation of crp gene expression in Escherichia coli. J Bacteriol 188:5935–5944
Brückner R, Titgemeyer F (2002) Carbon catabolite repression in bacteria: choice of the carbon source and autoregulatory limitation of sugar utilization. FEMS Microbiol Lett 209:141–148
Chandran SS, Yi J, Draths KM, von Daeniken R, Weber W, Frost JW (2003) Phosphoenolpyruvate availability and the biosynthesis of shikimic acid. Biotechnol Prog 19:808–814
Chávez-Béjar MI, Lara AR, López H, Hernández-Chávez G, Martinez A, Ramírez OT, Bolívar F, Gosset G (2008) Metabolic engineering of Escherichia coli for l-tyrosine production by expression of genes coding for the chorismate mutase domain of the native chorismate mutaseprephenate dehydratase and a cyclohexadienyl dehydrogenase from Zymomonas mobilis. Appl Environ Microbiol 74:3284–3290
De Anda R, Lara AR, Hernández V, Hernández-Montalvo V, Gosset G, Bolívar F, Ramírez OT (2006) Replacement of the glucose phosphotransferase transport system by galactose permease reduces acetate accumulation and improves process performance of Escherichia coli for recombinant protein production without impairment of growth rate. Metab Eng 8:281–290
Death A, Ferenci T (1994) Between feast and famine: endogenous inducer synthesis in the adaptation of Escherichia coli to growth with limiting carbohydrates. J Bacteriol 176:5101–5107
Decker K, Plumbridge J, Boos W (1998) Negative transcriptional regulation of a positive regulator: the expression of malT, encoding the transcriptional activator of the maltose regulon of Escherichia coli, is negatively controlled by Mlc. Mol Microbiol 27:381–390
De Lay N, Gottesman S (2009) The Crp-activated small noncoding regulatory RNA CyaR (RyeE) links nutritional status to group behavior. J Bacteriol 191:461–476
Deutscher J (2008) The mechanisms of carbon catabolite repression in bacteria. Curr Opin Microbiol 11:87–93
Deutscher J, Francke C, Postma PW (2006) How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in Bacteria. Microbiol Mol Biol Rev 70:939–1031
Dien BS, Nichols NN, Bothast RJ (2002) Fermentation of sugar mixtures using Escherichia coli catabolite repression mutants engineered for production of l-lactic acid. J Ind Microbiol Biotechnol 29:221–227
Escalante A, Calderón R, Valdivia A, De Anda R, Hernández G, Ramírez OT, Gosset G, Bolívar F (2010) Metabolic engineering for the production of shikimic acid in an evolved Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system. Microb Cell Fact 9:1–12
Ferenci T (2001) Hungry bacteria: definition and properties of a nutritional state. Environ Microbiol 3:605–611
Flores N, Xiao J, Berry A, Bolívar F, Valle F (1996) Pathway engineering for the production of aromatic compounds in Escherichia coli. Nat Biotechnol 14:620–623
Flores S, Gosset G, Flores N, de Graaf AA, Bolívar F (2002) Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase systemby 13C labeling and NMR spectroscopy. Metab Eng 4:124–137
Flores N, de Anda R, Flores S, Escalante A, Hernández G, Martínez A, Ramírez OT, Gosset G, Bolívar F (2004) Role of pyruvate oxidase in Escherichia coli strains lacking the phosphoenolpyruvate:carbohydrate phosphotransferase system. J Mol Microbiol Biotechnol 8:209–221
Flores N, Flores S, Escalante A, de Anda R, Leal L, Malpica R, Georgelis D, Gosset G, Bolívar F (2005a) Adaptation for fast growth on glucose by differential expression of central carbon metabolism and gal regulon in an Escherichia coli strain lacking the phophoenolpyruvate:carbohydrate phosphotransferase system. Metab Eng 7:70–87
Flores S, Flores N, de Anda R, González A, Escalante A, Sigala JC, Gosset G, Bolívar F (2005b) Nutrient scavenging stress response in an Escherichia coli strain lacking the phophpenolpyruvate:carbohydrate phosphotransferase system, as explored by gene expression profile analysis. J Mol Microbiol Biotechnol 10:51–63
Flores N, Leal L, Sigala JC, de Anda R, Escalante A, Martínez A, Ramírez OT, Gosset G, Bolivar F (2007) Growth recovery on glucose under aerobic conditions of an Escherichia coli strain carrying a phosphoenolpyruvate:carbohydrate phosphotransferase system deletion by inactivating arcA and overexpressing the genes coding for glucokinase and galactose permease. J Mol Microbiol Biotechnol 13:105–116
Gama-Castro S, Salgado H, Peralta-Gil M, Santos-Zavaleta A, Muñiz-Rascado L, Solano-Lira H, Jiménez-Jacinto V, Weiss V, García-Sotelo JS, López-Fuentes A, Porrón-Sotelo L, Alquicira-Hernández S, Medina-Rivera A, Martínez-Flores I, Alquicira-Hernández K, Martínez-Adame R, Bonavides-Martínez C, Miranda-Ríos J, Huerta A, Mendoza-Vargas A, Collado-Torres L, Taboada B, Vega-Alvarado L, Olvera M, Olvera L, Grande R, Morett E, Collado-Vides J (2010) RegulonDB (version 7.0): transcriptional regulation of Escherichia coli K-12 integrated within genetic sensory response units (Gensor Units). Nucl Acids Res. doi:10.1093/nar/gkq1110
Görke B, Rak B (1999) Catabolite control of Escherichia coli regulatory protein BglG activity by antagonistically acting phosphorylations. EMBO J 18:3370–3379
Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624
Gosset G (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate:sugar phosphotransferase system. Microb Cell Fact 4:14
Gosset G (2009) Production of aromatic compounds in bacteria. Curr Opin Biotechnol 20:651–658
Gosset G, Yong-Xiao J, Berry A (1996) A direct comparison of approaches for increasing carbon flow to aromatic biosynthesis in Escherichia coli. J Ind Microbiol 17:47–52
Hosono K, Kakuda H, Ichihara S (1995) Decreasing accumulation of acetate in a rich medium by Escherichia coli on introduction of genes on a multicopy plasmid. Biosci Biotechnol Biochem 59:256–261
Jackson DW, Simecka JW, Romeo T (2002) Catabolite repression of Escherichia coli biofilm formation. J Bacteriol 184:3406–3410
Johansson J, Balsalobre C, Wang SY, Urbonaviciene J, Jin DJ, Sondén B, Uhlin BE (2000) Nucleoid proteins stimulate stringently controlled bacterial promoters: a link between the cAMP–CRP and the (p)ppGpp regulons in Escherichia coli. Cell 102:475–485
Keasling JD (2010) Manufacturing molecules through metabolic engineering. Science 330:1355–1358
Keseler IM, Collado-Vides J, Santos-Zavaleta A, Peralta-Gil M, Gama-Castro S, Muñiz-Rascado L, Bonavides-Martínez C, Paley S, Krummenacker M, Altman T, Kaipa P, Spaulding A, Pacheco J, Latendresse M, Fulcher C, Sarker M, Shearer AG, Mackie A, Paulsen I, Gunsalus RP, Karp PD (2011) EcoCyc: a comprehensive database of Escherichia coli biology. Nucl Acids Res 39:D583–D590
Kimata K, Inada T, Tagami H, Aiba H (1998) A global repressor (Mlc) is involved in glucose induction of the ptsG gene encoding major glucose transporter in Escherichia coli. Mol Microbiol 29:1509–1519
Knop DR, Draths KM, Chandran SS, Barker JL, von Daeniken R, Weber W, Frost JW (2001) Hydroaromatic equilibration during biosynthesis of shikimic acid. J Am Chem Soc 123:10173–10182
Koo BM, Yoon MJ, Lee CR, Nam TW, Choe YJ, Jaffe H, Peterkofsky A, Seok Y-J (2004) A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J Biol Chem 279:31613–31621
Krämer M, Bongaerts J, Bovenberg R, Kremer S, Müller U, Orf S, Wubbolts M, Raeven L (2003) Metabolic engineering for microbial production of shikimic acid. Metab Eng 5:277–283
LaDucca RJ, Berry A, Chotani G, Dodge TC, Gosset G, Valle F, Liao JC, Yong-Xiao J, Power SD (1999) Metabolic pathway engineering of aromatic compounds. In: Demain AL, David JE (eds) Manual of industrial microbiology and biotechnology, 2nd edn. ASM Press, Washington, D. C., pp 605–615
Landis L, Xu J, Johnson RC (1999) The cAMP receptor protein CRP can function as an osmoregulator of transcription in Escherichia coli. Genes Dev 13:3081–3091
Lara AR, Caspeta L, Gosset G, Bolívar F, Ramírez OT (2008) Utility of an Escherichia coli strain engineered in the substrate uptake system for improved culture performance at high glucose and cell concentrations: an alternative to fed-batch cultures. Biotechnol Bioeng 99:893–901
Larson TJ, Ye SZ, Weissenborn DL, Hoffmann HJ, Schweizer H (1987) Purification and characterization of the repressor for the sn-glycerol 3-phosphate regulon of Escherichia coli K12. J Biol Chem 262:15869–15874
Lin EC (1976) Glycerol dissimilation and its regulation in bacteria. Annu Rev Microbiol 30:535–578
Mao XJ, Huo YX, Buck M, Kolb A, Wang YP (2007) Interplay between CRP–cAMP and PII–Ntr systems forms novel regulatory network between carbon metabolism and nitrogen assimilation in Escherichia coli. Nucl Acids Res 35:1432–1440
Martínez K, de Anda R, Hernández G, Escalante A, Gosset G, Ramírez OT, Bolívar F (2008) Coutilization of glucose and glycerol enhances the production of aromatic compounds in an Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system. Microb Cell Fact 7:1
Muñoz AJ, Hernández-Chavez G, de Anda R, Martínez A, Bolívar F, Gosset G (2011) Metabolic engineering of Escherichia coli for improving l-3,4-dihydroxyphenylalanine (l-DOPA) synthesis from glucose. J Ind Microbiol Biotechnol 38:1845–1852
Nam TW, Cho SH, Shin D, Kim JH, Jeong JY, Lee JH, Roe JH, Peterkofsky A, Kang SO, Ryu S, Seok YJ (2001) The Escherichia coli glucose transporter enzyme IICB(Glc) recruits the global repressor Mlc. EMBO J 20:491–498
Nam TW, Jung HI, An YJ, Park YH, Lee SH, Seok YJ, Cha SS (2008) Analyses of Mlc–IIBGlc interaction and a plausible molecular mechanism of Mlc inactivation by membrane sequestration. Proc Natl Acad Sci USA 105:3751–3756
Nichols NN, Dien BS, Bothast RJ (2001) Use of catabolite repression mutants for fermentation of sugar mixtures to ethanol. Appl Microbiol Biotechnol 56:120–125
Nishino K, Senda Y, Yamaguchi A (2008) CRP regulator modulates multidrug resistance of Escherichia coli by repressing the mdtEF multidrug efflux genes. J Antibiot 61:120–127
Park Y‑H, Lee BR, Seok Y‑J, Peterkofsky A (2006) In vitro reconstitution of catabolite repression in Escherichia coli. J Biol Chem 281:6448–6454
Plumbridge J (2001) DNA binding sites for the Mlc and NagC proteins: regulation of nagE, encoding the N-acetylglucosamine-specific transporter in Escherichia coli. Nucl Acids Res 29:506–514
Plumbridge J (2002) Regulation of gene expression in the PTS in Escherichia coli: the role and interactions of Mlc. Curr Opin Microbiol 5:187–193
Postma PW, Lengeler JW, Jacobson GR (1996) Phosphoenolpyruvate: Carbohydrate phosphotransferase systems. In: Neidhart FC (ed) Escherichia coli and Salmonella. Cellular and molecular biology. ASM Press, Washington, D. C., pp 1149–1174
Saier MH Jr, Ramseier TM, Reizer J (1996) Regulation of carbon utilization. In: Neidhart FC (ed) Escherichia coli and Salmonella. Cellular and molecular biology. ASM Press, Washington, D. C., pp 1325–1343
Schnetz K (1995) Silencing of Escherichia coli bgl promoter by flanking sequence elements. EMBO J 14:2545–2550
Schnetz K, Toloczyki C, Rak B (1987) Beta-glucoside (bgl) operon of Escherichia coli K-12: nucleotide sequence, genetic organization, and possible evolutionary relationship to regulatory components of two Bacillus subtilis genes. J Bacteriol 169:2579–2590
Sigala JC, Flores S, FloresN AC, de Anda R, Gosset G, Bolivar F (2009) Acetate metabolism in Escherichia coli strains lacking phosphoenolpyruvate: carbohydrate phosphotransferase system; evidence of carbon recycling strategies and futile cycles. J Mol Microbiol Biotechnol 16:224–235
Sinha S, Cameron AD, Redfield RJ (2009) Sxy induces a CRP-S regulon in Escherichia coli. J Bacteriol 191:5180–5195
Tchieu JH, Norris V, Edwards JS, Saier MH Jr (2001) The complete phosphotranferase system in Escherichia coli. J Mol Microbiol Biotechnol 3:329–346
Vanderpool CK (2007) Physiological consequences of small RNA-mediated regulation of glucose-phosphate stress. Curr Opin Microbiol 10:146–1451
Vanderpool CK, Gottesman S (2004) Involvement of a novel transcriptional activator and small RNA in post-transcriptional regulation of the glucose phosphoenolpyruvate phosphotransferase system. Mol Microbiol 54:1076–1089
Weissenborn DL, Wittekindt N, Larson TJ (1992) Structure and regulation of the glpFK operon encoding glycerol diffusion facilitator and glycerol kinase of Escherichia coli K-12. J Biol Chem 267:6122–6131
Yamada M, Saier MH (1988) Positive and negative regulators for glucitol (gut) operon expression in Escherichia coli. J Mol Biol 203:569–583
Yao R, Hirose Y, Sarkar D, Nakahigashi K, Ye Q, Shimizu K (2011) Catabolic regulation analysis of Escherichia coli and its crp, mlc, mgsA, pgi and ptsG mutants. Microb Cell Fact 10:67
Zhang Z, Gosset G, Barabote R, Gonzalez CS, Cuevas WA, Saier MH (2005) Functional interactions between the carbon and iron utilization regulators, Crp and Fur, in Escherichia coli. J Bacteriol 187:980–990
Acknowledgments
This work was supported by CONACYT Sector Salud 126793, Ciencia Básica 105782 grants, DGAPA-PAPIIT UNAM IN224709, IN202611, and IN206812 grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Escalante, A., Salinas Cervantes, A., Gosset, G. et al. Current knowledge of the Escherichia coli phosphoenolpyruvate–carbohydrate phosphotransferase system: peculiarities of regulation and impact on growth and product formation. Appl Microbiol Biotechnol 94, 1483–1494 (2012). https://doi.org/10.1007/s00253-012-4101-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4101-5