Abstract
Chemical surfactants may impact microbial cell surface properties, i.e., cell surface hydrophobicity (CSH) and cell surface charge, and may thus affect the uptake of components from non-aqueous phase liquids (NAPLs). This work explored the impact of Triton X-100, Igepal CA 630, and Tween 80 (at twice the critical micelle concentration, CMC) on the cell surface characteristics of Burkholderia cultures, Burkholderia cepacia (ES1, aliphatic degrader) and Burkholderia multivorans (NG1, aromatic degrader), when grown on a six-component model NAPL. In the presence of Triton X-100, NAPL biodegradation was enhanced from 21% to 60% in B. cepacia and from 18% to 53% in B. multivorans. CSH based on water contact angle (50–52°) was in the same range for both strains while zeta potential at neutral pH was −38 and −31 mV for B. cepacia and B. multivorans, respectively. In the presence of Triton X-100, their CSH increased to greater than 75° and the zeta potential decreased. This induced a change in the mode of uptake and initiated aliphatic hydrocarbon degradation by B. multivorans and increased the rate of aliphatic hydrocarbon degradation in B. cepacia. Igepal CA 630 and Tween 80 also altered the cell surface properties. For B. cepacia grown in the presence of Triton X-100 at two and five times its CMC, CSH increased significantly in the log growth phase. Growth in the presence of the chemical surfactants also affected the abundance of chemical functional groups on the cell surface. Cell surface changes had maximum impact on NAPL degradation in the presence of emulsifying surfactants, Triton X-100 and Igepal CA630.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Petroleum wastes pose a threat to the environment since they are recalcitrant and may potentially contain carcinogenic components. Bioremediation is considered as a cost-effective technology for treatment of petroleum hydrocarbons. Although biological treatment of petroleum hydrocarbon contaminants is well established, complete removal of petroleum hydrocarbons from a complex mixture is rarely achieved. Two major constraints in the process include bioavailability limitation due to the aqueous–non-aqueous biphasic nature of the system and lack of cultures that can simultaneously degrade the various group types present in a complex petroleum mixture (Churchill et al. 1999; Verma et al. 2006). Bioavailability refers to accessibility of substrate to the microorganisms. Bioavailability can be increased by increasing the concentration of hydrocarbons in the aqueous phase by mass transfer from the non-aqueous phase liquid (NAPL) and also by favoring attachment of the microorganisms to the NAPL phase for direct uptake (Mohanty and Mukherji 2008a; Zhao et al. 2011). While the transfer of hydrocarbons to the aqueous phase would tend to enhance the rate of degradation by any microorganism, attachment of microorganisms to the hydrocarbons would enhance degradation only if the culture is inherently capable of direct interfacial uptake of hydrocarbons and when they possess the necessary enzymes.
Enhancement in bioavailability has been attempted successfully by the use of chemical surfactants (Volkering et al. 1998; Van Hamme and Ward 2001). Surfactants enhance bioavailability by enhancing the mass transfer of hydrocarbons from the non-aqueous phase to the aqueous phase. Surfactants can enhance mass transfer by various mechanisms, such as emulsification, micellar solubilization, and facilitated transport. Research has been conducted extensively to explore various aspects involved in the transfer of hydrocarbons between phases, particularly with respect to the type of hydrocarbon (Bruheim et al. 1997; Grimberg et al. 1996), dose and type of surfactant used (Van Hamme and Ward 1999), and mechanism of partitioning of hydrocarbon into surfactant micelles (Guha and Jaffe 1996). Surfactants may also have a detrimental effect on bioremediation due to their inherent toxicity to microorganisms and due to enhancement in bioavailability of toxic components. Some surfactants are inherently recalcitrant and may accumulate in the environment. Thus, choice of appropriate surfactant type and dose based on the target pollutants and the microorganisms involved is essential. Surfactant-aided biodegradation may also be influenced by the inherent ability of the hydrocarbon-degrading microorganisms to utilize a specific hydrocarbon group type (aliphatic or aromatic) and the inherent mode of uptake of hydrocarbons by the microorganisms.
Studies conducted by Al-Tahhan et al. (2000) demonstrated that addition of a low concentration of rhamnolipid biosurfactant caused enhancement in cell surface hydrophobicity (CSH) of Pseudomonas aeruginosa as determined by the bacterial adhesion to hydrocarbon (BATH) assay. Adherence of Aeromonas hydrophilia to hydrocarbons measured in the BATH assay was shown to be a dynamic parameter that changed with time and was also affected by the surfactants, saponin, Triton-X 100, and rhamnolipids used in the study (Kaczorek et al. 2010). Such changes in adherence of hydrocarbon-degrading cultures to NAPLs may enhance the biodegradation of hydrocarbons through direct interfacial uptake. However, the BATH assay does not provide a true measure of CSH of bacterial cells. Contact angle of a water drop deposited on a bacterial mat provides a better measure of CSH. In addition to CSH, adhesion of microorganisms to NAPLs may also be affected by the cell surface charge (van Loosdrecht et al. 1987; Chakraborty et al. 2010). Decrease in electronegativity of the cell surface would facilitate the attachment of bacteria to hydrocarbons since both bacterial cells and hydrocarbon droplets carry a negative charge when suspended in water at neutral pH. The negative charge on hydrocarbon droplets dispersed in water is attributed to adsorbed ions, such as hydroxyl ion (OH−) from dissociation of water, bicarbonate ion (HCO −3 ) that is present in water at pH less than 7, chloride ion (Cl−) that is present in background electrolyte (KCl), and ionic impurities in the hydrocarbon (Medrzycka 1991). Alteration of cell surface properties in the presence of biosurfactants and simultaneous enhancement in hydrocarbon degradation have been demonstrated for biosurfactant-producing microorganisms (Hua et al. 2003; Zhang and Miller 1994). However, the impact of chemical surfactants in altering these cell surface properties and their influence on NAPL biodegradation has not been studied extensively.
This work attempted to test the hypothesis that growth in the presence of chemical surfactants induces changes in CSH, cell surface charge, and cell surface functional groups and thus affects NAPL degradation. Such changes may affect the mode of uptake of hydrocarbons from NAPL phases. The study was conducted using Burkholderia cultures capable of growth on hydrocarbons as sole substrate in the absence and presence of chemical surfactants, Triton X-100, Igepal CA 630, and Tween 80. The cultures were grown on synthetic NAPL composed of multiple hydrocarbons to ensure a well-defined system. Of the Burkholderia cultures chosen, Burkholderia cepacia (ES1) was capable of utilizing aliphatic hydrocarbons as sole substrate by direct interfacial uptake (Mohanty and Mukherji 2008a, b), while Burkholderia multivorans (NG1) was capable of utilizing the aromatic hydrocarbon, naphthalene (NAP) as sole substrate. Naphthalene uptake by B. multivorans possibly occurred after mass transfer into the aqueous phase since this culture did not demonstrate tendency to adhere to the NAPL phase (Chakraborty et al. 2010) and did not produce biosurfactants. Observed changes in cell surface properties were correlated with NAPL biodegradation observed in the presence of chemical surfactants. These results have important implications on surfactant-aided oil bioremediation.
Materials and methods
Source of chemicals and microorganisms
NAP (>99%), 1-methylnaphthalene (1-MN) (>98%), n-hexadecane (nC16) (>98%), n-octadecane (nC18) (>99%), n-nonadecane (nC19) (98%), and pyrene (PYR) (98%) were used for preparation of model non-aqueous phase liquid. Three non-ionic chemical surfactants, Triton X-100, Igepal CA 630, and Tween 80 of >99% purity were used. Igepal CA 630, 5α-androstane (internal standard), 1-MN, and PYR were procured from Sigma Aldrich, Mumbai, India. Triton X-100, the other petroleum hydrocarbons, and mineral media components were procured from SD Fine Chemicals, Pvt. Ltd., Mumbai, India. Tween 80 was procured from Sisco Research Laboratories, Mumbai, India. Dichloromethane (DCM) (Extrapure grade, >99.5%) used for extraction was procured from Merck India Ltd. (Mumbai, India).
B. cepacia (ES1) and B. multivorans (NG1) were used for surfactant-aided biodegradation of model NAPL. B. cepacia (ES1) is an aliphatic hydrocarbon-degrading strain originally isolated from Arabian Sea sediment using diesel as sole substrate (submitted to microbial culture collection, IMTECH, Chandigarh, India, MTCC 5332). B. multivorans (NG1) is a naphthalene-degrading strain, enriched and isolated from an oil-contaminated composite motor garage sample in Powai, Mumbai (submitted to IMTECH, Chandigarh, India, MTCC 11076). Based on 16S rRNA gene sequencing, the NG1 strain showed 99.8% match with B. multivorans. Chakraborty et al. (2010) reported the cell surface properties of these strains grown on three- to four-component model NAPLs in the absence of surfactants.
Experimental design
The impact of the three chemical surfactants (at twice the critical micelle concentration, CMC) on degradation of the NAPL and the cell surface properties (including hydrophobicity and charge) of both cultures was studied. The Burkholderia cultures were unable to use these surfactants as growth substrate. Controls without any surfactant were also set up. These studies were conducted after growing the cultures in the NAPL for 60 h (late log phase). Subsequently, the microorganism–surfactant combination resulting in highest degradation (B. cepacia–Triton X-100) was selected for growth and biodegradation studies conducted over 168 h by varying the surfactant dose from 0.5 to five times the CMC. The impact of surfactant concentration and growth phase on cell surface hydrophobicity and cell surface charge, respectively, was also studied under these conditions. Studies with B. cepacia were designed to determine if growth on NAPL in the presence of surfactants caused changes in the nature and abundance of cell surface functional groups.
Preparation of model NAPL
The model NAPL was prepared by mixing the petroleum hydrocarbons belonging to different group types. The mole fraction of each solid phase pure component was maintained as less than its fugacity ratio at room temperature (Mukherji et al. 1997). The model NAPL had the following composition (mole fraction): n-C16 (0.375), n-C18 (0.09), n-C19 (0.11), NAP (0.125), 1-MN (0.25), and PYR (0.05). The solid components were added to the liquid components and heated to 100 °C for 30 min to obtain a homogenous liquid mixture. The n-C16 and NAP mole fractions were in the ratio of 3:1, representing the aliphatic/aromatic components ratio typically found in diesel.
Culture growth on NAPL and degradation studies
The growth and degradation studies were both conducted in batch flasks (500 ml) containing 50 ml mineral media and 0.1% (v/v) NAPL amended with or without surfactant. The flasks were inoculated with 0.5 ml of culture suspension having an optical density of one. For inoculum preparation, B. cepacia (ES1) and B. multivorans (NG1) were grown on diesel (1% v/v) and naphthalene (1% w/v), respectively. The flasks were incubated in a rotary shaker at 120 rpm and 30 °C. The experiments were set up in duplicate and un-inoculated controls were also included. A pair of flasks was sacrificed at each time interval and these studies were conducted up to 168 h. In the growth studies, biomass was estimated using a 10-ml aliquot. It was centrifuged at 12,000 rpm for 15 min, the supernatant was discarded, the pellet was re-suspended in an equal volume of phosphate buffer, and the absorbance was measured at 600 nm. Direct measurement of absorbance was avoided since emulsification of NAPL by the surfactant may cause artifacts in growth determination. For these cultures, centrifugation of the cells could effectively recover the biomass from NAPL interfaces. Complete removal of emulsion from the tubes was required for accurate estimation of biomass. A correlation was developed to convert absorbance at 600 nm to dry weight of cells for each culture. A separate set of controls was set up for determining the emulsification of NAPL in the presence of the surfactants (2 CMC). In these controls, direct measurement of absorbance at 500 nm was made to determine emulsification of the NAPL (Mozaffar et al. 1994). For estimating NAPL degradation at any time, the residual NAPL was extracted with 1:1 DCM by centrifugation (Van Hamme and Ward 2001) from both control (no cultures) and experimental flasks with and without surfactants. The upper aqueous layer was removed by pipetting and the lower organic layer was passed through Na2SO4 to remove any remnant water droplets. For the stated protocol involving extraction with centrifugation using an equal volume of DCM and aqueous phase, much higher extraction efficiencies were obtained in a single extraction step compared to extraction with 1:10 DCM and aqueous phase (manual mixing) and six serial extractions (unpublished results). In these studies, the extraction efficiencies were determined by adding a known mass of NAPL and extracting it using both procedures. The extracted samples were diluted by a factor of two and internal standard (IS, 5α-androstane, 40 mg/l) was added prior to analysis. The controls were used for estimating the extraction loss (0 day control) and abiotic losses. The %loss values were based on mass of NAPL initially added to the flasks.
Analysis of NAPL components was performed using a gas chromatograph equipped with a flame ionization detector (Clarus 500, Perkin Elmer, USA) and Elite-5 capillary column (Perkin Elmer;) (L 30 m; ID 0.53 mm; film thickness 1.5 μm; stationary phase 5% diphenyl and 95% dimethylsiloxane). The carrier gas used was nitrogen and the injection volume was 1 μl. The instrument was operated in splitless mode and the injector and detector temperatures were set at 200 and 280 °C, respectively. The temperature program included an initial hold time of 1 min at an initial temperature of 50 °C followed by a ramp rate of 10 °C/min until 100 °C (hold time, 1 min), ramp rate of 8 °C/min until 250 °C (hold time, 1 min), and ramp rate of 10 °C/min until 260 °C (hold time, 2 min). The total run time was 29.75 min. The retention time (min) of the compounds NAP, 1-MN, nC16, nC18, nC19, IS, and PYR were 10.4, 12.7, 16.9, 19.6, 20.9, 22.7, and 24 min, respectively. External standards containing all the components were prepared, spiked with a fixed concentration of the IS (40 mg/l), and analyzed in the GC along with the unknowns. All samples and standards were analyzed in duplicate. Component concentration in each unknown sample was determined using standard curves for each component prepared using the external standards. Residual mass of NAPL was determined by summation of mass of all the components. The %loss reported was calculated based on the duplicate measurements, and the standard errors (SE) reported in Figs. 1 and 2 and Table 1 were determined by propagation of errors.
Determination of cell surface hydrophobicity and cell surface charge
CSH was determined by measurement of water contact angle on bacterial mats prepared on filter membranes (0.45 μm cellulose acetate). The contact angle was measured using a goniometer (Digidrop, GBX Instruments, France) (Mohanty and Mukherji 2008a). For preparation of the bacterial mats, cells were harvested, washed, and resuspended in deionized water to obtain unit optical density, and 15 ml of the culture suspension was filtered to prepare the bacterial cell mat. Ten measurements were made at various locations on each bacterial mat after following standard protocols for moisture standardization and drying.
Cell surface charge was determined by measurement of the zeta potential of the cell surface as described by Chakraborty et al. (2010) with minor modifications. The cells were harvested, washed, and resuspended in 1 mM KCl. The KCl solution was prepared in filtered deionized water (0.45 μm cellulose acetate filters) to avoid the presence of particulates. Absorbance of the cell suspension was adjusted to 0.1 and it was sonicated in a bath sonicator for 90 s to obtain a homogenous suspension. Zeta potential was measured after adjusting the pH (over the range 2–8) using a zeta potential analyser (Zeta Pals, Brookhaven Instruments Corporation, New York, NY, USA) that determined electrophoretic mobility based on phase analysis light scattering. Ten zeta potential measurements were made for each set at every pH.
The contact angle and zeta potential measurements were conducted by growing cells in two batch flasks under identical conditions before harvesting the cells and processing them. The SE indicated in Figs. 3 and 4 represents the pooled SE across the two sets, each consisting of ten replicate measurements. The zeta potential of the NAPL was also determined using the method described by Chakraborty et al. (2010).
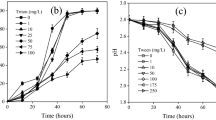
Water contact angle of a B. cepacia (ES1) and B. multivorans (NG1) and effect of surfactants; b B. cepacia (ES1) in different phases of growth in the presence of varying doses of Triton X-100. Cells were grown in two batch flasks under identical conditions and duplicate mats were prepared. Ten measurements were made on each bacterial mat. Standard error was determined for each mat and data were pooled to represent the error bar
Zeta potential of a B. cepacia (ES1) and b B. multivorans (NG1) in the presence of various surfactants; c B. cepacia (ES1) in different phases of growth in the presence of varying doses of Triton X-100. Cells were grown in two batch flasks under identical conditions before harvesting and processing them. Error bars represent the pooled standard errors across the two sets, each consisting of ten replicate measurements
Determination of cell surface functional groups
Functional groups on cell surfaces were determined by surface complexation modeling using data sets generated by potentiometric titration of the bacterial suspensions. Non-electrostatic three-site discrete modeling approach was adopted and the chemical speciation software FITEQL 4.0 was used.
Potentiometric titrations were conducted after appropriate cell preparation (Fein et al. 2005; Castro and Tufenkji 2007). The bacterial biomass was harvested, washed thrice, and resuspended in 1 mM KCl. Before commencing the titration, CO2 was removed by purging the cell suspension with N2 gas for 30 min. The KCl solution used for resuspension and NaOH solution were also purged with nitrogen. The biomass suspension (25 ml) was titrated with 0.1 N NaOH in an air-tight vessel while maintaining an inert atmosphere by purging with nitrogen. Titration was initiated by addition of acid (0.1 N HCl) to the biomass suspension until the pH was lowered to 2.5. Subsequently, aliquots of base (0.1 N NaOH) were added to vary the pH over the range 2.5–10. At every step, the volume of titrant added and the corresponding pH were recorded. After completion, the wet weight of the biomass was measured. The raw data collected from titration experiments were transformed as follows (Fein et al. 2005).
where Q = net charge (mmol/g), C a = concentration of acid added (mmol/l), C b = concentration of base added (mmol/l), [H+] = concentration of protons (mmol/l), [OH−] = concentration of hydroxyl ions (mmol/l), and m x = bacterial wet mass (g/l).
The set of values of pH and [C a − C b] (normalized per gram of biomass) was provided as input in FITEQL after accounting for volume variations. FITEQL was used for determining the pK a values of ionizable functional groups and their corresponding site concentration as described by Herbelin and Westall (1999). The entries in Table 2 represent the best fit values and the SE. The best fit values were inferred using the variance of the difference function Y, i.e., V(Y), computed by FITEQL. Variance values in the range of 0.1–20 is indicative of good fit to the experimental data.
Results
Impact of surfactants on degradation of NAPL
B. cepacia and B. multivorans could grow on the model NAPL provided as sole source of carbon and energy both in the presence and absence of surfactants. For batches with Triton X-100 and Igepal CA 630, the controls devoid of microorganisms depicted increase in absorbance over time due to emulsification. Tween 80 did not cause emulsification. Degradation of model NAPL using both the aliphatic degrader B. cepacia (ES1) and aromatic degrader B. multivorans (NG1) was determined over 60 h (late log phase), using each surfactant at a concentration of 2 CMC. The overall loss in the system with no surfactant, Triton X-100, Igepal CA 630, and Tween 80 were 30 ± 4%, 75 ± 3%, 56 ± 3% and 33 ± 4%, respectively, for B. cepacia and 23 ± 4%, 62 ± 3%, 61 ± 3% and 37 ± 4%, respectively, for B. multivorans. The biotic loss of NAPL at any time was estimated from the overall loss of NAPL after accounting for the extraction loss and additional abiotic loss over time. In the absence of any surfactant, the overall loss of model NAPL was higher for B. cepacia (ES1) compared to B. multivorans (NG1). The trend in biotic loss (overall loss−extraction and abiotic losses) for the system with surfactants was similar for the two cultures: B. cepacia ES1 (Triton X-100 (60 ± 5%) > Igepal CA 630 (45 ± 5%) > Tween 80 (21 ± 5%)) and B. multivorans (NG1) (Triton X-100 (53 ± 5%) > Igepal CA 630 (50 ± 5%) > Tween 80 (20 ± 6%)). Increase in surface area due to emulsification was responsible for enhanced biodegradation observed in the presence of Triton X-100 and Igepal CA 630. Component-wise degradation of the model NAPL revealed that naphthalene and 1-methylnaphthalene were utilized completely in all cases (Fig. 1a, b). In the absence of any surfactant, only low degradation of n-hexadecane was observed while the other n-alkanes were not degraded by B. cepacia. Presence of surfactants not only enhanced the utilization of aliphatic components by B. cepacia but also promoted the degradation of aliphatics in B. multivorans, a naphthalene degrader. The four-ring polynuclear aromatic hydrocarbon (PAH), pyrene, could not be degraded by either of the two cultures, irrespective of choice of surfactant. The extraction and abiotic losses of aromatic components present in the NAPL were higher than the aliphatic components both in the absence and presence of surfactants.
Biodegradation studies at varying doses of Triton X-100
NAPL biodegradation and culture growth in the Triton X-100–B. cepacia (ES1) system at varying surfactant concentrations is illustrated in Fig. 2. Growth studies of B. cepacia (ES1) revealed that there was a significant increase in biomass in the presence of Triton X-100 at increasing concentration. This was more distinct at lower concentration of Triton X-100 (0.5 CMC and 1 CMC) compared to higher concentrations (2 and 5 CMC). Growth of the organism was accompanied by decrease in pH possibly due to production of acidic intermediates or due to production of some acidic by-products. Increase in surfactant dose resulted in increased rate of biodegradation. For surfactant concentration in the range 0 to 1 CMC, the biotic loss at 60 and 168 h showed an increasing trend (Table 1). At surfactant concentration equal to CMC and above, the extent of biodegradation was comparable. Mass balance of the model NAPL at the 168th hr depicted biotic loss above 70% in the presence of Triton X-100 at concentrations equal to CMC and above. The rate of degradation was fastest in the presence of 5 CMC Triton X-100 followed by 2 CMC Triton X-100. Degradation rate in the presence of 0.5 and 1 CMC Triton X-100 was comparable. Naphthalene was utilized within 12 h. 1-Methylnaphthalene was utilized between 12 and 36 h at the various surfactant concentrations. The rate of depletion of naphthalene and 1-methylnaphthalene remained unaffected by the increase in surfactant dose. In the presence of Triton X-100, all the aliphatic components (including n-hexadecane) were continuously degraded throughout the study duration. The rate of utilization of the aliphatics increased with increase in surfactant dose. The extraction and abiotic losses of the aromatic components (naphthalene, 1-methyl naphthalene, and pyrene) were significantly higher compared to the aliphatic components, irrespective of Triton X-100 concentration.
Alteration in CSH and zeta potential during surfactant-aided biodegradation
The water contact angle of B. cepacia (ES1) and B. multivorans (NG1) grown on the model NAPL was measured after 60 h of growth in the presence of various surfactants. In the absence of any surfactant, the cell surface of both cultures was moderately hydrophobic as depicted by water contact angle in the range of 50–52° (Fig. 3a). In the presence of all the surfactants, the water contact angle of both cultures increased to greater than 70° except for B. cepacia (ES1) grown in the presence of Igepal CA 630. Water contact angle in this range denotes a highly hydrophobic cell surface (Mozes and Rouxhet 1987).
The change in CSH of B. cepacia (ES1) as a function of growth phase was studied in the absence and presence of the surfactant, Triton X-100. In the absence of Triton X-100, and at low Triton X-100 concentration (0.5 and 1 CMC), the cell surface was moderately hydrophobic and did not vary much throughout the period of degradation. At higher concentrations of Triton X-100, i.e., 2 and 5 CMC, the CSH increased significantly in the log growth phase between 36 and 60 h and decreased in the stationary phase (168 h) (Fig. 3b).
The zeta potential of B. cepacia (ES1) and B. multivorans (NG1) grown on model NAPL in the presence of various surfactants for 60 h was measured as a function of pH. In the absence of any surfactant, the zeta potential of B. cepacia (ES1) was −38 mV, and for B. multivorans (NG1) it was −31 mV at neutral pH (Fig. 4a, b). For B. cepacia (ES1), the electronegativity decreased in the presence of Triton X-100 whereas the electronegativity increased in the presence of Igepal CA 630 and Tween 80. For B. multivorans (NG1), the electronegativity decreased only marginally in the presence of Triton X-100 and Igepal CA 630.
The change in zeta potential at different phases of growth was studied at neutral pH for B. cepacia (ES1) in the absence and presence of the surfactant, Triton X-100 (Fig. 4c). The zeta potential of Burkholderia spp. varied significantly at different growth phases. At 60 h (log growth phase), the zeta potential in the presence of varying doses of Triton X-100 was less electronegative compared to that at 36 (mid-log) and 168 h (stationary phase). The net charge on the cell surface is a result of interaction of functional groups present on the cell surface, and since presence/abundance of these groups is metabolically regulated (Martienssen et al. 2001), some variation may be expected over the growth phase.
Alteration in cell surface functional groups during surfactant-aided biodegradation
An attempt was made to study the effect of surfactants on the functional groups present on the cell surface. The functional groups present on the cell surface were predicted by modeling the potentiometric titration data using the non-electrostatic model and discrete site approach. Three- and two-site modeling was attempted using FITEQL 4.0. The three-site model provided a better fit compared to the two-site model and this was clearly evident from the variance computed by FITEQL. The variance for the three-site models for all cases was within 16, while the variance for the two-site model was much higher, in the range of 45–115. Thus, the three-site model explains the protonation behavior better than the two-site model. The potentiometric titrations conducted on the cells of B. cepacia (ES1) grown in the absence and presence of surfactants are shown in Fig. 5. These titration curves indicate the influence of surfactants on the acid–base properties of the cells characterized by three distinct cell surface functional groups with defined dissociation constants. They suggest that deprotonation is observed for all cases in the high-pH range except for Tween 80. There is change in the net charge on the cell surface as pH varies even in the absence of any surfactant. The net negative charge was lowest for cultures grown in the presence of Triton X-100, intermediate for Igepal CA 630, and highest for Tween 80. pK a1 was in the range of 5.2–6.2, pK a2 was in the range of 7.1–8.3, and pK a3 was in the range of 10.1–10.9 (Table 2).
Discussions
The structurally similar surfactants Triton X-100 and Igepal CA 630 (octylphenoxy polyoxyethylene ethanol), varying only in the number of ethylene oxide (EO) units (average number of EO units is 9.5 in Triton X-100 and 9 in Igepal), had similar hydrophile–lipophile balance (HLB) number (13.5 and 13, respectively) but distinctly different CMC values of 0.23 and 0.083 mM, respectively. At twice the CMC concentration, both of these surfactants could emulsify the NAPL and cause a significant increase in NAPL biodegradation by B. cepacia and B. multivorans. Enhancement in biodegradation of model NAPL in the presence of Triton X-100 and Igepal CA 630 may be attributed to increase in surface area due to emulsification. In contrast, Tween 80, a polyethoxyethylene sorbitan monooleate with 20 EO units having an HLB number of 15 and CMC of 0.012 mM, did not cause emulsification of the NAPL and did not cause much enhancement in biodegradation of the NAPL in the presence of the Burkholderia cultures. Mohanty and Mukherji (2007) also observed enhanced degradation of aliphatic components present in diesel by B. cepacia (ES1) in the presence of Triton X-100 (2 CMC) in batch systems with 1% diesel. Degradation of 1% diesel was complete within 5 days in the presence of Triton X-100, unlike degradation without surfactant where complete mineralization did not occur even after 15 days.
B. cepacia (ES1) is an aliphatic degrader. It has been reported to degrade n-alkanes in the absence of surfactants in batch systems with 1% n-hexadecane and diesel and is reported to be capable of direct interfacial uptake (Mohanty and Mukherji 2008a). The negligible degradation of aliphatics in the absence of surfactant observed in this study is possibly due to the low initial NAPL volume (0.1% NAPL) used, which would correspondingly reduce the surface area available for direct interfacial uptake and enhance the lag phase. In this study, B. cepacia (ES1) was found to utilize naphthalene and 1-methylnaphthalene from the NAPL phase while earlier studies revealed its inability to utilize naphthalene as sole source of carbon and energy when present as solid crystals at a concentration of 1 mg/ml (Mohanty and Mukherji 2009). The low naphthalene mole fraction in the NAPL is likely to have reduced its aqueous solubility (Peters et al. 1997) and toxicity.
The naphthalene degrader B. multivorans (NG1) was found to utilize aliphatic hydrocarbons only in the presence of emulsifying surfactants, Triton X-100 and Igepal CA 630, and this was responsible for enhancement in overall degradation of the NAPL. Thus, these surfactants induced the utilization of aliphatics by B. multivorans. A significant change in the mode of uptake of B. multivorans (NG1) may have occurred in the presence of surfactant which enabled the aromatic degrader to utilize aliphatics. Although B. multivorans could degrade the two-ring PAHs, naphthalene and 1-methyl naphthalene, it was unable to degrade the four-ring PAH, pyrene, which is inherently more difficult to degrade. The presence of surfactants could not promote utilization of pyrene by these cultures, indicating the absence of enzymes required for pyrene degradation.
Adhesion of microorganisms to NAPL droplets is an important phenomenon involved in the direct uptake mechanism. This phenomenon is influenced by CSH of the microorganisms. Changes in CSH during petroleum hydrocarbon degradation in the presence of surfactants have been reported by various researchers (Zhao et al. 2011; Owsianiak et al. 2009; Hua et al. 2003; Zhang and Miller 1994). Zhang and Miller (1994) observed that the biosurfactant rhamnolipid increased the CSH of the slow degraders and this enhanced the rate of degradation of octadecane by two strains of P. aeruginosa. Owsianiak et al. (2009) reported that rhamnolipid and Triton X-100 modified the CSH (as determined by BATH assay) of a microbial consortia during biodegradation of diesel fuel, but this increase was not correlated with increase in hydrocarbon degradation. Zhao et al. (2011) illustrated different effects of rhamnolipid addition on phenanthrene degradation by a hydrophobic Bacillus subtilis strain and a hydrophilic P. aeruginosa strain. The former depicted reduction in hydrophobicity (as determined by nitrocellulose filter test) and increased lag in biodegradation, while the latter depicted increased hydrophobicity and enhanced biodegradation. These differences were attributed to adsorption of rhamnolipids on bacterial cell surfaces. In the present study, it was observed that the cell surface of both Burkholderia spp. was initially moderately hydrophobic, while in the presence of all surfactants they were moderately to highly hydrophobic. Correspondingly, enhancement in degradation was also observed in most cases, except for Tween 80. Changes in CSH with growth phase as observed in this study have also been reported for a Rhodococcus sp. during degradation of crude oil (Bredholt et al. 2002).
Very few studies have reported zeta potential of hydrocarbon degrading cultures. The zeta potential is the potential at the plane of shear at some distance away from the surface. It reflects the charge on the surface of the microorganism, and a higher negative zeta potential is indicative of a higher negative charge on the surface. Bastiens et al. (2000) investigated the charge on the cell surface of Spingomonas and Mycobacterium spp.-degrading PAHs; however, they did not report the pH at which zeta potential was measured. Zeta potential is significantly influenced by the pH of the solution such that at pH values close to neutrality the zeta potential is more electronegative and with decrease in pH the zeta potential is lowered. The zeta potential of B. cepacia and B. multivorans grown on model NAPLs composed of n-hexadecane, naphthalene, phenanthrene, and pyrene in the absence of any surfactant was reported by Chakraborty et al. (2010). Chakraborty et al. (2010) reported the zeta potential of B. cepacia as less than −5 mV, whereas in this study it was −38 mV at neutral pH. This change in zeta potential is due to the change in composition of NAPL used as growth substrate in the two studies. However, although the NAPL composition differed, the zeta potential of B. multivorans observed at neutral pH (−31 mV) was comparable to that reported by Chakraborty et al. (2010), i.e., −20 to −38 mV for the various NAPLs. No studies have reported changes in zeta potential in the presence of surfactants.
The isoelectric point (IEP) of the cell surface of Burkholderia spp. was calculated based on the zeta potential values obtained as a function of pH. IEP refers to the pH at which the charge on any surface is zero. The IEP of B. cepacia (ES1) and B. multivorans (NG1) in the absence of surfactants was 2.4 and 2.0, respectively. Only a marginal variation in IEP was observed in the presence of the chemical surfactants, i.e., the IEP range was 2.3–2.6 for B. cepacia (ES1) and 2.0–2.7 for B. multivorans (NG1). Martienssen et al. (2001) reported the IEP of several bacterial cultures isolated from wastewater treatment plants to be in the range of 3–3.8. Van der Wal et al. (1997) reported the IEP of five Gram-positive bacterial cultures which were also in the above range, except Rhodococcus erythropolis A177, which demonstrated an IEP ≤2.0.
The acid–base properties of a bacterial cell are known to be altered by several environmental factors. Comparing the ranges for pK a1, pK a2, and pK a3 with those obtained from literature, we conclude that pK a1 corresponds to the acidity constant of carboxyl (RCOOH/RCOO−)/phosphoryl (RH2PO4/RHPO −4 ) groups, pK a2 corresponds to phosphoryl group (RHPO −4 /RPO 2−4 ), and pK a3 corresponds to hydroxyl (ROH/RO−)/amine (RNH +3 /RNH2) groups, respectively (Cox et al. 1999; Fein et al. 1997; Dittrich and Sibler 2005; Jiang et al. 2004). The net charge on the cell surface is determined by the site concentration of the functional groups. For B. cepacia (ES1), the functional group denoted by pK a3 was the most abundant. Identification of this functional group can be confirmed by analyzing the IEP obtained from the zeta potential profiles together with the deprotonation constant (Hong and Brown 2006). The observed pK a3 could either be due to hydroxyl group or amine group. The IEP for this culture is in the range of 2.3–2.7. At pH higher than the IEP, the net charge on the cell surface is negative while at lower pH the net charge is positive. The first two pK a values correspond to groups that are negatively charged at neutral pH. Uncertainty in the identity of these groups will not impact the overall charge, while identity of pK a3 will affect the overall charge distribution with respect to pH. At neutral pH, hydroxyl groups are uncharged but amine groups carry a positive charge. To determine the total charge, it is important to know the distribution of these two groups independently. If pK a3 is entirely due to hydroxyl groups, IEP would be zero and B. cepacia (ES1) would be negatively charged over the entire pH range, while if pK a3 is entirely amine the IEP would be much higher. The observed low IEP in the range 2–2.5 can be observed if the functional group corresponding to pK a3 is predominantly hydroxyl (OH−)/phenolic group rather than amine group.
The results of this study further illustrate that surfactants can impact the acidity constants of the functional groups (Table 2). In the presence of emulsifying surfactants, Triton X-100 and Igepal CA 630, the total site concentrations were decreased and this corresponds to a significant decrease in the site concentration of pK a3. Conversely, in the case of non-emulsifying surfactant, Tween 80, the total site concentration was increased and this too corresponds to a significant increase in the site concentration of pK a3. These site concentrations are however lower compared to the average site concentration obtained for 36 bacterial pure cultures, as compiled by Borrok et al. (2005), i.e., 320 ± 10 μmol/g. However, the site densities for specific oil-degrading cultures have not been reported so far.
Although biodegradation is affected by the enzymatic capabilities of microorganisms, bioavailability can play a significant role in biodegradation of petroleum hydrocarbons from NAPLs. Surfactants cause alteration not only in NAPL distribution (emulsification or micellar solubilization) but also in the cell surface properties as illustrated in this study. The process of emulsification increases the NAPL surface area, thereby increasing the mass transport of components out of the NAPL. Further, this also provides greater surface area for attachment and direct interfacial uptake. In the latter case, changes in the cell surface hydrophobicity and zeta potential of the bacterial cells involved have important implications. If a surfactant reduces the cell surface hydrophobicity of the culture and increases the negative zeta potential, then biodegradation will not increase even when the surfactant promotes emulsification. In the other process, surfactant micelles are formed which trap the NAPL components within the hydrophobic core. The micelle-solubilized hydrocarbons are either partly or fully bioavailable (Grimberg et al. 1996); hence, degradation would be increased. When this uptake mechanism is dominant, the changes in cell surface hydrophobicity and zeta potential will not affect biodegradation significantly. Thus, changes in cell surface characteristics caused by Tween 80 had no relevance on NAPL biodegradation.
The zeta potential on the NAPL used in this study was found to be −45.2 mV at pH 6. At neutral pH, n-hexadecane is also reported to carry a negative charge (Stachurski and Michalek 1996). Significant electrostatic repulsion may be expected since both NAPLs and bacterial cultures used in this study carry a negative charge. Chakraborty et al. (2010) demonstrated a trend indicating exponentially decreasing adherence to n-hexadecane as zeta potential became more negative for these Burkholderia cultures grown on three- and four-component NAPLs in the absence of surfactants. For the emulsifying surfactants Triton X-100 and Igepal CA 630, increase in hydrophobicity and decrease in negative zeta potential together correlated with increased biotic loss. In the presence of Triton X-100, a simultaneous increase in cell surface hydrophobicity and decrease in the negative zeta potential caused maximum biodegradation by both B. cepacia (ES1) and B. multivorans (NG1). Biodegradation of the model NAPL by B. cepacia (ES1) grown in the presence of Igepal CA 630 was lower than in the presence of Triton X-100 since the increase in hydrophobicity was only marginal. However, biodegradation was still higher than in the absence of any surfactant since Igepal CA 630 caused a marked reduction in negative charge on the cell surface. B. multivorans (NG1) demonstrated comparable biodegradation of the NAPL in the presence of both Triton X-100 and Igepal CA 630 since the hydrophobicity and surface charge were comparable in both cases. It appears that in the presence of these surfactants B. multivorans changed its mode of uptake. Thus, while in the absence of surfactants it only utilized naphthalene after mass transfer into the aqueous phase, in the presence of emulsifying surfactants that favorably altered its cell surface properties it adhered and utilized aliphatic hydrocarbons by direct interfacial uptake. Further investigations are required to explore if this phenomenon is prevalent among other aromatic hydrocarbon degraders.
References
Al-Tahhan RA, Sandrin RT, Bodour AA, Maier RM (2000) Rhamnolipid-induced removal of polysaccharide from Pseudomonas aeruginosa: effect on cell surface properties and interaction with hydrophobic substrates. Appl Environ Microbiol 66:3262–3268
Bastiens L, Springael D, Wattiau P, Harms H, deWachter R, Verachtert H, Diels L (2000) Isolation of adherent polycyclic aromatic hydrocarbons (PAH) degrading bacteria using PAH-sorbing carriers. Appl Environ Microbiol 66:1834–1843
Borrok D, Turner BJ, Fein JB (2005) A universal surface complexation framework for modeling proton binding onto bacterial surfaces in geologic settings. Am J Sci 305:826–853
Bredholt H, Bruheim P, Potocky M, Eimhjellen K (2002) Hydrophobicity development, alkane oxidation and crude-oil emulsification in a Rhodococcus species. Can J Microbiol 48:295–304
Bruheim P, Bredholt H, Eimhjellen K (1997) Bacterial degradation of emulsified crude oil and the effect of various surfactants. Can J Microbiol 43:17–22
Castro FD, Tufenkji N (2007) Relevance of nontoxigenic strains as surrogates for Escherichia coli 0157:H7 in groundwater contamination potential: role of temperature and cell acclimation time. Environ Sci Technol 41:4332–4338
Chakraborty S, Mukherji S, Mukherji S (2010) Surface hydrophobicity of petroleum hydrocarbon degrading Burkholderia strains and their interactions with NAPLs and surfaces. Colloids Surf B 78:101–108
Churchill SA, Harper JP, Churchill PF (1999) Isolation and characterization of a Mycobacterium species capable of degrading three- and four- ring aromatic and aliphatic hydrocarbons. Appl Environ Microbiol 65:549–552
Cox SJ, Smith DS, Warren LA, Ferris FG (1999) Characterizing heterogenous bacterial surface functional groups using discrete affinity spectra for proton binding. Environ Sci Technol 33:4514–4521
Dittrich M, Sibler S (2005) Cell surface groups of two picocyanobacteria strains studied by zeta potential investigations, potentiometric titration and infrared spectroscopy. J Colloid Interface Sci 286:487–495
Fein JB, Daughney CJ, Yee N, Davies T (1997) A chemical equilibrium model for metal adsorption onto bacterial surfaces. Geochim Cosmochim Acta 61:3319–3328
Fein JB, Boily J, Yee N, Lewis DG, Turner BF (2005) Potentiometric titrations of Bacillus subtilis cells to low pH and a comparison of modeling approaches. Geochim Cosmochim Acta 69:1123–1132
Grimberg SJ, Stringfellow WT, Aitken MD (1996) Quantifying the biodegradation of phenanthrene by Pseudomonas stutzeri P16 in the presence of nonionic surfactant. Appl Environ Microbiol 62:2387–2392
Guha S, Jaffe PR (1996) Bioavailability of hydrophobic compounds partitioned into the micellar phase of nonionic surfactants. Environ Sci Technol 30:1382–1391
Herbalin AL, Westall JC (1999) A Computer Program for Determination of Chemical Equilibrium Constants from Experimental Data. In FITEQL 4.0, Department of Chemistry, Oregon State University, Corvallis, Oregon 97331
Hong Y, Brown DG (2006) Cell surface acid–base properties of Escherichia coli and Bacillus brevis and variation as a function of growth phase, nitrogen source and C:N ratio. Colloids Surf B 50:112–119
Hua Z, Chen J, Lun S, Wang X (2003) Influence of biosurfactants produced by Candida antarctica on surface properties of microorganism and biodegradation of n-alkanes. Water Res 37:4143–4150
Jiang W, Saxena A, Song B, Ward BB, Beveridge TJ, Myneni SCB (2004) Elucidation of functional groups on gram positive and gram negative bacterial surfaces using infrared spectroscopy. Langmuir 20:11433–11442
Kaczorek E, Urbanowicz M, Olszanowski A (2010) The influence of surfactants on cell surface properties of Aeromonas hydrophila during diesel oil biodegradation. Colloids Surf B 81:363–368
Martienssen M, Reichel O, Kohlweyer U (2001) Surface properties of bacteria from different wastewater treatment plants. Acta Biotechnol 21:207–225
Medrzycka KB (1991) The effect of particle concentration on zeta potential in extremely dilute solutions. Colloid Polym Sci 269:85–90
Mohanty G, Mukherji S (2007) Effect of an emulsifying surfactant on diesel degradation by cultures exhibiting inducible cell surface hydrophobicity. J Chem Technol Biotechnol 82:1004–1011
Mohanty G, Mukherji S (2008a) Enhancement of NAPL bioavailability by induction of cell surface hydrophobicity in Exiguobacterium aurantiacum and Burkholderia cepacia. Indian J Biotechnol 7:295–306
Mohanty G, Mukherji S (2008b) Biodegradation rate of diesel range n-alkanes by bacterial cultures Exiguobacterium aurantiacum and Burkholderia cepacia. Int Biodeter Biodegrad 61:240–250
Mohanty S, Mukherji S (2009) Elucidation of petroleum hydrocarbon degradation by Burkholderia cepacia (ES1) in model systems and effect of nonionic chemical surfactants. J Environ Res Dev 3:654–662
Mozaffar Z, Weete JD, Dute R (1994) Influence of surfactants on an extracellular lipase from Pythium ultimum. J Am Oil Chem Soc 71:75–79
Mozes N, Rouxhet PG (1987) Methods for measuring hydrophobicity of microorganisms. J Microbiol Methods 6:99–112
Mukherji S, Peters CA, Weber WJ Jr (1997) Mass transfer of polynuclear aromatic hydrocarbons from complex DNAPL mixtures. Environ Sci Technol 31:416–423
Owsianiak M, Szulc A, Chrzanowski L, Cyplik P, Bogacki M, Olejnik-Schmidt AK, Heipeiper HJ (2009) Biodegradation and surfactant-mediated biodegradation of diesel fuel by 218 microbial consortia are not correlated to cell surface hydrophobicity. Appl Microbiol Biotechnol 84:545–553
Peters CA, Mukherji S, Knightes CD, Weber WJ Jr (1997) Phase stability of multicomponent NAPLs containing PAHs. Environ Sci Technol 31:2540–2546
Stachurski J, Michalek M (1996) The effect of the ζ potential on the stability of a non-polar oil-in-water emulsion. J Colloid Interface Sci 184:433–436
Van der Wal A, Norde W, Zehnder AJB, Lyklema J (1997) Determination of the total charge in the cell walls of gram-positive bacteria. Colloids Surf B 9:81–100
Van Hamme JD, Ward OP (1999) Influence of chemical surfactants on the biodegradation of crude oil by a mixed bacterial culture. Can J Microbiol 45:130–137
Van Hamme JD, Ward OP (2001) Physical and metabolic interactions of Pseudomonas sp. strain JA5-B45 and Rhodococcus sp. strain F9-D79 during growth on crude oil and effect of a chemical surfactant on them. Appl Environ Microbiol 67:4874–4879
Van Loosdrecht MCM, Lyklema J, Norde W, Schraa G, Zehnder AJB (1987) Electrophoretic mobility and hydrophobicity as a measure to predict the initial steps of bacterial adhesion. Appl Environ Microbiol 53:1898–1901
Verma S, Bhargava R, Pruthi V (2006) Oily sludge degradation by bacteria from Ankleshwar, India. Int Biodeter Biodegrad 57:207–213
Volkering F, Breure AM, Rulkens WH (1998) Microbiological aspects of surfactant use for biological soil remediation. Biodegradation 8:401–417
Zhang Y, Miller R (1994) Effect of Pseudomonas rhamnolipid biosurfactant on cell hydrophobicity and biodegradation of octadecane. Appl Environ Microbiol 60:2101–2106
Zhao Z, Selvam A, Wong JWC (2011) Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and biodegradation of phenanthrene. Bioresour Technol 102:3999–4007
Acknowledgements
This work was partly supported by the Society of Environmental Toxicology and Chemistry (SETAC)/Procter & Gamble Company Global Fellowship for Doctoral Research in Environmental Science and partly by a sponsored project from Department Science and Technology, New Delhi. The McGill–India Research Initiative (MISRI) scheme provided travel funds for interactions and discussions with Prof. Subhasis Ghoshal, Department of Civil Engineering and Applied Mechanics, and Prof. Nathalie Tufenkji, Department of Chemical Engineering, McGill University, Montreal Canada. Dr. Suresh Korpole, scientist, IMTECH Chandigarh, is acknowledged for the identification of B. multivorans NG1 based on 16S rRNA gene sequencing. Student support was initially provided by Grant No. 9187(441)/06-EMR-1, Council for Scientific and Industrial Research, New Delhi, India. The authors acknowledge the Centre for Research in Nanotechnology and Science–Sophisticated Analytical Instrumentation Facility (CRNTS-SAIF) and Centre for Nanoelectronics, IIT, Bombay, for providing facilities for zeta potential and contact angle measurements, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohanty, S., Mukherji, S. Alteration in cell surface properties of Burkholderia spp. during surfactant-aided biodegradation of petroleum hydrocarbons. Appl Microbiol Biotechnol 94, 193–204 (2012). https://doi.org/10.1007/s00253-011-3703-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3703-7