Abstract
Two heterotrophic As(III)-oxidizing bacteria, SPB-24 and SPB-31 were isolated from garden soil. Based on 16S rRNA gene sequence analysis, strain SPB-24 was closely related to genus Bordetella, and strain SPB-31 was most closely related to genus Achromobacter. Both strains exhibited high As(III) (15 mM for SPB-24 and 40 mM for SPB-31) and As(V) (>300 mM for both strains) resistance. Both strains oxidized 5 mM As(III) in minimal medium with oxidation rate of 554 and 558 μM h−1 for SPB-24 and SPB-31, respectively. Washed cells of both strains oxidized As(III) over broad pH and temperature range with optimum pH 6 and temperature 42°C for both strains. The As(III) oxidation kinetic by washed cells showed K m and V max values of 41.7 μM and 1,166 μM h−1 for SPB-24, 52 μM and 1,186 μM h−1 for SPB-31. In the presence of minimal amount of carbon source, the strains showed high As(III) oxidation rate and high specific arsenite oxidase activity. The ability of strains to resist high concentration of arsenic and oxidize As(III) with highest rates reported so far makes them potential candidates for bioremediation of arsenic-contaminated environment.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Inorganic arsenic exists in two major forms: the reduced form, arsenite-As(III), and the oxidized form, arsenate-As(V) (Neff 1997). Though it is only the 20th most abundant element in the earth's crust (NRC 1977), arsenic contamination of ground waters and soils is a serious environmental hazard and health problem in many parts of the world, mainly in Bangladesh. Arsenic in the drinking water poses the greatest threat to human health. In Bangladesh concentrations of arsenic in ground water are as high as 1,000 μg l−1 (Nickson et al. 2000). About 50–60 million people are exposed to arsenic-contaminated drinking water, and thousands of cases of arsenicosis are diagnosed every year (Chowdhury 2004).
Microbial transformation of arsenic has great implications on its geochemical cycling in the environment because different forms of arsenic vary in solubility, mobility, bioavailability, and toxicity (Smedley and Kinniburgh 2002). As(III) oxidation is a potential detoxification mechanism which allows microorganisms to tolerate high levels of As(III). Oxidation of As(III) to As(V) is used both for detoxification and energy generation which can also impact the mobility and speciation of arsenic in environment (Stolz et al. 2002). Biological As(III) oxidation, therefore, is being studied as a new alternative to the use of chemical oxidants and as the basis for bioremediation of systems where As(III) is a pollutant because As(V) can be immobilized onto strong adsorbents (Battaglia-Brunet et al. 2002). Isolation of more than 50 phylogenetically diverse As(III)-oxidizing strains distributed among more than 13 genera from various environments are reported so far (Stolz et al. 2010). These include both heterotrophic As(III) oxidizers and the more recently described chemolithoautotrophic As(III) oxidizers (Oremland and Stolz 2003). Chemolithoautotrophic As(III) oxidizers use the energy and reducing power from As(III) oxidation during CO2 fixation and cell growth under both aerobic (Santini et al. 2000) and nitrate-reducing conditions (Oremland et al. 2002; Rhine et al. 2006). However, all the As(III)-oxidizing bacteria reported so far are isolated from the arsenic-contaminated environments such as soils, groundwater, sediments, hot springs, and mine tailings. This article reports the isolation, physiological characterization, and As(III) oxidation potential of the heterotrophic bacterial strains. The strains were further phylogenetically characterized and the involvement of arsenite oxidase enzyme in As(III) oxidation was studied. Laboratory studies of isolated strains throw insight on their potential application for the bioremediation of arsenic-contaminated water.
Material and methods
Enrichment, isolation, and screening of As(III)-oxidizing bacteria
Garden soil was collected from the campus of University of Pune, Pune, India. Soil sample (3 g) was inoculated in the 250-ml conical flask containing 27 ml Tris–mineral medium (TMM) with low phosphate content (Mergeay et al. 1985), supplemented with 0.04% yeast extract, 20 mM sodium acetate (TMMA), and 5 mM of As(III). Flask was incubated on orbital shaker at 30°C for 5 days, and 3 ml of this enrichment culture was inoculated in fresh medium. This procedure was repeated twice and arsenic-resistant bacteria were isolated by plating enrichment cultures on tryptic soya agar (TSA) 0.1× containing 5 mM of As(III). Morphologically different colonies were isolated and purified by repeated sub-culturing on TSA 0.1× with 5 mM As(III). The cultures were routinely maintained on TSA (0.1×) with 1 mM of As(III). The glycerol stocks (20%) were also prepared and maintained at −20°C. As(III)-resistant strains obtained were screened for their As(III)-oxidation ability by using silver nitrate (AgNO3) screening method (Lett et al. 2001). The strains were grown on TSA 0.1× with 1 mM As(III) for 4 days at 30°C. The plates were flooded with AgNO3 solution (0.1 M). Brown precipitate around the colony indicated positive As(III) oxidation reaction. The colonies showing positive As(III) oxidation were further studied.
Identification of isolated strains
The strains SPB-24 and SPB-31 were grown in Luria Bertani broth to exponential phase. The cells were separated by centrifugation and DNA was extracted via enzymatic lysis using extraction buffer (100 mM Tris–HCl, pH 8.0, 100 mM EDTA, and 1.5 M NaCl) containing proteinase K (10 mg ml−1). The isolated DNA was purified, and polymerase chain reaction (PCR) amplification of 16S rRNA gene sequences was carried out using the primers F27 (5′-CCAGAG TTT GAT CMT GGC TCA G-3′) and R1525XP (5′-TTC TGC AGT CTA GAA GGA GGT GWT CCA GCC-3′) (Integrated DNA Technologies, Inc. USA). Each reaction mixture contained approximately 10 ng of DNA; 2.5 mM MgCl2; 1× PCR buffer (Bangalore Genei, Bangalore, India); 200 μM each dCTP, dGTP, dATP, and dTTP; 2 pmol of each forward and reverse primer; and 1 U of Taq DNA polymerase (Bangalore Genei, Bangalore, India) in a final volume of 20 μl. The PCR was performed using the Eppendorf Mastercycler gradient system with a cycle of 94°C for 5 min; 30 cycles of 94°C, 60°C, and 72°C for 1 min each; and final extension at 72°C for 10 min, and the mixture was held at 4°C. The amplified PCR products were checked by electrophoresis and purified by PEG–NaCl method. The purified PCR product was sequenced using Big Dye terminator kit (Applied Biosystems Inc., Foster city, CA). The 16S rDNA sequences were searched for homology by using the NCBI-Blast2-Nucleotide Database Query program. Phylogenetic analysis of 16S rDNA sequences were performed using MEGA version 4 software (Tamura et al. 2007). Phylogenetic tree was constructed using the neighbor-joining distance method based on p distance. A total of 100 bootstrap replications were calculated. The 16S rRNA gene sequences of strain SPB-24 and SPB-31 were deposited in GenBank/EMBL/DDBJ under the accession numbers JN208922 and JN208923, respectively.
Determination of resistance to As(III) and As(V)
Resistance of strain SPB-24 and SPB-31 for As(V) and As(III) was determined by growing them in TMMA liquid medium amended with increasing concentrations of As(V) (from 0, 25, 50, 75, 100, 150, 200, 250 to 300 mM) or As(III) (from 0, 5, 10, 15, 20, 25, 30, 35, 40, 45 to 50 mM). Two flasks for each concentration were inoculated with appropriate cell suspension grown in TMMA without arsenic to obtain initial optical density of approximately 0.02. The growth was evaluated by measuring the OD600 after 72 h incubation at 30°C.
Arsenic transformation by SPB-24 and SPB-31
To test the ability of the strains to reduce As(V) or oxidize As(III) in minimal medium, the strains were grown overnight in TMMA without arsenic and then inoculated (1%) into flasks containing 20 ml of TMMA either with 2 mM of As(V) or 2 mM As(III). The flasks were incubated in dark on rotary shaker with 140 rpm at 30°C. Control flasks without cells were incubated to check abiotic transformation of arsenic. At each sampling time, suspension was removed to measure cell growth and to determine As(V) and As(III) concentration. For further growth and As(III) oxidation experiments, strains were grown to exponential phase in TMMA with 1 mM As(III). The culture was inoculated (1%) into TMMA with 2, 5, and 10 mM of As(III) concentrations. The incubation and sample analysis were performed as described above. Utilization of different carbon sources by strain SPB-24 and SPB-31 was tested. For this, strains were grown overnight in TMMA and then washed twice with normal saline solution. The cells were resuspended into normal saline solution and were inoculated into TMM each with 20 mM of different carbon source and growth was measured. The different carbon sources tested include: glucose, sodium gluconate, sodium citrate, sodium lactate, sodium succinate, malate, and methanol.
Arsenic quantification
The concentration of arsenic was determined by spectrophotometric method described by Cummings et al. (1999). To determine concentration of As(V), 100 μl of appropriately diluted sample was added in 100 μl of acidifying solution (24 mM HCl) and to determine As(III) concentration, a second sample was oxidized in 100 μl oxidizing solution (5 mM KIO3 and 48 mM HCl) for 10 min. A 100-μl of the acidified and oxidized sample was then added separately to 900 μl of the reaction mixture containing ammonium molybdate (6 g l−1), ascorbic acid (10·8 g l−1), potassium antimonyl tartrate (0·136 g l−1), and concentrated H2SO4 (67·3 ml l−1). All reagents were prepared in milli-Q water and stored as a separate solution. Fresh reaction mixture was prepared every time and used within 6 h. Samples were heated in a water bath at 78°C for 10 min and placed on ice for 5 min. The absorbance at 865 nm was compared to acidified As(V) and oxidized As(III) standards. Blanks of milli-Q water were used to calibrate the spectrophotometer. Standard curves were prepared for concentrations of (0–100 μM) for both As(V) and As(III). The difference between oxidized and unoxidized samples represented the concentration of As(III).
Washed cells experiment: effect of pH, temperature, and initial cell density on As(III) oxidation by washed cells
The strains were grown overnight in TMMA with 1 mM As(III), and cells were harvested by centrifugation at 10,000×g and 4°C for 15 min and washed twice with saline (0.85% NaCl) solution. The cells were resuspended in saline and added to 20 ml of different buffer solutions to give same absorbance for each experiment. Different buffer solutions (50 mM) used were: pH 4, 5 (acetate buffer); pH 6 (2-(N-morpholino) ethanesulfonic acid, MES buffer); pH 7, 8, 9 (tris–HCl buffer) and pH 10 (glycine–NaOH buffer). As(III) was added at final concentration of 1.33 mM and incubated at 30°C for 1 h. The effect of temperature on As(III) oxidation by washed cells was studied in MES buffer, pH 6.0. The washed cells were suspended in buffer to give same absorbance for each strain, and 20 ml of this suspension was added to flasks. The flasks were incubated at 8°C, 20°C, 30°C, 37°C, 42°C, and 50°C for 30 min for equilibration. Once the cells got acclimatized at that temperature, As(III) was added into each flask to final concentration of 1.33 mM, and samples were withdrawn after 1 h for arsenic quantification. Similarly, effect of different cell density on As(III) oxidation rate was studied by suspending the washed cells into 20 ml of MES buffer, pH 6. The cell densities were adjusted to absorbance of 0.1 to 0.5 at 600 nm. The flasks were incubated at 30°C on shaker (140 rpm), and samples were removed for arsenic quantification after 1 h. Samples were immediately preserved by freezing at −20°C until further analysis. Arsenic quantification was performed as described above.
Effect of growth condition on As(III) oxidation rate
Oxidation of As(III) by washed cells of both the strains grown in different concentration of yeast extract were tested. Strains were grown in 200 ml of (1) TMM containing 0.04% yeast extract as carbon source, (2) TMM with 0.1% yeast extract as carbon source, and (3) TMMA (which contained 0.04% yeast extract and 20 mM acetate as carbon source). As(III) was added to all flasks at final concentration of 1 mM. Strains were grown to stationary phase, and cells were harvested by centrifugation at 10,000×g and 4°C for 15 min and washed twice with MES buffer, pH 6.0. The cells were suspended in 20 ml of MES buffer (pH 6), and As(III) was added to final concentration of 1.33 mM. The flasks were incubated at 30°C for 1 h, and samples were withdrawn after every 10 min for arsenic quantification.
Effect of initial As(III) concentration on oxidation
Effect of initial concentration of As(III) on its oxidation rate was studied for both strains. The washed cells were prepared as described above and resuspended in MES buffer 50 mM, pH 6. Ten milliliter of this suspension was added to different flasks and As(III) was added to give initial concentrations of 0.05, 0.1 0.15, 0.2, 0.25, 0.3, 0.35, and 0.4 mM. Flasks were incubated at 30°C for 5 min. The reaction was stopped by boiling the sample for 10 min, the volume was adjusted to the original level, in order to avoid the effect of evaporation and concentration of As(V) was determined as described above.
Induction of arsenite oxidase system
To check if the arsenite oxidase system was constitutive or inducible, strains were first grown in TMMA without arsenic and sub-cultured three times in same medium. After third subculture, cells were inoculated separately into TMMA without arsenic, with 1 mM of As(V) and with 1 mM As(III). After overnight incubation at 30°C, cells were centrifuged at 10,000×g at 4°C for 15 min and washed twice with saline solution. The cells were suspended in 20 ml of MES buffer (pH 6), and As(III) was added to final concentration of 1.33 mM. The flasks were incubated at 30°C for 2 h. Samples were removed after every 30 min, and arsenic concentration was determined as described earlier.
Arsenite oxidase assay
The strains SPB 24 and SPB 31 were grown in different media as described above in presence of 1 mM As(III). After 16 h, cells were collected by centrifugation followed by washing with 50 mM tris–HCl buffer, pH 8.0. Cells were resuspended in 2 ml of same buffer containing 1 mg ml−1 lysozyme and 0.5 mM phenylmethylsulfonyl fluoride (PMSF). Cells were incubated at 37°C for 2 h and disrupted by sonication (5 cycles of 1 min each with an interval of 5 min for cooling). Cell debris was removed by centrifugation at 10,000×g at 4°C for 30 min. Protein concentration was determined by Bradford method (Bradford 1976) with bovine serum albumin as standard. Arsenite oxidase enzyme assay was performed as described by Anderson et al. (1992). Reduction of 60 μM 2,4-dichlorophenolindophenol (DCIP) was monitored at 600 nm for 5 min in the presence of 200 μM As(III) in 50 mM MES buffer, pH 6.0 at 30°C. The assay was initiated by the addition of enzyme.
Statistical analysis
Data were expressed as mean±S.D. and analyzed by one-way analysis of variance (ANOVA) with Dunnett's multiple comparisons test. Readings were considered significant when P was ≤0.05.
Results
Isolation, identification, and characterization of As(III) oxidizing strains
The As(III) oxidizing bacteria were isolated by enrichment culture technique. Out of 31 different bacterial strains isolated from enrichment culture, only two strains, SPB-24 and SPB-31, showed positive As(III) oxidation reaction by silver nitrate screening method. Other isolates showing negative As(III) oxidation reaction were discarded and strain SPB-24 and SPB-31 were further studied. Both strains were Gram-negative and short rods. Strain SPB-24 was non-motile, whereas strain SPB-31 was motile. Strains were identified by sequencing 16S rDNA, and their phylogenetic analysis was performed. Both strains belonged to β-proteobacteria group and strain SPB-24 showed 97% similarity to Bordetella sp. d16 (Genbank accession number HQ652589) while strain SPB-31 showed 99% similarity to Achromobacter xylosoxidans (Genbank accession number AF531768). Strain SPB-24 and SPB-31 are deposited in National Collection of Industrial Microorganisms (NCIM), Pune, India with the accession numbers NCIM 5428 and NCIM 5429, respectively. In phylogenetic analysis (Fig. 1), SPB-24, together with Bordetella sp. d16, Bordetella sp. e3, Bordetella sp. p23, formed different branch from other Bordetella species. SPB-31 was phylogenetically most closely related to A. xylosoxidans strain IR-826. Both strains showed high resistance towards As(III) and As(V). Growth of SPB-24 was observed in presence of 15 mM As(III) but not in 20 mM of As(III) and that of SPB-31 was observed in presence of 40 mM of As(III) but not in 45 mM of As(III). Both strains grew in presence of 300 mM As(V). Utilization of different carbon sources by strains was tested and both strains used citrate, lactate, and succinate. Gluconate and malate was used by SPB-31 but not by SPB-24. Both strains did not use glucose and methanol as carbon source.
Neighbor-joining phylogenetic tree of 16S rRNA gene sequence of strains SPB-24 and SPB-31, shown in bold face, and most closely related species. Scale bar indicates the number of base substitutions per site. Numbers above nodes represent bootstrap confidence values obtained with 100 resamplings. The GenBank accession numbers for the corresponding sequences are given before the strain name
Growth and As(III) oxidation by strain SPB-24 and SPB-31
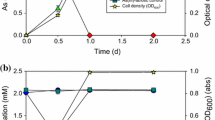
Growth and As(III) oxidation ability was tested in minimal medium containing 2, 5, and 10 mM of As(III). The growth of strain SPB-24 was not significantly affected by the presence of 2 and 5 mM As(III) (Fig. 2). The doubling time of strain SPB-24 in absence of As(III) was 42 min and in presence of 2, 5, and 10 mM As(III), it was 46, 50 and 130 min respectively showing significant increase in doubling time at 10 mM As(III). The growth of strain SPB-31 was not significantly affected by the presence of 2, 5, and 10 mM As(III) (Fig. 2). The doubling time in absence and presence of 2, 5, and 10 mM As(III) were 41, 41, 54, and 60 min, respectively. Both strains showed rapid oxidation of As(III) in minimal medium (Fig. 3). Strain SPB-24 oxidized 2 and 5 mM As(III) completely within 8 and 12 h, respectively, while strain SPB-31 required 10 and 12 h for complete oxidation of 2 and 5 mM of As(III), respectively. Both the strains began As(III) oxidation at the start of exponential phase, and it was complete before the end of exponential phase, it was also observed that cell density continued to increase even after complete oxidation of As(III). The As(III) oxidation rate for SPB-24 was 375 μM h−1 at 2 mM As(III) concentration and 554 μM h−1 at 5 mM As(III) concentration. The strain SPB-31 showed oxidation rate of 300 and 558 μM h−1 at 2 and 5 mM As(III) concentrations, respectively. For both strains, reduction of As(V) was not observed and chemolithotrophic growth was not supported by As(III). However, growth and As(III) oxidation was observed when TMM was supplemented with small amount of yeast extract (0.04%). Moreover, they oxidized As(III) with the same efficiency as in TMMA but optical density (600 nm) of the cultures of both the strains did not increased beyond 0.2 (data not shown).
Effect of pH, temperature, and initial cell density on As(III) oxidation by washed cells
Strains SPB-24 and SPB-31 oxidized As(III) between pH 4 to 10 (Fig. 4a). Both strains showed maximum oxidation at pH 6 and also oxidized As(III) efficiently at alkaline pH. Strain SPB-24 showed 89% and 56% As(III) oxidation at pH 8 and 9, respectively, while at pH 10, it showed 26% As(III) oxidation. For strain SPB-31, 92% and 89% oxidation was observed at pH 8 and 9, respectively, and oxidation was reduced to 46% at pH 10. At pH 5, 64% and 59% oxidation was observed for SPB-24 and SPB-31, respectively. At pH 4, strain SPB-24 showed significant reduction in oxidation (13%), while strain SPB-31 showed 31% oxidation activity. As(III) oxidation by washed cells of strain SPB-24 and SPB-31 was tested at temperature range from 8°C to 50°C (Fig. 4b). Strain SPB-24 showed relative As(III) oxidation of 20%, 54%, 83%, 89%, 100%, and 74% at 8°C, 20°C, 30°C, 37°C, 42°C, and 50°C, respectively, while relative As(III) oxidation for strain SPB-31 was 24%, 53%, 82%, 91%, 100%, and 78% at 8°C, 20°C, 30°C, 37°C, 42°C, and 50°C, respectively. The maximum oxidation by washed cells was observed at 42°C for both the strains.
Effect of a pH and b temperature on oxidation of As(III) by washed cells of SPB-24 and SPB-31. Different buffers were used to check the effect of pH. The effect of temperature was tested in 50 mM MES buffer, pH 6.0. The cell density used in both experiment was 109 cells/ml. Data are represented as mean±S.D., n = 4. The highest As(III) oxidation observed at particular pH and temperature was considered as 100%, and other values were calculated relative to highest oxidation
Effect of initial cell density (OD 600 nm) on As(III) oxidation rate was studied for strain SPB-24 and SPB-31, and it was observed that the rate of As(III) oxidation was directly proportional to the initial cell density (Fig. 5). The strain SPB-24 showed oxidation rate of 0.20 mM h−1 at initial cell density 0.1, and it increased up to 0.92 mM h−1 at initial cell density of 0.5 OD (600 nm). On the other hand, for SPB-31, oxidation rates of 0.14 and 0.56 mM h−1 were observed at initial cell density of 0.1 and 0.5, respectively.
Effect of initial As(III) concentration on As(III) oxidation
Kinetic data were obtained for the As(III) oxidation by washed cells of strain SPB-24 and SPB-31 (Fig. 6). Both strains showed high As(III) oxidation rates (V max 1,166 and 1,186 μM h−1 for SPB-24 and SPB-31, respectively). There was no significant difference in V max values of both strains but K m value of SPB-24 was lower (40.7 μM) than SPB-31 (52 μM).
As(III) oxidation rates as a function of initial As(III) concentration for a SPB-24 and b SPB-31. Rate data were fit to the Lineweaver Burk plot to estimate the kinetic parameters K m and V max. Assay was performed in 4 ml assay volumes (109 cells ml−1). Data points are average of two independent experiments
Induction of arsenite oxidase system
Arsenite oxidase system of SPB-24 and SPB-31 was induced by both the forms of arsenic, As(III), and As(V). The As(III) oxidation was not observed when cells were grown without arsenic (Fig. 7). However, oxidation was higher (40% and 55% for strain SPB-24 and SPB-31, respectively) when cells were grown in presence of As(III) than when grown in presence of As(V) (22% and 18% for strain SPB-24 and SPB-31, respectively).
Effect of growth condition on As(III) oxidation rate
The strains SPB-24 and SPB-31 showed similar As(III) oxidation rates when grown in TMMA or TMM with 0.04% yeast extract, although cell density was higher when cells were grown in TMMA than when grown in TMM with 0.04% yeast extract alone (data not shown). Therefore, experiments were conducted to check the effect of concentration of carbon source (as yeast extract and acetate) on As(III) oxidation rate and arsenite oxidase activity of both strains. Washed cells of both the strains showed maximum oxidation rate when grown in presence of 0.04% yeast extract as sole carbon source (Fig. 8a). Washed cells of strain SPB-24 showed oxidation rate of 3.38 ± 0.66 mM h−1 when grown in presence of 0.04% yeast extract compared to 1.62 ± 0.31 mM h−1 when grown in presence of 0.1% yeast extract (about 50% reduction in As(III) oxidation rate). When cells were grown in presence of TMM with 0.04% yeast extract and 20 mM acetate, As(III) oxidation rate was reduced to 16%. Similar results were obtained for strain SPB-31 with maximum As(III) oxidation rate observed when cells were grown in presence of 0.04% yeast extract (2.12 ± 0.23 mM h−1). About 25% and 82% reduction in As(III) oxidation rate was observed when cells were grown in the presence of 0.1% yeast extract and in the presence of 0.04% yeast extract and 20 mM acetate, respectively.
Effect of growth condition on a As(III) oxidation rate and b specific activity of arsenite oxidase of SPB-24 and SPB-31. YE = yeast extract, data were expressed as mean±S.D., n = 3 and analyzed by one-way analysis of variance (ANOVA) with Dunnett's multiple comparisons test. Readings were considered significant when P was ≤0.05. *P < 0.05; ***P < 0.0001
Arsenite oxidase activity
Maximum arsenite oxidase activity was observed when cells were grown in the presence of 0.04% yeast extract as the only carbon source (12.6 and 12.2 nM min−1 mg−1 protein for SPB-24 and SPB-31, respectively) (Fig. 8b). About 35% and 28% reduction in arsenite oxidase activity of SPB-24 and SPB-31, respectively, was observed when cells were grown in presence of 0.1% yeast extract as sole carbon source . Presence of 0.04% yeast extract and 20 mM acetate as carbon source resulted in 80% and 73% decrease in arsenite oxidase activity of SPB-24 and SPB-31, respectively.
Discussion
Most of the As(III) oxidizing bacteria reported are isolated from the arsenic-contaminated environment such as gold mine (Santini et al. 2000), aquatic environment (Weeger et al. 1999; Oremland et al. 2002), ground water (Fan et al. 2008), and hot creek (Salmassi et al. 2002). The strains SPB-24 and SPB-31 were isolated from the garden soil which is not likely to be contaminated with arsenic. This suggests that As(III) oxidizing bacteria are widespread and not specifically found in the arsenic-rich contaminated environments. The strains isolated in this study belong to the genus Bordetella and Achromobacter. Achromobacter species having As(III) oxidation ability have been reported previously (Santini et al. 2002; Fan et al. 2008; Cai et al. 2009). Strain NT-10 which is closely related to Bordetella genus has been reported by Santini et al. (2002), but it was not characterized in detail. The strain SPB-24 is therefore the first Bordetella species characterized in detail with respect to arsenic resistance and As(III) oxidation potential. Phylogenetic analysis of 16S rDNA sequence of SPB-24 showed that it was distantly placed from the other Bordetella species, and thus is more likely to be a novel species. However, further studies are required to determine correct phylogenetic position of this strain.
The resistance levels of As(III) and As(V) obtained for strains SPB-24 and SPB-31 were high compared to other As(III) oxidizing bacteria reported previously, and therefore these strains can be classified as highly arsenic-resistant strains (Hedges and Baumberg 1973). Although strains were isolated from the soil with no previous history of arsenic contamination, they showed very high resistance towards both As(III) and As(V), indicating that the level of bacterial arsenic resistance is independent of arsenic content of the soil, in accordance with other similar studies (Achour et al. 2007; Bachate et al. 2009; Jackson et al. 2005). The strains grew rapidly in the presence of As(III) with very short lag phase. The doubling time of strain SPB-24 (46 min) and SPB-31 (41 min) in minimal medium containing 2 mM As(III) was very low compared to other As(III) oxidizing bacteria like Herminiimonas arsenicoxydans strain ULPAs1 for which doubling time was 1.5 h in the presence of 1 mM of As(III) (Weeger et al. 1999), while for Agrobacterium tumifaciens AOL 15, it was 1.6 h in the presence of 500 μM of As(III) (Salmassi et al. 2002). Some bacteria oxidize As(III) in late exponential phase or early stationary phase (Salmassi et al. 2002; Phillips and Taylor 1976), but strains SPB-24 and SPB-31 oxidized As(III) in exponential phase, suggesting the presence of two component signal transduction mechanism (Cai et al. 2009). Both strains oxidized As(III) with remarkably high rates at 2 and 5 mM As(III) concentration. The strains isolated in the present study oxidized even 10 mM As(III) completely within 24 h (data not shown). Cai et al. (2009) reported As(III) oxidation rates of 52.9 and 59.1 μM h−1 for Achromobacter sp. SY8 and Pseudomonas sp. TS44, respectively, while the strains GW1 and GW4 showed As(III) oxidation rates of 270.2 and 110.2 μM h−1 (Fan et al. 2008). To our knowledge, the As(III) oxidation rates obtained for SPB-24 and SPB-31 are highest reported so far. Table 1 shows the comparison of As(III) resistance level and the overall As(III) oxidation rates of strain SPB-24 and SPB-31 with heterotrophic As(III) oxidizing bacteria described previously. Strain SPB-24 and SPB-31 did not appear to grow chemolithotrophically with As(III) as electron donor, consistent with previously characterized As(III) oxidizing strains closely related to genus Bordetella (Santini et al. 2002) and belonging to genus Achromobacter (Fan et al. 2008; Cai et al. 2009) and suggests that both strains resist As(III) by detoxification mechanism.
To assess the potential of bacterial cells to use in bioremediation of arsenic-contaminated water, experiments were conducted on washed cells in buffer. Washed cells of strain SPB-24 and SPB-31 showed rapid oxidation of 1.33 mM As(III) in buffer. In both the cases, the resting cells oxidized As(III) to As(V) without any lag phase. Both strains can oxidize As(III) over broad range of pH and temperature. Oxidation of As(III) by washed cells has been reported by other researchers (Weeger et al. 1999; Salmassi et al. 2002), but oxidation was not tested at different pH values, therefore results were not directly compared. Growth and As(III) oxidation by strain GM1 at low temperature (4°C) was reported by Osborne et al. (2010), and this is the only strain able to grow and oxidize As(III) at low temperature. Washed cells of strain SPB-24 and SPB-31 oxidized As(III) efficiently at low temperature (8°C and 20°C); however, growth was not tested at these temperatures. The ability of strains to oxidize As(III) over broad pH and temperature range and taking into account their high oxidation rates, makes these strains potential candidates for remediation of arsenic-contaminated water. Moreover, average pH and temperature of the ground water in tropical countries like India and Bangladesh falls in the range tested in this study (Smedley and Kinniburgh 2002). However, further experiments are required to assess the potential of these strains for bioremediation of arsenic-contaminated water. The rate of transformation of As(III) to As(V) was directly proportional to and dependent on the cell density. These results are in accordance with other As(III) oxidizing bacteria (Turner 1949; Weeger et al. 1999; Mokashi and Paknikar 2002).
Although constitutive expression of arsenite oxidase has been reported in few strains (Kashyap et al. 2006; Duquense et al. 2008; Osborne et al. 2010; Lieutaud et al. 2010), it is inducible in most of the As(III) oxidizing bacteria. Strains SPB-24 and SPB-31 did not show constitutive expression of arsenite oxidase. Washed cells of strain SPB-24 and SPB-31 oxidized As(III) when grown in the presence of As(III) or As(V) but not when grown in absence of arsenic, indicating that arsenite oxidase activity is not constitutive and was induced by both arsenic forms. These results are in agreement with those obtained by Weeger et al. (1999) for H. arsenicoxydans strain ULPAs1 which showed As(III) oxidation activity only when grown in presence of As(III) and As(V), and higher activity was observed when cells were grown in the presence of As(III) than in As(V). Similar results were observed for strain SPB-24 and SPB-31, indicating that As(III) is a strong inducer of arsenite oxidase system.
Santini et al. (2000) reported As(III)-oxidizing strain NT-26 which grows chemolithotrophically using As(III) as energy source. This strain showed high arsenite oxidase activity when grown without any organic carbon source. Addition of yeast extract (organic carbon source) resulted in decrease in the arsenite oxidase activity. Interestingly, strain SPB-24 and SPB-31, which were unable to grow chemolithotrophically in the presence of As(III), showed high As(III) oxidation rate and specific arsenite oxidase activity when grown in media with minimal concentration of carbon source. This suggests that arsenite oxidase is overexpressed in stressed (nutrient-limiting) conditions. However, detailed genomic and proteomic studies are necessary to understand the physiology of these strains in As(III) stressed and nutrient-limiting conditions. The rate of As(III) oxidation obtained for washed cells of the strain SPB-24 and SPB-31 to our knowledge, are highest reported so far compared to other As(III) oxidizing bacteria. Kinetic study showed that strain SPB-24 has higher affinity towards As(III) than SPB-31, and K m and V max values of both strains were different from those observed for other strains, like for Agrobacterium tumifaciens, K s and V max values were 3.4 ± 2.2 μM and 1.81 ± 0.58 × 10−12 μM cell−1 min−1 (Salmassi et al. 2002), while for Hydrogenobaculum strain, K m value of 89.1 μM and V max value of 41.7 nmol h−1 was reported (Donahoe-Christiansen et al. 2004).
The results, thus obtained, have characterized the arsenic-resistant bacterial strains Bordetella sp. SPB-24 and A. xylosoxidans SPB-31 able to tolerate high concentrations of As(III) and As(V). The strains were able to oxidize As(III) to As(V) when grown in minimal medium with high oxidation rates. The study also demonstrates the inducibility of the arsenite oxidase and its involvement in As(III) oxidation. Further studies of these strains with respect to genomic and proteomic analysis in arsenic stress and nutrient stress as well as feasibility to use them in bioremediation of arsenic-contaminated water are in progress.
References
Achour AR, Bauda P, Billard P (2007) Diversity of arsenite transporter genes from arsenic-resistant soil bacteria. Res Microbiol 158:128–137
Anderson GL, Williams J, Hille R (1992) The purification and characterization of arsenite oxidase from Alcaligenes faecalis, a molybdenum containing hydroxylase. J Biol Chem 267:23674–23682
Bachate SP, Cavalca L, Andreoni V (2009) Arsenic resistant bacteria isolated from agricultural soils of Bangladesh and characterization of arsenate reducing strains. J Appl Microbiol 107:145–156
Battaglia-Brunet F, Dictor M-C, Garrido F, Crouzet C, Morin D, Dekeyser K, Clarens M, Baranger P (2002) An As(III)-oxidizing bacterial population: selection, characterization, and performance in reactors. J Appl Microbiol 93:656–667
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Branco R, Francisco R, Chung AP, Morais PV (2009) Identification of an aox system that requires cytochrome c in the highly arsenic-resistant bacterium Ochrobactrum tritici SCII24. Appl Environ Microbiol 75:5141–5147
Cai L, Rensing C, Li X, Wang G (2009) Novel gene clusters involved in arsenite oxidation and resistance in two arsenite oxidizers: Achromobacter sp. SY8 and Pseudomonas sp. TS44. Appl Microbiol Biotechnol 83:715–725
Campos VL, Valenzuela C, Yarza P, Kämpfer P, Vidal R, Zaror C, Mondaca M-A, Lopez-Lopez A, Rosselló-Móra R (2010) Pseudomonas arsenicoxydans sp nov., an arsenite-oxidizing strain isolated from the Atacama desert. Syst Appl Microbiol 33:193–197
Chowdhury AMR (2004) Arsenic crisis in Bangladesh. Sci Am 291:87–91
Connon SA, Koski AK, Neal AL, Wood SA, Magnuson TS (2008) Ecophysiology and geochemistry of microbial arsenic oxidation within a high arsenic, circumneutral hot spring system of the Alvord Desert. FEMS Microbiol Ecol 64:117–128
Cummings DE, Caccavo F, Fendorf S, Rosenzweig RF (1999) Arsenic mobilization by the dissimilatory Fe(III)-reducing bacterium Shewanella alga BrY. Environ Sci Technol 33:723–729
Donahoe-Christiansen J, D'Imperio S, Jackson CR, Inskeep WP, McDermott TR (2004) Arsenite-oxidizing Hydrogenobaculum strain isolated from an acid-sulfate-chloride geothermal spring in Yellowstone National Park. Appl Environ Microbiol 70:1865–1868
Duquense K, Lieutaud A, Ratouchniak J, Muller D, Lett M, Bonnefoy V (2008) Arsenite oxidation by a chemoautotrophic moderately acidophilic Thiomonas sp.: from the strain to gene study. Environ Microbiol 10:228–237
Fan H, Su C, Wang Y, Yao J, Zhao K, Wang Y, Wang G (2008) Sedimentary arsenite-oxidizing and arsenate-reducing bacteria associated with high arsenic groundwater from Shanyin, Northwestern China. J Appl Microbiol 105:529–539
Gihring TM, Druschel GK, McCleskey RB, Hamers RJ, Banfield JF (2001) Rapid arsenite oxidation by Thermus aquaticus and Thermus thermophilus: field and laboratory investigations. Environ Sci Technol 35:3857–3862
Handley KM, Héry M, Lloyd JR (2009) Marinobacter santoriniensis sp. nov., an arsenate-respiring and arsenite-oxidizing bacterium isolated from hydrothermal sediment. Int J Syst Evol Microbiol 59:886–892
Hedges RW, Baumberg S (1973) Resistance to arsenic compounds conferred by a plasmid transmissible between strains of Escherichia coli. J Bacteriol 115:459–460
Jackson CR, Dugas SL, Harrison KG (2005) Enumeration and characterization of arsenate-resistant bacteria in arsenic free soils. Soil Biol Biochem 37:2319–2322
Kashyap DR, Botero LM, Franck WL, Hassett DJ, McDermott TR (2006) Complex regulation of arsenite oxidation in Agrobacterium tumefaciens. J Bacteriol 188:1081–1088
Lett M-C, Paknikar K, Lihvremont D (2001) In: Ciminelli VST, Garcia O (eds) Ion hydrometallurgy—fundamentals, technology and sustainable development, part B. Elsevier Science, New York
Liao VH-C, Chu YJ, Su YC, Hsiao SY, Wei CC, Liu CW, Liao CM, Shen WC, Chang FJ (2011) Arsenite-oxidizing and arsenate-reducing bacteria associated with arsenic-rich groundwater in Taiwan. J Contam Hydrol 123:20–29
Lieutaud A, van Lis R, Duval S, Capowiez L, Muller D, Lebrun R, Lignon S, Fardeau ML, Lett MC, Nitschke W, Schoepp-Cothenet B (2010) Arsenite oxidase from Ralstonia sp. 22: characterization of the enzyme and its interaction with soluble cytochromes. J Biol Chem 285:20433–20441
Macur RE, Jackson CR, Botero LM, Mcdermott TR, Inskeep WP (2004) Bacterial populations associated with the oxidation and reduction of arsenic in an unsaturated soil. Environ Sci Technol 38:104–111
Mergeay M, Nies D, Schlegel HG, Gerits J, Charles P, Gijsegem FV (1985) Alcaligenes eutrophus CH34 is a facultative chemolithotroph with plasmid-bound resistance to heavy metals. J Bacteriol 162:328–334
Mokashi SA, Paknikar KM (2002) Arsenic (III) oxidizing Microbacterium lacticum and its use in the treatment of arsenic contaminated groundwater. Lett Appl Microbiol 34:258–262
National Research Council (NRC) (1977) Arsenic. National Academy of Sciences, Washington, p 16
Neff JM (1997) Ecotoxicology of arsenic in marine environment. Environ Toxicol Chem 16:917–927
Nickson RT, McArthur JM, Ravenscroft P, Burgess WG, Ahmed KM (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Oremland RS, Stolz JF (2003) The ecology of arsenic. Science 300:939–944
Oremland RS, Hoeft SE, Santini JM, Bano N, Hollibaugh RA, Hollibaugh JT (2002) Anaerobic oxidation of arsenite in Mono Lake water and by a facultative, arsenite oxidizing chemoautotroph, strain MLHE-1. Appl Environ Microbiol 68:4795–4802
Osborne TH, Jamieson HE, Hudson-Edwards KA, Nordstrom DK, Walker SR, Ward SA, Santini JM (2010) Microbial oxidation of arsenite in a subarctic environment: diversity of arsenite oxidase genes and identification of a psychrotolerant arsenite oxidizer. BMC Microbiol 10:205–212
Phillips SE, Taylor ML (1976) Oxidation of arsenite to arsenate by Alcaligenes faecalis. Appl Environ Microbiol 32:392–399
Rhine ED, Phelps CD, Young LY (2006) Anaerobic arsenite oxidation by novel denitrifying isolates. Environ Microbiol 8:899–908
Salmassi TM, Venkateswaren K, Satomi M, Nealson KH, Newman DK, Hering G (2002) Oxidation of arsenite by Agrobacterium albertimagni, AOL15, sp. nov., isolated from hot creek, California. Geomicrobiol J 19:53–66
Santini JM, Sly L, Schnagl RD, Macy JM (2000) A new chemolithoautotrophic arsenite-oxidizing bacterium isolated from a gold mine: phylogenetic, physiological and preliminary biochemical studies. Appl Environ Microbiol 66:92–97
Santini JM, Sly LI, Wen A, Comrie D, Wulf-Durand P, Macy JM (2002) New arsenite-oxidizing bacteria isolated from Australian gold mining environments-phylogenetic relationships. Geomicrobiol J 19:67–76
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Stolz J, Basu P, Oremland R (2002) Microbial transformation of elements: the case of arsenic and selenium. Int Microbiol 5:201–207
Stolz JF, Basu P, Oremland RS (2010) Microbial arsenic metabolism: new twists on an old poison. Microbe 5:53–59
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Turner AW (1949) Bacterial oxidation of arsenite. Nature 164:76–77
Weeger W, Lievremont D, Perret M, Lagarde F, Hubert J-C, Leroy M, Lett M-C (1999) Oxidation of arsenite to arsenate by a bacterium isolated from an aquatic environment. BioMetals 12:141–149
Yoon IH, Chang JS, Lee JH, Kim KW (2009) Arsenite oxidation by Alcaligenes sp. strain RS-19 isolated from arsenic-contaminated mines in the Republic of Korea. Environ Geochem Health 31:109–117
Acknowledgements
The author SPB would like to thank University Grants Commission (UGC), New Delhi, India for D. S. Kothari post-doctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bachate, S.P., Khapare, R.M. & Kodam, K.M. Oxidation of arsenite by two β-proteobacteria isolated from soil. Appl Microbiol Biotechnol 93, 2135–2145 (2012). https://doi.org/10.1007/s00253-011-3606-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3606-7