Abstract
The genus Pycnoporus forms a cosmopolitan group of four species belonging to the polyporoid white-rot fungi, the most representative group of homobasidiomycetes causing wood decay. Pycnoporus fungi are listed as food- and cosmetic-grade microorganisms and emerged in the early 1990s as a genus whose biochemistry, biodegradation and biotechnological properties have since been progressively detailed. First highlighted for their original metabolic pathways involved in the functionalization of plant cell wall aromatic compounds to yield high-value molecules, e.g. aromas and antioxidants, the Pycnoporus species were later explored for their potential to produce various enzymes of industrial interest, such as hydrolases and oxidases. However, the most noteworthy feature of the genus Pycnoporus is its ability to overproduce high redox potential laccase—a multi-copper extracellular phenoloxidase—as the predominant ligninolytic enzyme. A major potential use of the Pycnoporus fungi is thus to harness their laccases for various applications such as the bioconversion of agricultural by-products and raw plant materials into valuable products, the biopulping and biobleaching of paper pulp and the biodegradation of organopollutants, xenobiotics and industrial contaminants. All the studies performed in the last decade show the genus Pycnoporus to be a strong contender for white biotechnology. In this review, we describe the properties of Pycnoporus fungi in relation to their biotechnological applications and potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The world of fungi offers a fascinating and seemingly endless wealth of biological diversity and forms a valuable resource. Almost 75,000 species of filamentous fungi are known to date, but there may be more than five million (Blackwell 2011). White-rot filamentous fungi form an important ecological group; they cause selective removal of lignin from wood and so have a high potential for biotechnological processes, particularly for lignocellulosic feedstock biorefinery applications. Among these fungi, the Polyporales group, including the genus Pycnoporus, is the most representative order of saprotrophic homobasidiomycetes causing wood decay, and its high lignocellulolytic potential is recognized (Alexopoulos et al. 1996). Pycnoporus is a genus closely related to Trametes, morphologically similar in all its characters, except for the conspicuous bright reddish-orange colour of its basidiocarp (Ryvarden 1991). This colour arises from the synthesis of various pigments of the phenoxazin-3-one type, including cinnabarin, tramesanguin and cinnabarinic acid (Sullivan and Henry 1971). Pycnoporus is morphologically characterized by an annual, sessile to effused-reflexed basidiocarp, a dimitic or trimitic hyphal system, generative hyphae with clamps, clavate 4-sterigmate basidia and cylindrical, slightly curved, hyaline smooth basidiospores (Ryvarden and Gilbertson 1994). Historically, four species were discerned based on their morphological characters (pore size of basidiocarp and basidiospore shape) and their distribution areas (Nobles and Frew 1962; Ryvarden and Johansen 1980): (1) Pycnoporus cinnabarinus, a common species distributed especially in the Northern Hemisphere, (2) Pycnoporus puniceus, a rare species distributed in Africa, India, Malaysia and New Caledonia (characterized by a basidiocarp with large irregular pores of 1–3/mm), (3) Pycnoporus sanguineus, a common species distributed in tropical and subtropical regions and (4) Pycnoporus coccineus, distributed in the countries bordering the Indian and Pacific Oceans. The Pycnoporus fungi are heterothallic homobasidiomycetes with a tetrapolar mating system (Nobles and Frew 1962). The basidiomycete life cycle (Fig. 1) shows a sexual development from haploid basidiopores, which produce, upon germination, a hypha with one nucleus per cell (monokaryon). Two monokaryotic strains with different mating alleles can fuse and produce a dikaryotic mycelium—characterized by clamp connections—in which the two parental nuclei do not fuse during the vegetative growth. Vegetative growth is maintained until environmental conditions induce fruit-body formation. After karyogamy and meiosis within the basidia, four uninucleate spores are produced and give rise to monokaryotic cell lines (Herpoël et al. 2000; Lomascolo et al. 2002).
Scheme of Pycnoporus life cycle: clamp connection (a), dikaryotic mycelium (b), fruit-body-like structure (c), basidia with basidiospores (d), monokaryotic cell line (e); solid line, sexual reproduction; dashed line, asexual reproduction. Adapted from Herpoël et al. (2000)
In the early 1980s, the species Phanerochate chrysosporium was historically the first white-rot model, characterized by a secondary metabolism pattern triggered by nitrogen limitation. Research was then gradually extended to other organisms, among which Pycnoporus emerged, in the middle of 1990s, as a genus whose biochemistry, biodegradation and biotechnological properties have since been progressively detailed. The exploration and use of the metabolic capacities of the genus Pycnoporus holds great promise for biotechnological applications in view of three main characteristics: (1) its ability to produce various copper and iron metalloenzymes involved in the transformation of plant cell wall aromatic compounds (Moukha et al. 1999; Halaouli et al. 2005), (2) original metabolic pathways involved in the functionalization of these cell wall aromatics to yield high added-value compounds including aromas and antioxidants (Lesage-Meessen et al. 1997; Estrada Alvarado et al. 2003) and (3) its ability to produce fruit-body structures and monokaryotic cell lines in laboratory culture conditions—very rare among basidiomycetes—enabling genetic improvement by both classical genetics and genetically modified-organism (GMO) methods (Alves et al. 2004): the isolation of monokaryotic cell lines offers an alternative to the construction of GMOs providing a simpler genetic and biochemical system than the dikaryotic state, the haploid state being easier to handle for mutagenesis and further transformations. Moreover, the enzymes from a non-GMO could also enjoy food-grade status if required for food or cosmetic applications.
The Pycnoporus fungi are white-rot basidiomycetes listed as food- and cosmetic-grade microorganisms. Pycnoporus fungi are not edible fungi in Europe, but they belong to the traditional pharmacopeia of countries in Africa and South America for the treatment of various illnesses and skin lesions (Smânia et al. 2003). In addition, cinnabarin from Pycnoporus fungi has been shown to display antiviral and antibacterial activities against undesirable food bacteria (Smânia et al. 2003) and human pathogenic bacteria such as Klebsiella pneumoniae and Salmonella typhi (Smânia et al. 1995). An active compound with Leishmanicidal activity was also isolated from P. sanguineus (Correa et al. 2006).
Physiologically and biotechnologically, the Pycnoporus fungi are easy to cultivate at laboratory and pilot-plant scales. High yields of biomass can be obtained on the following carbohydrate substrates: starch, malt extract, maltose, methyl cellulose, sucrose, dextrose and malt extract broth supplemented with yeast extract and/or phospholipids (Holler and Brooks 1980; Oddou et al. 1999). The glycolytic pathway and pentose-phosphate shunt both seem to be operative in Pycnoporus, although the glycolytic pathway is preponderant (Hirono et al. 1978). Pycnoporus species have been successfully grown, fully submerged, in 20- to 200-l packed-bed bioreactors (Lonergan et al. 1993, 1995) and in mechanically agitated 2- and 15-l fermentors (Stentelaire et al. 2000; Georis et al. 2003). The fungus was insensitive to fluctuations in both pH (although no buffering was used) and temperature and was capable to produce biomass rapidly. These properties fit Pycnoporus to industrial applications. Among basidiomycetes, members of the genus Pycnoporus have been shown especially to produce various enzymes of industrial interest, including hydrolases such as xylanase and β-glucosidase (Esposito et al. 1993), invertase (Quiroga et al. 1995), and α-amylase (De Almeida Siqueira et al. 1997). However, the most obviously useful feature of the genus Pycnoporus is its ability to overproduce high redox potential laccase—a multi-copper extracellular phenoloxidase— as the predominant ligninolytic enzyme (Eggert et al. 1996a; Lomascolo et al. 2003). A major promise of the Pycnoporus fungi lies in the use of their laccases for a variety of applications such as the bioconversion of agricultural by-products and raw plant materials into valuable products, the biopulping and biobleaching of paper pulp, and the biodegradation of organopollutants, xenobiotics and industrial contaminants. All the studies performed in this last decade support the genus Pycnoporus as a strong contender for white biotechnology. This review is the first to summarize these findings.
The genus Pycnoporus as a cell factory for the production of a wide range of enzymes
Hydrolases
Interest in the enzyme activities of Pycnoporus began thirty years ago when the first studies of carboxyl proteinase, chitinase and β-N-acetylhexosaminidase were published by Japanese authors (Table 1). The first enzyme, the carboxyl proteinase Ia from P. coccineus, was characterized by Ichishima et al. (1980): its major splitting sites, in the B chain of insulin, were Ala-Leu, His-Leu and Phe-Phe. The substrate specificity of this trypsinogen-activating carboxyl proteinase was especially investigated with angiotensin and proangiotensin (Kumagai et al. 1981). A serine carboxypeptidase was then isolated and purified from P. sanguineus (Ichishima et al. 1983) and displayed a preference for neutral aliphatic residues and glutamic acid in the penultimate peptide position.
Basidiomycetous fungi have been shown to have significant glycosyl hydrolytic potential and Pycnoporus is no exception (Gomez-Alarcon et al. 1989; Esposito et al. 1993). A chitinase and an N-acetyl-β-glucosaminidase, both enzymes required for the complete hydrolysis of chitin, were purified from P. cinnabarinus extracellular fluid (Ohtakara 1988). The P. cinnabarinus chitinase hydrolysed chitin acting on chitooligosaccharides, the mode of action being endo-type, predominantly hydrolysing the second β-N-acetylglucosaminide linkage from the non-reducing end (Ohtakara 1988). The β-N-acetylhexosaminidase identified in P. cinnabarinus hydrolysed chitooligosaccharides at the non-reducing end to N-acetylglucosamine, in an exo-type mode (Ohtakara et al. 1981a, b). Biotechnological applicability of Pycnoporus hydrolytic enzymes was further demonstrated in the case of glycosidases such as α- and β-galactosidases, α-amylase and β-glucosidase (Table 1). These enzymes are of industrial interest in sugar beet, starch and soymilk processing, in the dairy and fruit product industries and in structural studies of glycoproteins. The α-galactosidase from P. cinnabarinus was especially studied by Ohtakara et al. (1984), Mitsutomi et al. (1985), Ohtakara and Mitsutomi (1987), Mitsutomi and Ohtakara (1988) and Mitsutomi et al. (1991). This enzyme hydrolyses α-galactosyl linkages in oligo- and polysaccharides but also displays a galactosyltransferase activity (Mitsutomi and Ohtakara 1988). This highly thermostable α-galactosidase was purified and immobilized on colloidal chitin with glutaraldehyde (Mitsutomi et al. 1985) or on chitosan beads (Ohtakara and Mitsutomi 1987), keeping biochemical properties similar to the native enzyme. The immobilized enzyme was then successfully used for hydrolysis of raffinose in sugar beet molasses in a 30-day continuous process. Later, Mitsutomi et al. (1991) found that the P. cinnabarinus α-galactosidase was able to synthesize galactooligosaccharides, specifically the trisaccharides raffinose, planteose and 3Gα-galactosyl sucrose, from the condensation of galactose and sucrose. Such trisaccharides were expected to be utilized as a growth factor for bifidobacteria. In 1995, Quiroga et al. described, for the first time, the isolation and purification of a β-fructofuranosidase (or invertase) from P. sanguineus. The enzyme was able to attack sucrose, raffinose, stachyose, inulin and levan, sucrose being the preferred substrate (Table 1). Very recently, a novel exo-polygalacturonase, PGase I, was isolated from P. sanguineus grown on citrus fruit pectin (Quiroga et al. 2009), as an exocellular enzyme releasing galacturonic acid as its principal hydrolysis product. Such a pectinase has potential applications in the fruit, paper and textile industries, but also in protoplast fusion technology and plant pathology (Quiroga et al. 2009). Cellulolytic and hemicellulolytic activities were notably evidenced in P. sanguineus and P. cinnabarinus. An acidic α-d-mannosidase, stable up to 60°C, was isolated from liquid cultures of P. sanguineus (Ichishima et al. 1985) and was shown to specifically cleave the 1,2-α-linked side-chain of α-mannan. When cultured on wheat straw solid medium, P. sanguineus exhibited exo-glucanase activity on microcrystalline cellulose (Avicel), β-glucosidase activity on cellobiose and xylanase activity on oat spelt and birchwood xylans (Quiroz-Castañeda et al. 2009). These enzymatic activities resisted incubation for 1 h at high temperatures (up to 80°C) and were stable in the pH range of 2–8. Thermostable xylanase production (300–3,700 U/l) was obtained from P. cinnabarinus grown on natural substrates (cellulose powder, maize and wheat bran and sugar beet pulp) as carbon sources and increased in the presence of Tween 80 as surfactant (Sigoillot et al. 2002).
It is worth noting that the hydrolytic enzymes characterized from Pycnoporus generally exhibited high thermal stability, broad pH range activity and remarkable potential in various biotechnological applications. Surprisingly, these results were never taken further by genetic and molecular studies.
Oxidative enzymes
Laccase
Laccases (p-diphenol/oxygen oxidoreductases, EC 1.10.3.2) are multi-copper oxidases containing one type-1 copper, the redox potential of which determines the substrates to be oxidized, and three other copper atoms transferring the electrons to O2, further reduced to water. They catalyse the one-electron oxidation of a wide range of compounds including di-, substituted and polyphenols and di- and aromatic amines to form free radicals, which in turn can produce dimers, oligomers and polymers (Baldrian 2006). In the genus Pycnoporus, laccases are extracellular monomeric glycoproteins, produced in both submerged cultures (Eggert et al. 1996a; Lomascolo et al. 2003) and solid-state fermentation of agro-residues (Meza et al. 2006; Vikineswary et al. 2006). Laccase production depends on the cultivation conditions: carbon and nitrogen sources and concentrations (Eugenio et al. 2009), addition of surfactants such as Tween 80 (Gomez-Alarcon et al. 1989) and the presence of inducers and of metal ions such as copper (Hoshida et al. 2005). One of the most effective method for regulating and increasing Pycnoporus laccase production is the addition of an appropriate inducer to the medium, which may be aromatic (ferulic acid or 2,5-xylidine) or aliphatic (ethanol, methanol or dimethylsulfoxide), and lignocellulosic agro-residues (Herpoël et al. 2000; Jones et al. 2001; Lomascolo et al. 2002, 2003; Alves Garcia et al. 2006; Valeriano et al. 2009). Likewise, significant Pycnoporus intra-genus and intra-species diversity in laccase production has been reported. Wild Pycnoporus strains originating from various geographical areas, especially tropical habitats, have been shown to produce up to 17,000 U/l laccase, i.e. 65 mg/l (Lomascolo et al. 2002; Uzan et al. 2010). A monokaryotic strain, P. cinnabarinus CIRM-BRFM 137, was further identified as an outstanding overproducer of laccase (266,600 U/l, i.e. 1 g/l) in the presence of ethanol as inducer (Lomascolo et al. 2003). These activities were measured at pH 4 with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid (ABTS)) as substrate.
Until now, two genes of laccases, named lac1 (or lcc3-1) and lac 2 (lcc3-2), have been isolated from P. cinnabarinus and P. sanguineus (Eggert et al. 1998; Temp et al. 1999; Alves Garcia et al. 2006, 2007), but Lac 1 seemed to be the isoenzyme mainly expressed in induced culture conditions (Antorini et al. 2002; Lomascolo et al. 2003). Several Pycnoporus laccase genes and cDNAs have been cloned and sequenced (NCBI accession numbers AB072703, AB072704, AF025481, AF123571, AF152170, AF 170093, AY147188, AY458017, AY510604, FJ513007, FJ858749, FJ 858750 and FJ585751). These genes are DNA fragments of about 2.1 kbp interrupted by ten introns. The ORFs consist of 11 exons corresponding to sequences of about 1,600–1,900 nucleotides coding for proteins of 518 amino acids (aa) containing a signal peptide of 21 aa. The expression of laccase genes depends on the culture conditions, and a large panel of isoenzymes have been described in the literature (for an overview, see Table 1). The properties of Pycnoporus laccases are generally close to those of the high redox potential laccases from Trametes spp. More specifically, the main characteristics of Pycnoporus laccases are: molecular weight between 57 and 80 kDa, isoelectric point generally acidic but ranging from 3 to 7, Michaelis constant (ABTS as substrate) in the range of 26–239 μM (Table 1). Compared with other white-rot fungal enzymes, Pycnoporus laccases display useful biochemical features suitable for biotechnological applications, including high activity optimal temperature (50–65°C), high thermostability and tolerance towards salts and organic solvents (Table 1). Laccases are generally classified into three groups according to the redox potential of the type-1 copper centre: low (ca. 0.4–0.5 V), medium (ca. 0.5–0.6 V) or high (ca. 0.7–0.8 V) (Xu et al. 1996). Pycnoporus laccases obviously belong to the group with the highest redox potential, with values of 0.72–0.8 V (Sigoillot et al. 2004; Uzan et al. 2010). Also, the immobilization of Pycnoporus laccases on magnetic particles has been successfully achieved (Jiang et al. 2005; Whang et al. 2008). The immobilized enzymes exhibited remarkably improved catalytic capacity and stability properties for various parameters, such as pH, temperature, re-use and storage time, promising economic advantages for large-scale biotechnological applications.
Heterologous expression of the Pycnoporus laccase gene has been successfully performed in heterologous eucaryotic hosts including the yeasts Pichia pastoris, Yarrowia lipolytica and Saccharomyces cerevisiae (Otterbein et al. 2000; Hoshida et al. 2005; Mazdak et al. 2005; Romano et al. 2007) and the filamentous fungi Aspergillus niger and Aspergillus oryzae (Record et al. 2002; Hoshida et al. 2005). In addition, an efficient transformation and expression system was developed for P. cinnabarinus (Alves et al. 2005). This was used to transform the monokaryotic strain CIRM-BRFM 44 with the homologous lac1 gene (Alves et al. 2005). The yields ranged from a few mg per litre in yeasts to about 100 mg/l in A. niger and 1 g/l in P. cinnabarinus.
Tyrosinase
Tyrosinases (monophenol, o-diphenol:oxygen oxidoreductase, EC 1.14.18.1) are type-3 copper proteins involved in the initial step of melanin synthesis. These enzymes catalyse both the ortho-hydroxylation of monophenols and the subsequent oxidation of the resulting ortho-diphenols into reactive o-quinones, which evolve spontaneously to produce intermediates that associate in dark brown pigments. In fungi, tyrosinases are generally associated with the formation and stability of spores, in defence and virulence mechanisms, and in browning and pigmentation. First characterized from the edible mushroom Agaricus bisporus while addressing undesirable enzymatic browning problems during postharvest storage, tyrosinases were found more recently in several other fungi, with useful insights into molecular and genetic characteristics and reaction mechanisms, highlighting their very promising properties for biotechnological applications (Halaouli et al. 2006a). These applications remain limited because native fungal tyrosinases are generally intracellular and produced in low quantities. Halaouli et al. (2005) showed for the first time that several Pycnoporus strains were able to produce tyrosinase. The strain P. sanguineus CBS 614.73 was identified as the best tyrosinase producer, with tyrosinase production of 45.4 and 163.6 U/g protein per day for monophenolase and diphenolase, respectively. This tyrosinase was monomeric and intracellular; it was purified and characterized (Table 1) and stood apart from other source tyrosinases so far reported in its N-terminal amino acid sequence, kinetic parameters and thermal stability. This tyrosinase proved effective in the synthesis of natural antioxidants and in protein cross-linking (Halaouli et al. 2005). The corresponding tyrosinase-encoding gene (2,204 bp) and cDNA (1,857 nucleotides) were cloned from P. sanguineus CBS 614.73. This gene consisted of seven exons and six introns and encoded a predicted protein of 68 kDa, exceeding the mature tyrosinase by 23 kDa (C-terminal proteolytic cleavage). P. sanguineus tyrosinase cDNA was over-expressed in A. niger, under the control of the strong and constitutive glyceraldehyde-3-phosphate-dehydrogenase promoter. The glucoamylase preprosequence of A. niger was used to target the secretion. This construction enabled the production, for the first time, of a fully active recombinant tyrosinase in the extracellular medium of A. niger in a yield of ca. 20 mg l−1 (Halaouli et al. 2006b).
Cellobiose dehydrogenase
Cellobiose dehydrogenase (CDH) is an extracellular oxidoreductase that contains both a protoporphyrin-IX-based heme and a flavin prosthetic group. It is a bifunctional enzyme containing a cellulose-binding domain. It was suggested that CDH could degrade cellulose, hemicellulose and lignin and could participate in wood degradation by white-rot fungi via the generation of highly reactive hydroxyl radicals involved in demethylation of lignin. A CDH gene was cloned from P. cinnabarinus for the first time by Moukha et al. (1999). mRNA-encoding P. cinnabarinus CDH was shown to be induced by cellulose and relatively repressed by cellobiose or glucose in the culture medium. The corresponding protein (92 kDa) was further purified and characterized by Sigoillot et al. (2002) (Table 1).
Others
A partial sequence of a lignin peroxidase-like gene was amplified for the first time in the strain P. sanguineus CBS 614.73 (Pointing et al. 2005). Several partial lip- and mnp-like sequences were obtained from P. cinnabarinus in the context of a classification study of peroxidases in the orders Hymenochaetales and Polyporales (Morgenstern et al. 2010). However, members of the genus Pycnoporus have never yet been shown to produce any detectable peroxidase during cultivation on a defined growth medium. However, almost all studies on lignin-modifying enzymes produced by Pycnoporus spp. have been performed in more or less synthetic liquid media which is not the most favourable condition to detect peroxidase-type activity. Very recently, Liers et al. (2011) have demonstrated the presence of manganese-oxidizing peroxidase activities for P. cinnabarinus during beech-wood colonization in solid-state microcosme cultures.
Biotechnological applications
The genus Pycnoporus as biological tool for aromatic compound functionalization: utilization and transformation of cell wall aromatic compounds into high added-value products
In the past the only natural sources of flavours were plants. However, active components often occur in tiny amounts and only in exotic plants, making isolation difficult and products expensive. The white-rot basidiomycetes, and especially the genus Pycnoporus, represent major biotechnological agents for generating, de novo or by bioconversion, natural aromas for industry when grown in standard media or in the presence of precursors (Asther et al. 1998; Lomascolo et al. 2002). The high demand for natural aroma makes vanillin production by biotransformation a viable alternative to natural and chemical sources. Ferulic acid (4-hydroxy 3-methoxycinnamic acid), a component of plant cell walls with a chemical structure close to that of vanillin, is efficient as a precursor for the production of vanillin. Among 300 food-grade strains of basidiomycetes, the wild dikaryotic strain I-937 of P. cinnabarinus was selected for its ability to produce 64 mg/l vanillin after 6 days of culture from 300 mg/l ferulic acid, with a molar yield of 27.5% (Gross et al. 1991). The metabolism of ferulic acid into vanillin by P. cinnabarinus I-937 has been established. Vanillic acid, the major degradation product of ferulic acid, is produced by the loss of two carbon atoms from the propenoic chain, and is further metabolized into methoxyhydroquinone through an oxidative decarboxylation or into vanillin and vanillyl alcohol through reductive pathways (Falconnier et al. 1994) (Fig. 2). The major intermediates of [5-2H]-ferulic acid biotransformation by P. cinnabarinus have been identified; the phenyl propenoic side-chain degradation of ferulic acid appears to be analogous to fatty acid β-oxidation (Krings et al. 2001). The biotransformation yield of vanillin from ferulic acid was lowered by three unwanted by-pass pathways (Fig. 2, pathways 1, 2 and 3) that could be successfully controlled by: (1) the selection of monokaryotic laccase-deficient P. cinnabarinus strains, using formal genetics (Lesage-Meessen et al. 1996), (2) the addition of cellobiose to fungal cultures to channel the flow of vanillic acid through the reductive pathway (inhibition of pathway 2) (Lesage-Meessen et al. 1997) and (3) the addition of a selective adsorbent (a hydrophobic cross-linked polystyrene copolymer resin named XAD-2), to trap vanillin before its bioconversion into vanillyl alcohol (Stentelaire et al. 1998). In these conditions, vanillin concentration reached 500 mg/l, with a molar yield of 47%, and vanillin can be easily recovered by desorption from resin using ethyl alcohol (Stentelaire et al. 2000). In addition, a mechanistic model for vanillin production from vanillic acid by P. cinnabarinus grown in a 2-l bioreactor was established to predict the evolution of the variables during the growth and biotransformation phases (inoculum, mode and addition time of precursor and cellobiose, aeration and XAD-2 addition). The complete procedure finally led to the setting-up of a simulation model for the process, and the results were supported by the data from five cultures of P. cinnabarinus (Bernard et al. 1999). High-density cultures of P. cinnabarinus were tested for the optimization of ferulic acid bioconversion into vanillin. A sixfold increase in biomass using glucose-phospholipid mixture as carbon source instead of maltose allowed 760 mg/l vanillin to be produced from ferulic acid in 15 days with a molar yield of 61% (Oddou et al. 1999).
Joint work by various laboratories (EC contract FAIR CT 96–1099) allowed complete processes to be set up to produce natural vanillin from abundant, cheap European agro-industrial by-products (less than 0.2 €/t dry wt.), rich in ferulic acid, such as sugar beet pulp (residues from the sugar industry containing 0.8% dry wt. ferulic acid) and maize brans (residue from the starch industry containing up to 5.5% dry wt. ferulic acid) (Lesage-Meessen et al. 2002). A new two-step process for the production of pure vanillin from autoclaved maize bran has been designed with a limited number of steps involving Aspergillus niger and P. cinnabarinus. For process economy, two strategies were defined using autoclaved maize bran (Fig. 3). In the first one, the potentialities of A. niger grown on sugar beet pulp to produce high levels of polysaccharide-degrading enzymes, including feruloyl esterases, and to transform ferulic acid into vanillic acid were successfully combined for the release of free ferulic acid from autoclaved maize bran (Bonnin et al. 2002). Vanillic acid was then recovered and efficiently transformed into vanillin by the monokaryotic strain of P. cinnabarinus MUCL 39533: 767 mg/l of biotechnological vanillin could be produced in the presence of cellobiose and XAD-2 resin after a 14-day bioconversion with a molar yield of 71% (Fig. 3a). In the second strategy, 3-day-old high-density cultures of P. cinnabarinus MUCL 39533 were fed with the autoclaved maize bran as a ferulic acid source and A. niger culture filtrate as an extracellular enzyme source. Under these conditions, P. cinnabarinus was shown to biotransform free ferulic acid released from the autoclaved maize bran by A. niger enzymes directly into 584 mg/l vanillin on day 11 with a molar yield of 22% (Fig. 3b). These patented processes (Lesage-Meessen et al. 1995; Bonnin et al. 2000), involving physical, enzymatic and fungal treatments, allowed natural crystalline vanillin, characterized by X-ray diffraction, to be produced from autoclaved maize bran with no purification step (Lesage-Meessen et al. 2002). Such vanillin may be considered “natural” according to the European and US legislations, i.e. originating from a natural source and obtained by enzymatic or fungal transformation (EC directive 88/388, OJ N0. L 184, 15 July 1988). Zheng et al. (2007) developed a similar process that would convert ferulic acid prepared from waste residues of rice bran oil into vanillin using the combination of the biotransformation potentials of A. niger and P. cinnabarinus, previously described. The yield of vanillin reached 2.8 g/l when 5 g/l glucose and 25 g of HZ802 resin were added to the bioconversion medium.
In addition, P. cinnabarinus was shown to be able to convert p-coumaric acid, a hydroxycinnamic acid found covalently esterified to polysaccharides and lignin in plant cell walls, to p-hydroxybenzaldehyde, a component of high organoleptic value present in natural vanilla bean extracts (Estrada Alvarado et al. 2001). The use of a phospholipid-enriched medium induced high-density fungal cultures, which produced 155 mg/l p-hydroxybenzaldehyde on culture day 13 with a molar yield of 26%. Based on the different metabolites identified, metabolic pathways of p-coumaric acid were suggested. As previously suggested for ferulic acid (Falconnier et al. 1994; Krings et al. 2001), an oxidative side-chain degradation of p-coumaric acid led to p-hydroxybenzoic and protocatechuic acids, which were then reduced to their corresponding aldehydes and alcohols. Additionally, a reductive pathway of p-coumaric acid occurred, leading to 3-(4-hydroxyphenyl)-propanol as the terminal product, as already reported in Ischnoderma benzoinum (Krings et al. 1996).
Among aromatic compounds of industrial interest, benzaldehyde (bitter almond aroma) is, like vanillin, widely used in the food and cosmetic industries. The strain P. cinnabarinus MUCL 39533 was shown to produce, in a 2-l bioreactor, 100 mg/l benzaldehyde from l-phenylalanine as precursor (Lomascolo et al. 1999). The addition of HP20 resin, a styrene divinylbenzene copolymer highly selective for benzaldehyde, proved an efficient strategy to enhance benzaldehyde production up to 790 mg/l. Described as the organoleptic flavour of orange blossom and wood strawberry, methyl anthranilate (o-aminobenzoic acid methyl ester) is widely used in the food and perfumery industries, but there is no readily available source of this compound, owing to its extremely low concentrations in plants. P. cinnabarinus has been reported to produce methyl anthranilate de novo in culture conditions combining low nitrogen concentration, maltose as carbon source and uncontrolled culture pH (Gross et al. 1990).
Over the past 25 years, in view of the high demand for natural ingredients, particular attention has been directed to the antioxidant properties of hydroxycinnamic acids, due to their occurrence in nature and their radical scavenging activity. The antioxidant activity of natural phenolic acids depends on the number and relative position of the hydroxyl groups on the ring, which give them reducing properties and hydrogen-donating abilities (Rice-Evans et al. 1996). The cinnamic acids dihydroxylated in the 3,4 position, such as caffeic acid, have a higher radical-scavenging ability than monophenolics like p-coumaric acid. Fungal transformation of p-coumaric acid into caffeic acid, potentially a strong antioxidant, has been evidenced in P. cinnabarinus cultures grown with high p-coumaric acid feeding rates. Thus feeding 450 mg/l p-coumaric acid to P. cinnabarinus cultures grown on glucose medium resulted in the production of 257 mg/l caffeic acid with a molar yield of 21%. Under these conditions, a p-coumaric hydroxylating pathway rarely described in fungi was induced at the expense of p-hydroxybenzaldehyde (Estrada Alvarado et al. 2003).
Lignin degradation, pretreatment of lignocellulosic biomass, pulp and paper applications
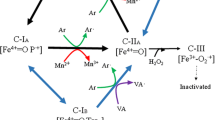
The lignin polymer is highly recalcitrant towards chemical and biological degradation due to its molecular architecture, where different phenolic and non-phenolic units form a complex three-dimensional network linked by a variety of ether and carbon–carbon bonds. The non-phenolic substructures represent about 90% of total lignin and are more recalcitrant to degradation than the phenolic ones. Lignin removal is thus a central aspect in industrial uses of lignocellulosic biomass, such as bioethanol production and manufacture of cellulose-based chemicals and materials, including paper. Geng and Li (2002) showed that P. cinnabarinus was capable of oxidatively degrading both phenolic and non-phenolic lignins. The authors evidenced that after incubation of the fungus and lignin preparations for 3 months, over 40% of the non-phenolic lignin and about 70% of the phenolic lignin substructures were degraded. The degradation rate of phenolic lignins was greatly enhanced by the presence of hydroxyl groups and was faster than that of the non-phenolic lignins. According to the studies of Eggert et al. (1997) and Bermek et al. (1998), laccase is essential for lignin degradation and pulp bleaching by P. cinnabarinus. However, Pycnoporus laccase alone cannot oxidize non-phenolic lignin model compounds in vitro (Eggert et al. 1996b; Uzan et al. 2010). For a while, 3-hydroxyanthranilic acid had been thought to be the natural mediator acting in vivo but it remains a controversial question (Li et al. 2001) until now. Uzan et al. (2010) described the oxidation of monomeric and dimeric non-phenolic lignin model compounds such as veratryl alcohol and adlerol by P. sanguineus laccase, testing different natural and synthetic redox mediators at different pH. 1-Hydroxybenzotriazole (HBT), a synthetic mediator, was the most efficient redox mediator and allowed 100% oxidation of veratryl alcohol into veratraldehyde and 86% oxidation of adlerol into adlerone. Given these findings, Pycnoporus fungi are microorganisms of interest for the pretreatment of recalcitrant lignocellulosic biomass, which is the most important step required to remove lignin, and hold potential in utilizing plant residues for the saccharification of cellulosics and the synthesis of biofuels. The biological delignification of tropical lignocellulosic feeedstocks from Prosopsis juliflora and Lantana camara was carried out with P. cinnabarinus under solid-state fermentation conditions with a pre-treatment scalability up to 500 g of substrate (Gupta et al. 2011). The fungal fermentation with 10 g of substrate optimally delignified P. juliflora by 11.9% and L. camara by 8.4% and enriched their holocellulose content by 3.3% and 4.9%, respectively.
Through their enzyme panel, basidiomycetes, and especially the genus Pycnoporus, are also able to degrade the lignin content of wood pulp, and this feature is very promising for the paper pulp industry, as it could allow a substantial reduction in the use of chlorine-containing bleaching agents, thereby reducing their environmental impact. In this context, Herpoël et al. (2002) have developed a biotechnological process using a xylanase and a P. cinnabarinus laccase for the delignification of wheat straw pulp. The pulp was delignified by about 47% (compared with untreated pulp). A reduction in the consumption of chlorine chemicals in the bleaching sequence, and a subsequent diminution of the chemical oxygen demand (COD) by about 60%, has also been demonstrated. Enzymatically treated pulp was bleached to 69% ISO brightness by a treatment sequence without addition of chlorinated compounds. To determine the economic feasibility of such an enzymatic process, Sigoillot et al. (2004) produced a recombinant Pycnoporus laccase by heterologous expression in A. niger. The same promising results (75% delignification of wheat straw kraft pulp) were obtained, showing that a recombinant enzyme-based technology could be considered for reducing process costs. These types of process included redox mediators such as HBT and are called laccase mediator systems (LMS) (Fig. 4). Successful flax alkaline pulp delignification and bleaching could also be achieved by using wild laccases from P. cinnabarinus in the presence of HBT as a mediator (Camarero et al. 2004). Up to 20% ISO brightness increase was attained after laccase-HBT treatment, and a decrease in the kappa number from 9 to 3 was simultaneously observed. P. cinnabarinus laccase plus HBT gave the best selectivity in lignin removal, determined by the ratio between the decrease in kappa number and pulp viscosity. It is noteworthy that HBT is one of the best laccase redox mediator but (1) it is relatively expensive in terms of bulk applications (such as in the pulp and paper sector), and (2) it can be converted into toxic/mutagenic products during laccase catalysis. The current challenge is now to find cheap and natural mediators. For instance, natural mediators such as syringaldehyde and acetosyringone have been found to perform well for dye decolorization by Pycnoporus laccase (Camarero et al. 2005), opening the way to eco-friendly (and potentially cheap) treatments. In another study, an LMS, formed by a P. sanguineus fluid enriched in laccase and acetosyringone as mediator proved to be an effective bleaching booster of a Eucalyptus globulus kraft pulp totally chlorine-free sequence (Eugenio et al. 2010). This LMS enabled the reduction of hydrogen peroxide load from 94% to 87.4% and the increase in the final brightness from 51% to 59% ISO, compared with conventional bleaching. Ravalason et al. (2009) performed the fusion of a family 1 carbohydrate-binding module (CBM) of A. niger cellobiohydrolase B to the laccase of P. cinnabarinus CIRM-BRFM 137 for efficient softwood kraft pulp biobleaching. The chimeric enzyme laccase-CBM was able to bind to a cellulosic substrate and, to a greater extent, to softwood kraft pulp. Addition of a CBM was shown to greatly improve the delignification capabilities of the laccase in the presence of HBT as mediator. In addition, chlorine dioxide reduction using 5 U of chimeric enzyme/g of pulp was almost twice than that observed using 20 U of P. cinnabarinus laccase/g of pulp. Conferring a carbohydrate-binding capability to the laccase could significantly enhance the biobleaching properties of the enzyme (Ravalason et al. 2009).
In order to develop a new integrated process, Meza et al. (2006) combined (1) the production of laccase, an enzyme usable to bleach paper pulp, (2) the delignification of sugarcane bagasse, a residue of sugar production that can be recycled in the paper industry and (3) the depollution of gaseous effluents such as ethanol, known to be a strong laccase inducer (Lomascolo et al. 2003). Ethanol vapour was blown up through a vapour phase bioreactor packed with bagasse and inoculated with the high redox potential laccase from P. cinnabarinus CIRM-BRFM 137. After 28 days, bagasse delignification reached 12%. The fungus-treated bagasse was pulped and refined. An improvement of 35% in the mechanical characteristics (tensile index, breaking strength) of the paper sheets was obtained from biotreated bagasse, with a reduction of 50% in the energy necessary to delignify the pulp. All these results show that enzymatic treatment using Pycnoporus has great potential for pulp biobleaching.
Bioremediation, decolourization and detoxification of industrial effluents
Pycnoporus fungi secrete laccases that present a low specificity with regard to substituted aromatic structures and are thus of great interest in wastewater decolourization and detoxification (Thurston 1994). The use of Pycnoporus laccase-based techniques for these processes can make bioremediation cost-effective and environmentally friendly. Pycnoporus fungi especially demonstrated their ability to decolourize several classes of dyes. Schliephake et al. (1993) first showed the degradation of the diazo dye Chicago Sky Blue by P. cinnabarinus in a packed-bed reactor. The degradation was due to the laccase produced by the strain P. cinnabarinus tested (Schliephake et al. 2000). Several studies highlighted the efficiency of Pycnoporus laccases to degrade azo, triphenylmethane and anthraquinonic dyes (Pointing et al. 2000). Total degradation of reactive blue 38, acid blue 74, reactive blue 19, aniline blue and reactive black 5 was obtained with the P. cinnabarinus CIRM-BRFM 137 laccase in the presence of different synthetic or natural mediators (Camarero et al. 2005). Trovaslet et al. (2007) showed high percentages of decolourization obtained by P. cinnabarinus and P. sanguineus laccases on several dyes such as acid blue 62, acid orange 7 and acid yellow 36 without the addition of a redox mediator (respectively, 80%, 60% and 52% decolourization). Uzan et al. (2010) elected to test the ability of purified laccases from P. coccineus and P. sanguineus to decolourize a varied range of polycyclic dyes, including, for the first time, Poly-R 478 (a surrogate substrate for lignin biodegradation). The authors showed decolourization even in the absence of a redox mediator. The decolourization of the anthraquinone dye remazol brilliant blue R (RBBR) was consistent with the results of Lu et al. (2007) that showed 94% of RBBR decolourization after incubation at 40°C with a P. sanguineus laccase without additional redox mediator. Natural mediators, such as syringaldehyde and acetosyringone, were also found to favour dye decolourization by Pycnoporus laccases (Camarero et al. 2005), opening the way to eco-friendly treatments.
Olive oil mill wastewater (OOMW) are pollutant by-products of the olive oil industry. The fungus P. coccineus was selected among a wide range of white-rot fungi for its ability to grow on OOMW, without any additional carbon source, strongly decreasing colour and COD in this industrial effluent (Jaouani et al. 2003) as an alternative to physico-chemical methods of detoxification. Oxidation of monomeric phenolic compounds in the different OOMW fractions by the P. coccineus laccase could produce radicals leading to polymerization (Jaouani et al. 2005). Similar results have been reported for a P. coccineus laccase immobilized on the acrylic epoxy-activated Eupergit C resin (Berrio et al. 2007). Gel filtration profiles of the OOMW treated with this enzyme showed both degradation and polymerization of the phenolic compounds.
Abilities of the white-rot basidiomycete Pycnoporus are also very extensive and very attractive in oxidation of several compounds such as polycyclic aromatic hydrocarbons and halogenated derivatives to decrease the toxicity, mutagenicity and carcinogenic properties of these substances. In this context, biotransformation of benzo[a]pyrene in a bench-scale reactor using laccase of P. cinnabarinus has been studied (Rama et al. 1998). The authors showed that benzo[a]pyrene concentration was strongly decreased within 3 h in the presence of purified laccase and ABTS as mediator; after 24 h of incubation, most (95%) of this compound was converted into 1,6-, 3,6- and 6,12-quinones in a 2:1:1 ratio. These quinoid-free metabolites proved to be a substrate for microbial populations and were mineralized to carbon dioxide.
Polyvinyl alcohol, a synthetic polymer used in the paper coating and textile industries, can also be degraded by P. cinnabarinus (Larking et al. 1999). Another study carried out by Hundt et al. (2000) showed the potential of Pycnoporus to biotransform triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether), an antimicrobial compound used in deodorants, soaps and dentifrices. After 48–72 h of incubation in the culture medium of P. cinnabarinus, triclosan began to disappear from the supernatant. The metabolites produced were substantially less cytotoxic than triclosan.
The presence of industrial effluents containing heavy metals poses serious environmental problems as they are toxic even at low concentration. White-rot basidiomycetes, and especially the genus Pycnoporus, have been used for the removal of heavy metals from aqueous solutions, because of their high tolerance towards these compounds and other adverse conditions (such as low pH), high cell wall binding capacity and high intracellular metal uptake capacity (Yahaya et al. 2009). The following studies suggest that Pycnoporus can be used as a low cost, efficient and easily regenerated biosorbent (Zulfadhly et al. 2001) for heavy metals that occur in the environment either naturally (e.g. Cu), or as a result of human activities (e.g. Cd, Hg or Pb). For instance, Mashitah et al. (1999) investigated the capacity of P. sanguineus to remove copper ions from aqueous solutions. The authors clearly demonstrated that the sorption capacity for copper increased up to about 10 mg/g of biomass. Yahaya et al. (2009) further showed that copper removal could be improved by the immobilization of P. sanguineus cells on alginate beads. Lead Pb(II) biosorption onto immobilized cells of P. sanguineus has also been studied (Azila et al. 2008). At pH 4 and 10 g/l of biosorbent, the immobilized cells of this fungus can remove 97.7% of 200 mg/l of Pb(II) ions. Similarly, Mashitah et al. (2008) have investigated the biosorption of cadmium (II) ions from aqueous solution onto immobilized cells (alginate beads) of P. sanguineus in a batch system. It reached 1.36 mg of Cd/g of biomass at pH 6 and 3 g of biomass loading.
Biopolymer synthesis
A recent study was carried out using Pycnoporus laccase as a catalyst to develop new potential natural active ingredients from rutin (quercetin-3-rutinoside, one of the best-known naturally occurring flavonoid glycosides) for cosmetic applications (Uzan et al. 2011). Rutin bioconversion reached about 67% for Pycnoporus laccases after 24 h incubation. New flavonoid oligomers were synthesized such as dimers and trimers of rutin with one or two ortho-quinone moieties or none. These innovative oligorutins, suitable for cosmetic applications, provided some protection against oxidative and inflammatory damage (superoxide radical scavenging activity, inhibitory effects on the cyclooxygenase COX-2 and the human matrix metalloproteinase 3 MMP-3). Laccase-catalysed derivatization may offer a promising route to new medically valuable structures. In addition, laccase from P. cinnabarinus can derivatize azoles with hydroxyl benzoic acid methyl or ethyl esters, leading to oligomer products with more and stronger biological activities (antimicrobial activites and stronger cytotoxicity) than the corresponding monomers (Hahn et al. 2010).
Oxidative enzymes from Pycnoporus (laccase from P. cinnabarinus and tyrosinase from P. sanguineus) were also successfully used for the synthesis of biopolymers suitable for the food industry from, for instance, agro-residues such as sugar beet pulp or cereal brans. Ferulic acid has been found ester linked to cell wall polysaccharides in sugar beet pectins or cereal brans such as arabinofuranose residues in maize bran (Saulnier and Thibault 1999). Consequently, these polysaccharide chains are able to gel through ferulic acid covalent cross-linking with oxidizing systems such as laccases. P. cinnabarinus laccase was used for the oxidative gelling of sugar beet pectins (Micard and Thibault 1999), soluble wheat arabinoxylans (Figueroa-Espinoza and Rouau 1998) and maize bran arabinoxylans (de Wilde et al. 2008). In the latter case, the gels were heatproof and resistant to freezing down to −20°C as well as to moderate acidic conditions (de Wilde et al. 2008). The P. sanguineus CBS 614.73 tyrosinase has been shown to be very effective in the cross-linking of casein, chosen as a model protein substrate for food applications (Halaouli et al. 2005). The use of these enzymes is eco-friendly and food-compatible, since only O 2 (i.e. air and agitation) being required for the cross-linking process.
Conclusions
Pycnoporus fungi have become model lignolytic basidiomycetes whose physiological and biochemical properties have been studied in detail, mainly in liquid media but also in solid-state fermentations. These fungi have demonstrated a significant potential in both white and green biotechnologies. This review is the first attempt to collect the scattered information on Pycnoporus species, spread throughout the literature in over 30 years and provides an exhaustive treatment focused on biochemical and biotechnological issues. The Pycnoporus genus comes out as a promising microorganism of choice applicable to enzyme biotechnologies, specifically as a producer of various hydrolase and oxidase activities and as a robust biodegradation agent. The lignolytic system of this group is composed mainly of high redox potential blue laccases, easy to produce and purify on a large scale. The remarkable properties of these laccases make the enzymes suitable for a variety of applications such as the bioconversion of agricultural by-products and raw plant materials into valuable products, the biopulping and biobleaching of paper pulp, and the biodegradation of organopollutants, xenobiotics and industrial contaminants. In addition, classical and molecular genetic engineering of the genus Pycnoporus is now well established in laboratory conditions, opening the way to applicable industrial processes.
References
Alexopoulos CJ, Mims CW, Blackwell M (1996) Phylum Basidiomycota order Aphyllophorales, polypores, Chantharelles, tooth fungi, coral fungi and corticioids. In: Harris D (ed) Introductory Mycology, 4th edn. New York, USA, Wiley and sons Inc, pp 563–597
Alves Garcia T, Fontes Santiago M, José Ulhoa C (2006) Properties of laccases produced by Pycnoporus sanguineus induced by 2,5-xylidine. Biotechnol Lett 28:633–636
Alves Garcia T, Fontes Santiago M, José Ulhoa C (2007) Studies on the Pycnoporus sanguineus CCT-4518 laccase purified by hydrophobic interaction chromatography. Appl Microbiol Biotechnol 75:311–318
Alves AMCR, Record E, Lomascolo A, Sigoillot J-C, Asther M, Wosten H (2005) Method for overproducing a specific recombinant protein with P. cinnabarinus monokaryotic strains. European patent WO2005073381
Alves A, Record E, Lomascolo A, Scholtmeijer K, Asther M, Wessels JGH, Wosten HAB (2004) Highly efficient production of laccase in the basidiomycete Pycnoporus cinnabarinus. Appl Env Microbiol 70:6379–6384.
Antorini M, Herpoël-Gimbert I, Choinowski T, Sigoillot JC, AstherM WK, Piontek K (2002) Purification, crystallisation and X-ray diffraction study of fully functional laccases from two lignolytic fungi. Biochim Biophys Acta 1594:109–114
Asther M, Lomascolo A, Mi A, Moukha S, Lesage-Meessen L (1998) Metabolic pathways of biotransformation and biosynthesis of aromatic compounds for the flavour industry by the basidiomycete Pycnoporus cinnabarinus. Mycologia Neotropical Aplicada 11:69–76
Azila YY, Mashitah MD, Bhatia S (2008) Process optimization studies of lead (Pb(II)) biosorption onto immobilizs cells of Pycnoporus sanguineus using response surface methodology. Bioresour Technol 99:8549–8552
Baldrian P (2006) Fungal laccases—occurrence and properties. FEMS Microbiol Rev 30:215–242
Bermek H, Li H, Eriksson KEL (1998) Laccase-less mutants of the white-rot fungus Pycnoporus cinnabarinus cannot delignify kraft pulp. J Biotechnol 66:117–124
Bernard O, Bastin G, Stentelaire C, Lesage-Meessen L, Asther M (1999) Mass balance modelling of vanillin production from vanillic acid by cultures of the fungus Pycnoporus cinnabarinus in bioreactors. Biotechnol Bioeng 65:558–571
Berrio J, Plou FJ, Ballesteros A, Martinez AT, Martinez MJ (2007) Immobilization of Pycnoporus coccineus laccase on Eupergit C: stabilization and treatment of olive oil mill wastewaters. Biocatal Biotransform 25:130–134
Blackwell M (2011) The fungi: 1, 2, 3 …5.1 million species ? Am J Bot 98:426–438
Bonnin E, Lesage-Meessen L, Asther M, Thibault J-F (1999) Enhanced bioconversion of ferulic acid into vanillin by the use of natural cellobiose. J Sci Food Agric 79:484–486
Bonnin E, Lesage-Meessen L, Stentelaire C, Asther M, Thibault J-F (2000) Method for obtaining dollar I (A. niger) cultures and their uses for producing ferulic acid and vanillic acid. European Patent EP1171574
Bonnin E, Saulnier L, Brunel M, Marot C, Lesage-Meessen L, Asther M, Thibault JF (2002) Release of ferulic acid from agroindustrial by-products by the cell wall-degrading enzymes produced by Aspergillus niger I-1472. Enzyme Microb Technol 31:1000–1005.
Camarero S, Garcia O, Vidal T, Colom J, del Rio CJ, Gutierrez AM, Gras J, Monje R, Martinez MJ, Martinez AT (2004) Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microb Technol 35:113–120
Camarero S, Ibarra D, Martinez MJ, Martinez A (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71:1775–1784
Camarero S, Canas AI, Nousiainen P, Record E, Lomascolo A, Martinez MJ, Martinez AT (2008) p-hydroxycinnamic acids as natural mediators for laccase oxidation of recalcitrant compounds. Environ Sci Technol 42:6703–6709
Correa E, Cardona D, Quinones W, Torres F, Franco AE, Vélez ID, Robledo S, Echeverri F (2006) Leishmanicidal activity of Pycnoporus sanguineus. Phytotherap Res 20:497–499
Dantan-Gonzalez E, Vite-Vallejo O, Martinez-Anaya C, Mendez-Sanchez M, Gonsalez MC, Palomares LA, Mallol JF (2008) Production of two novel laccase isoforms by a thermotolerant strain of Pycnoporus sanguineus isolated from an oil-polluted tropical habitat. Int Microbiol 11:163–169
De Almeida Siqueira EM, Mizuta K, Giglio JR (1997) Pycnoporus sanguineus: a novel source of α-amylase. Mycol Res 2:188–190
De Wilde C, Uzan E, Zhou Z, Kruus K, Andberg M, Buchert J, Record E, Asther M, Lomascolo A (2008) Transgenic rice as novel production system for Melanocarpus and Pycnoporus laccases. Transgenic Res 17:515–527
Eggert C, Temp U, Dean JFD, Eriksson KEL (1995) Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEBS Lett 376:202–206
Eggert C, Temp U, Eriksson KEL (1996a) The lignolytic system of the white-rot fungus Pycnoporus cinnabarinus: purification and characterization of the laccase. Appl Environ Microbiol 62:1151–1158
Eggert C, Temp U, Dean JFD, Eriksson KEL (1996b) A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett 391:144–148
Eggert C, Temp U, Dean JFD, Eriksson KEL (1997) Laccase is essential for lignin dégradation by the white-rot fungus Pycnoporus cinnabarinus. FEBS Lett 407:89–92
Eggert C, Lafayette P, Temp U, Eriksson K-EL, Dean JFD (1998) Molecular analysis of a laccase gene from the white-rot fungus Pycnoporus cinnabarinus. Appl Environ Microbiol 6:1766–1772
Esposito E, Innocentini-Mei LH, Ferraz A, Canhos VP, Duran N (1993) Phenoloxidases and hydrolases from Pycnoporus sanguineus (UEC-2050 strain): applications. J Biotechnol 29:219–228
Estrada Alvarado I, Lomascolo A, Navarro D, Delattre M, Asther M, Lesage-Meessen L (2001) Evidence of a new biotransformation pathway of p-coumaric acid into p-hydroxybenzaldehyde in Pycnoporus cinnabarinus. Appl Microbiol Biotechnol 57:725–730
Estrada Alvarado I, Navarro D, Record E, Mi A, Asther M (2003) Fungal biotransformation of p-coumaric acid into caffeic acid by Pycnoporus cinnabarinus: an alternative for producing a strong natural antioxidant. World J Microbiol Biotechnol 19:157–160
Eugenio ME, Carbajo JM, Martin JA, Gonzalez AE, Villar JC (2009) Laccase production by Pycnoporus sanguineus under different culture conditions. J Basic Microbiol 49:433–440
Eugenio ME, Santos SM, Carbajo JM, Martin JA, Martin-Sampedro R, Gonzales AE, Villar JC (2010) Kraft pulp biobleaching using an extracellular enzymatic fluid produced by Pycnoporus sanguineus. Bioresour Technol 101:1866–1870
Falconnier B, Lapierre C, Lesage-Meessen L, Yonnet G, Brunerie P, Colonna Ceccaldi B, Corrieu G, Asther M (1994) Vanillin as a product of ferulic acid biotransformation by the white-rot fungus Pycnoporus cinnabarinus I-937: identification of metabolic pathways. J Biotechnology 37:123–132
Figueroa-Espinoza MC, Rouau X (1998) Oxidative cross-linking of pentosans by a fungal laccase and horseradish peroxidase: mechanism of linkage between feruloylated arabinoxylans. Cereal Chem 75:259–265
Geng X, Li K (2002) Degradation of non-phenolic lignin by the white-rot fungus Pycnoporus cinnabarinus. Appl Microbiol Biotechnol 60:342–346
Georis J, Lomascolo A, Camarero S, Dorgeo V, Herpoël I, Asther M, Martinez AT, Dauvrin T (2003) Pycnoporus cinnabarinus laccases: an interesting tool for food applications. Commun Agric Appl Biol Sci 68:263–266
Gomez-Alarcon G, Saiz-Jimenez C, Lahoz R (1989) Influence of tween 80 on the secretion od some enzymes in stationary cultures of the white-rot fungus Pycnoporus cinnabarinus. Microbios 60:183–192
Gross B, Yonnet G, Picque D, Brunerie P, Corrieu G, Asther M (1990) Production of methylanthranilate by the basidiomycete Pycnoporus cinnabarinus (Karst). Appl Microbiol Biotechnol 34:387–391
Gross B, Asther M, Corrieu G, Brunerie P (1991) Production de vanilline par bioconversion de précurseurs benzéniques. European Patent no. 0453368A
Gupta R, Mehta G, Pal Khasa Y, Chander Kuhad R (2011) Fungal delignification of lignocellulosic biomass improves the saccharification of cellulosics. Biodegradation 22:797–804
Hahn V, Mikolash A, Wende K, Bartrow H, Lindequist U, Schauer F (2010) Derivatization of the azloe I-aminobenzotriazole using laccase of Pycnoporus cinnabarinus and Myceliophtora thermophila: influence of methanol on the reaction and biological evaluation of the derivative. Biotechnol Appl Biochem 56:43–48
Halaouli S, Mi A, Kruus K, Guo L, Hamdi M, Sigoillot J-C, Asther M, Lomascolo A (2005) Characterization of a new tyrosinase from Pycnoporus species with high potential for food technological applications. J Appl Microbiol 98:332–343
Halaouli S, Asther M, Sigoillot J-C, Hamdi M, Lomascolo A (2006a) A review. Fungal tyrosinases: new prospects in molecular characteristics, bioengineering, and biotechnological applications. J Appl Microbiol 100:219–232
Halaouli S, Record E, Casalot L, Hamdi M, Sigoillot J-C, Asther M, Lomascolo A (2006b) Cloning and characterization of a tyrosinase gene from the white-rot fungus Pycnoporus sanguineus, and overproduction of the recombinant protein in Aspergillus niger. Appl Microbiol Biotechnol 70:580–589
Herpoël I, Moukha S, Lesage-Meessen L, Sigoillot C, Asther M (2000) Selection of Pycnoporus cinnabarinus strains for laccase production. FEMS Microbiol Lett 183:301–306
Herpoël I, Jeller H, Fang G, Petit-Conil M, Bourbonnais R, Robert J-L, Asther M, Sigoillot J-C (2002) Efficient enzymatic delignification of wheat straw pulp by a sequential xylanase-laccase treatment. J Pulp Paper Sci 28:67–71
Hirono E, Zancan GT, Amaral D (1978) Glucose metabolism in Pycnoporus cinnabarinus. Can J Microbio 24:620–622
Holler JR, Brooks JC (1980) Nutritional studies of Pycnoporus cinnabarinus. Mycologia 72:329–337
Hoshida H, Fujita T, Murata K, Kubo K, Akada R (2005) Copper-dependent production of Pycnoporus coccineus extracellular laccase in Aspergillus oryzae and Saccharomyces cerevisiae. Biosci Biotechnol Biochem 69:1090–1097
Hundt K, Martin D, Hammer E, Jonas U, Kindermann MK, Schauer F (2000) Transformation of triclosan by Trametes versicolor and Pycnoporus cinnabarinus. Appl Environ Microbiol 66:4157–4160
Ichishima E, Kumagai H, Tomoda K (1980) Substrate specificity of carboxyl proteinase from P. coccineus, a wood-deteriorating fungus. Current Microbiol 3:333–337
Ichishima E, Yoshimura K, Tomoda K (1983) Acid carboxypeptidase from a wood-deteriorating basidiomycete, Pycnoporus sanguineus. Phytochem 22:825–829
Ichishima E, Ito Y, Takeuchi M (1985) 1,2-α-d-Mannosidase drom a wood-rotting basidiomycete, Pycnoporus sanguineus. Phytochem 24:2835–2837
Jaouani A, Sayadi S, Vanthournhout M, Penninckx M (2003) Potent fungi for decolourization of olive oil mill wastewater. Enzyme Microb Technol 33:802–809
Jaouani A, Guillen F, Penninckx MJ, Martinez A, Martinez MJ (2005) Role of Pycnoporus coccineus laccase in the degradation of aromatic compounds in olive oil mill wastewater. Enzyme Microb Technol 36:478–486
Jiang D-S, Long S-Y, Huang J, Xiao H-Y, Zhou J-Y (2005) Immobilization of Pycnoporus sanguineus laccase on magnetic chitosan microspheres. Biochem Eng J 25:15–23
Jones CL, Baker WL, lonergan GT (2001) Dimethylsulfoxide elevates extracellular laccase (phenol oxidase) activity of Pycnoporus cinnabarinus grown on a low nutritional newspaper medium. J Chem Technol Biotechnol 76:494–500
Krings U, Hinz M, Berger RG (1996) Degradation of [2H]phenylalanine by the basidiomycete Ischnoderma benzoinum. J Biotechnol 51:123–129.
Krings U, Pilawa S, Theobald C, Berger RG (2001) Phenyl propenoic side chain degradation of ferulic acid by Pycnoporus cinnabarinus—elucidation of metabolic pathways using [5-2H]-ferulic acid. J Biotechnol 85:305–314
Kumagai H, Matsue M, Majima E, Tomoda K, Ichishima E (1981) Carboxyl proteinase from the wood deteriorating basidiomycete Pycnoporus coccineus: substrate specificity with oxidized insulin peptide B1 B6 and B15 B24, angiotensin and proangiotensin. Agric Biol Chem 45:981–985
Larking DM, Crawford RJ, Christie GBY, Lonergan GT (1999) Enhanced degradation of polyvinyl alcohol by Pycnoporus cinnabarinus after pretreatment with Fenton’s reagent. Appl Environ Microbiol 65:1798–1800
Lesage-Meessen L, Delattre M, Haon M, Asther M (1995) Method for obtaining vanillic acid and vanillin by bioconversion by an association of filamentous microorganisms. European Patent WO9608576
Lesage-Meessen L, Delattre M, Haon M, Thibault J-F, Colonna Ceccaldi B, Brunerie P, Asther M (1996) A two-step bioconversion process for vanillin production from ferulic acid combining Aspergillus niger and Pycnoporus cinnabarinus. J Biotechnol 50:107–113
Lesage-Meessen L, Haon M, Delattre M, Thibault J-F, Colonna-Ceccaldi B, Asther M (1997) An attempt to channel the transformation of vanillic acid into vanillin by controlling methoxyhydroquinone formation in Pycnoporus cinnabarinus. Appl Microbiol Biotechnol 47:393–397
Lesage-Meessen L, Lomascolo A, Bonnin E, Thibault J-F, Buleon A, Roller M, Mi A, Record E, Colonna Ceccaldi B, Asther M (2002) A biotechnological process involving filamentous fungi to produce natural crystalline vanillin from maize bran. Appl Biochem Biotechnol 102–103:141–153
Li K, Horanyi PS, Collins R, Phillips RS, Eriksson KEL (2001) Investigation of the role of 3-hydroxyanthranilic acid in the degradation of lignin by white-rot fungus Pycnoporus cinnabarinus. Enzyme Microbiol Technol 28:301–307
Liers C, Arnstadt T, Ullrich R, Hofrichter M (2011) Patterns of lignin degradation and oxidative enzyme secretion by different wood- and litter-colonizing basidiomycetes and ascomycetes grown on beech-wood. FEMS Microbiol Ecol. doi:10.1111/j.1574-6941.2011.01144.x
Litthauer D, Jansen van Vuuren M, van Tonder A, Wolfaardt FW (2007) Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SSC 108). Enzyme Microb Technol 40:563–568
Lomascolo A, Lesage-Meessen L, Labat M, Navarro D, Asther M (1999) Enhanced benzaldehyde formation by a monokaryotic strain of Pycnoporus cinnabarinus using a selective solid adsorbent in the culture medium. Can J Microbiol 45:653–657
Lomascolo A, Cayol JL, Roche M, Guo L, Robert JL, Record E, Lesage-Meessen L, Ollivier B, Sigoillot J-C, Asther M (2002) Molecular clustering of Pycnoporus strains from various geographic origins and isolation of monokaryotic strains for laccase hyperproduction. Mycol Res 106:1193–1203
Lomascolo A, Record E, Herpoël-Gimbert I, Delattre M, Robert JL, Georis J, Dauvrin T, Sigoillot JC, Asther M (2003) Overproduction of laccase by a monokaryotic strain of Pycnoporus cinnabarinus using ethanol as inducer. J Appl Microbiol 94:618–624
Lonergan GT, Schliephake K, Jones C, Mainwaring DE (1993) The growth of the white-rot fungus, Pycnoporus cinnabarinus, in a packed-bed bioreactor. Biotechnology 4:239–242
Lonergan GT, Panow A, Jones CL, Schliephake K, Ni CJ, Mainwaring DE (1995) Physiological and biodegradative behaviour of the white-rot fungus, Pycnoporus cinnabarinus in a 200 litre packed-bed bioreactor. Biotechnology 5:107–111
Lu L, Zhao M, Zhang B-B, Yu S-Y, Bian X-J, Wang W, Wang Y (2007) Purification and characterization of laccase from Pycnoporus sanguineus and decolorization of an anthraquinone dye by the enzyme. Appl Microbiol Biotechnol 74:1232–1239
Mashitah MD, Zulfadhly Z, Bhatia S (1999) Ability of Pycnoporus sanguineus to remove copper ions from acqueous solution. Art Cells Blood Subs Immob Biotech 27:429–433
Mashitah MD, Azila YY, Bhatia S (2008) Biosorption of cadmium (II) ions by immobilized cells of Pycnoporus sanguineus from aqueous solution. Bioresour Technol 99:4742–4748
Mazdak C, Otterbein L, Chamkha M, Moukha S, Asther M, Gaillardin C, Beckerich J-M (2005) Heterologous production of a laccase from the basidiomycete Pycnoporus cinnabarinus in the dimorphic yeast Yarrowia lipolytica. FEMS Yeast Res 5:635–646
Meza JC, Sigoillot JC, Lomascolo A, Navarro D, Auria R (2006) New process for fungal delignification of sugar-cane bagasse and simultaneous production of laccase in a vapor phase bioreactor. J Agric Food Chem 54:3852–3858
Micard V, Thibault J-F (1999) Oxidative gelation of sugar-beet pectins: use of laccases and hydration properties of the cross-linked pectins. Carbohydr Polym 39:265–273
Mitsutomi M, Ohtakara A (1988) Isolation and identification of oligosaccharides produced from raffinose by transgalactosylation reaction of thermostable α-galactosidase from Pycnoporus cinnabarinus. Agric Biol Chem 52:2305–2311
Mitsutomi M, Uchida Y, Ohtakara A (1985) Immobilization of thermostable α-galactosidase from Pycnoporus cinnabarinus on chitin and some properties of the immobilized enzme. J Ferment Technol 63:325–329
Mitsutomi M, Honda J, Ohtakara A (1991) Enzymatic synthesis of galactooligosaccharides by the condensation action of thermostable α-galactosidase from Pycnoporus cinnabarinus. Nippon Shokuhin Kogyo Gakkaishi 38:722–728
Molina S, Rencoret J, del Rio JC, Lomascolo A, Record E, Martinez AT, Gutierrez A (2008) Oxidative degradation of model lipids representative for main pulp lipophilic extractives by the laccase-mediator system. Appl Microbiol Biotechnol 80:211–222
Morgenstern I, Robertson DL, Hibbett DS (2010) Characterization of three mnp genes of Fomitiporia mediterranea and report of additional class II peroxidases in the order Hymenochaetales. Appl Env Microbiol 76:6431–6440
Moukha SM, Dumonceaux TJ, Record E, Archibald FS (1999) Cloning and analysis of Pycnoporus cinnabarinus cellobiose dehydrogenase. Gene 234:23–33
Nishizawa Y, Nakabayashi K, Shinagawa E (1995) Purification and characterization of laccase from white-rot fungus Trametes sanguinea M85-2. J Ferment Bioeng 80:91–93
Nobles MK, Frew BP (1962) Studies in wood-inhabiting hymenomycetes. V. The genus Pycnoporus Karst. Can J Bot 40:987–1016
Oda Y, Adachi K, Aita I, Ito M, Aso Y, Igarashi H (1991) Purification and properties of laccase excreted by Pycnoporus coccineus. Agric Biol Chem 55:1393–1395
Oddou J, Stentelaire C, Lesage-Meessen L, Asther M, Colonna Ceccaldi B (1999) Improvement of ferulic acid bioconversion into vanillin by use of high-density cultures of Pycnoporus cinnabarinus. Appl Microbiol Biotechnol 53:1–6
Ohtakara A (1988) Chitinase and β-N-Acetylhexosaminidase from Pycnoporus cinnabarinus. Methods Enzymol 161:462–470
Ohtakara A, Mitsutomi M (1987) Immobilization of thermostable α-galactosidase from Pycnoporus cinnabarinus on chitosan beads and is application to the hydrolysis of raffinose in beet sugar molasses. J Ferment Technol 65:493–498
Ohtakara A, Yoshida M, Murakami M, Izumi T (1981a) Purification and characterization of β-N-acetylhexosaminidase from Pycnoporus cinnabarinus. Agric Biol Chem 45:239–247
Ohtakara A, Hayashi N, Mitsutomi M (1981b) Purification and some properties of acid β-galactosidase from Pycnoporus cinnabarinus. J Ferment Technol 59:325–328
Ohtakara A, Mitsutomi M, Nakamae E (1982) Mode of hydrolysis of chito-oligosaccharides with Pycnoporus cinnabarinus β-N-acetylhexosaminidase: application of high-performance liquid chromatography. Agric Biol Chem 46:293–295
Ohtakara A, Mitsutomi M, Uchida Y (1984) Purification and enzymatic properties of α-galactosidase from Pycnoporus cinnabarinus. Agric Biol Chem 48:1319–1327
Otterbein L, Record E, Longhi S, Asther M, Moukha S (2000) Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris. Eur J Biochem 267:1619–1625
Pointing SB, Jones EBG, Vrijmoed LLP (2000) Optimization of laccase production by Pycnoporus sanguineus in submerged liquid cultures. Mycologia 92:139–144
Pointing SB, Pelling AL, Smith JD, Hyde KD, Reddy CA (2005) Screening of basidiomycetes and xylariouceaous fungi for lignin peroxidase and laccase gene-specific sequences. Mycol Res 109:115–124
Quiroga EN, Vattuone MA, Sampietro AR (1995) Purification and characterization of the invertase from Pycnoporus sanguineus. Biochim Biophys Acta 1251:75–80
Quiroga EN, Sgariglia MA, Molina CF, Sampietro DR, Soberon JR, Vattuone MA (2009) Purification and characterization of an exo-polygalacturonase from Pycnoporus sanguineus. Mycol Res 113:1404–1410
Quiroz-Castañeda RE, Balcázar-López E, Dantán-Gonzáles E, Martinez A, Folch-Mallol J, Martinez Anaya C (2009) Characterization of cellulolytic activities of Bjerkandera adusta and Pycnoporus sanguineus on solid wheat straw medium. Electronic J Biotechnol 12:1–8
Rama R, Mougin C, Boyer FD, Kollmann A, Malosse C, Sigoillot JC (1998) Biotransformation of benzo[a]pyrene in bench scale reactor using laccase of Pycnoporus cinnabarinus. Biotechnol Lett 20:1101–1104
Ravalason H, Herpoel-Gimbert I, Record E, Bertaud F, Grisel S, de Weert S, van den Hondel CAMJJ, Asther M, Petit-Conil M, Sigoillot JC (2009) Fusion of a family 1 carbohydrate binding module of Aspergillus niger to the Pycnoporus cinnabarinus laccase for efficient softwood kraft pulp biobleaching. J Biotechnol 142:220–226
Record E, Punt PJ, Chamkha M, Labat M, van den Hondel CAMJJ, Asther M (2002) Expression of the Pycnoporus cinnabarinus laccase gene in Aspergillus niger and characterization of the recombinant enzyme. Eur J Biochem 269:602–609
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med 20:933–956
Romano I, Calandrelli V, Dipasquale L, Nicolaus B, Lama L (2007) Purification and characterization of Pycnoporus sanguineus MUCL38531 laccase expressed in methylotrophic yeast Pichia pastoris. J Biotechnol 131: S120.
Ryvarden L (1991) Genera of polypores, nomenclature and taxonomy. Synopsis Fungorum 5. Fungiflora, Oslo
Ryvarden L, Gilbertson RL (1994) Meripilus—Tyromyces. European Polypores, part 2. Fungiflora, Oslo
Ryvarden L, Johansen I (1980) A preliminary polypore flora of East Africa. Synopsis Fungorum 5. Fungiflora, Oslo
Saulnier L, Thibault J-F (1999) Ferulic acid and diferulic acids as components of sugar-beet pectins and maize bran heteroxylans. J Sci Food Agric 79:396–402
Schliephake K, Lonergan GT, Jones CL, Mainwaring DE (1993) Decolourisation of a pigment plant effluent by Pycnoporus cinnabarinus in a packed-bed bioreactor. Biotechnol Lett 15:1185–1188
Schliephake K, Mainwaring DE, Lonergan GT, Jones IK, Baker WL (2000) Transformation and degradation of the disazo dye Chicago Sky Blue by a purified laccase from Pycnoporus cinnabarinus. Enzyme Microb Biotechnol 27:100–107
Sigoillot JC, Petit-Conil M, Herpoël I, Joseleau JP, Ruel K, Kurek B, de Choudens C, Asther M (2001) Energy saving with fungal enzymatic treatment of industrial poplar alkaline peroxide pulps. Enzyme Microb Technol 29:160–165
Sigoillot C, Lomascolo A, Record E, Robert JL, Asther M, Sigoillot J-C (2002) Lignocellulolytic and hemicellulolytic system of Pycnoporus cinnabarinus: isolation and characterization of a cellobiose dehydrogenase and a new xylanase. Enzyme Microb Technol 31:876–883
Sigoillot C, Record E, Belle V, Robert J-L, Levasseur A, Punt PJ, van den Hondel CAMJJ, Fournel A, Sigoillot J-C, Asther M (2004) Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol 64:346–352
Smânia A, Delle Monache F, Smânia EFA, Gil ML, Benchetrit LC, Cruz FS (1995) Antibacterial activity of a substance produced by the fungus Pycnoporus sanguineus (Fr.) Murr. J Ethnoparmacol 45:177–181
Smânia A, Marques CJS, Smânia EFA, Zanetti CR, Carobrez SG, Tramonte R, Loguercio-Leite C (2003) Toxicity and antiviral activity of cinnabarin obtained from Pycnoporus sanguineus (Fr.) Murr. Phytother Res 17:1069–1072
Stentelaire C, Lesage-Meessen L, Delattre M, Haon M, Sigoillot J-C, Colonna Ceccaldi B, Asther M (1998) By-passing of unwanted vanillyl alcohol formation using selective adsorbents to improve vanillin production with Phanerochaete chrysosporium. World J Microbiol Biotechnol 14:285–287
Stentelaire C, Lesage-Meessen L, Oddou J, Bernard O, Bastin G, Colonna Ceccaldi B, Asther M (2000) Design of a fungal bioprocess for vanillin production from vanillic acid at scalable level by Pycnoporus cinnabarinus. J Biosci Bioeng 89:223–230
Sullivan G, Henry ED (1971) Occurrence and distribution of phenoxazinone pigments in the genus Pycnoporus. J Pharmaceutical Sci 60:1097–1098
Temp U, Zierold U, Eggert C (1999) Cloning and characterization of a second laccase gene from the lignin-degrading basidiomycete Pycnoporus cinnabarinus. Gene 236:169–177
Thurston CF (1994) The structure and function of fungal laccases. Microbiol 140:19–26
Trovaslet M, Enaud E, Guiavarc’h Y, Corbisier A-M, Vanhulle S (2007) Potential of a Pycnoporus sanguineus laccase in bioremediation of wastewater and kinetic activation in the presence of an anthraquinonic acid dye. Enzyme Microb Technol 41:368–376
Uzan E, Nousiainen P, Balland V, Sipila J, Piumi F, Navarro D, Asther M, Record E, Lomascolo A (2010) High redox potential laccases from the lignolytic fungi Pycnoporus coccineus and P. sanguineus suitable for white biotechnology: from gene cloning to enzyme characterization and applications. J Appl Microbiol 108:2199–2213
Uzan E, Portet B, Lubrano C, Milesi S, Favel A, Lesage-Meessen L, Lomascolo A (2011) Pycnoporus laccase-mediated bioconversion of rutin to oligomers suitable for biotechnology applications. Appl Microbiol Biotechnol 90:97–105
Valeriano VS, Silva AMF, Santiago MF, Bara MTF, Garcia TA (2009) Production of laccase by Pycnoporus sanguineus using 2,5-xylidine and ethanol. Braz J Microbiol 40:790–794
Vikineswary S, Abdullah N, Renuvathani M, Sekaran M, Pandey A, Jones EBG (2006) Productivity of laccase in solid substrate fermentation of selected agro-residues by Pycnoporus sanguineus. Bioresour Technol 97:171–177
Whang F, Guo C, Liu H-Z, Liu C-Z (2008) Immobilization of Pycnoporus sanguineus laccase by metal affinity adsorption on magnetic chelator particles. J Chem Technol Biotechnol 83:97–104
Whang Z-X, Cai Y-J, Liao X-R, Tao G-J, Li Y-Y, Zhang F, Zhang D-B (2010) Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp. SYBC-L1. Process Biochem 45:1720–1729
Xu F, Shin W, Brown SH, Wahleithner JA, Sundaram UM, Solomon EI (1996) A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity and stability. Biochim Biophys Acta 1292:303–311
Yahaya YA, Mashitah MD, Bhatia S (2009) Biosorption of copper (II) onto immobilized cells of Pycnoporus sanguineus from acqueous solution: equilibrium and kinetic studies. J Hazard Mater 161:189–195
Zheng LR, Zheng P, Sun ZH, Bai YB, Wang J, Guo XF (2007) Production of vanillin from waste residue of rice bran oil by Aspergillus niger and Pycnoporus cinnabarinus. Biores Technol 98:1115–1119
Zulfadhly Z, Mashitah MD, Bhatia S (2001) Heavy metals removal in fixed-bed column by the macro fungus Pycnoporus sanguineus. Environ Pollut 112:463–470
Acknowledgements
This work was funded by the Commission of the European Communities through the BIORENEW project (NMP2-CT-2006-026456 “White Biotechnology for added-value products from renewable plant polymers: design of tailor-made biocatalysts and new Industrial bioprocesses”).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lomascolo, A., Uzan-Boukhris, E., Herpoël-Gimbert, I. et al. Peculiarities of Pycnoporus species for applications in biotechnology. Appl Microbiol Biotechnol 92, 1129–1149 (2011). https://doi.org/10.1007/s00253-011-3596-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3596-5