Abstract
A strain Rhodococcus sp. OCT 10 DSM 45596T, exhibiting 99.9% of 16S rDNA identity with Rhodococcus wratislaviensis NCIMB 13082, was isolated from a soil sample. The strain completely mineralised 2-chlorotoluene, 2-bromotoluene, o-xylene, benzyl alcohol and benzoate. In contrast, 2-fluorotoluene was only partially mineralised. By GC-MS and 1H-NMR analyses, 4-chloro-3-methylcatechol was identified as the central intermediate in the degradation pathway of 2-chlorotoluene. It was further degraded by enzymes of the meta cleavage pathway. Catechol 1,2-dioxygenase and chlorocatechol 1,2-dioxygenase as the initial enzymes of the ortho cleavage pathways were not detectable under these conditions. Furthermore, neither formation nor oxidation of 2-chlorobenzylic alcohol, 2-chlorobenzaldehyde, or 2-chlorobenzoate was observed, thereby excluding side chain oxidation activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2000, the worldwide production of monochlorotoluene isomers was about 130 kt per year (/a). With a share of 55–65 kt/a as isomeric mixtures and about 5,000 t/a as pure compound, 2-chlorotoluene as an ecotoxic substance was the main isomer (BUA 1989; Bohnet and Ullmann 2002; BMU 2001).
2-Chlorotoluene is used as educt for the production of o-cresol, agro-chemicals, flame retardants, dyes, varnish and pigments, textile additives, pharmaceuticals, adhesives, polymers and resins, air fresheners, drain cleaners and optical brighteners. Furthermore, it is used as solvent for agro-chemicals, in heavy metal industry, paint thinners, heat conductible oils as well as condenser liquids (BUA 1989; Bohnet and Ullmann 2002; BMU 2001; Scorecard 2009).
The widespread use led to substantial emission of 2-chlorotoluene. Today, 2-chlorotoluene can be found in soil, groundwater and surface waters, especially near to fields or industrial sites using 2-chlorotoluene (Antonious 2004; CSCCR 1999; Maltseva et al. 1994; Martí et al. 2005; McCulloch 1992; Nikolaou et al. 2002; Nishio et al. 2001; O'Brien et al. 1997). Its use as cleaning agent as well as solvent led to detection even in residual room air and convenience food (Buhamra 1998; Heikes et al. 1995; Weisel 2006).
Organisms capable of mineralising 3-chlorotoluene, 4-chlorotoluene or di- and trihalogenated monocyclic aromatics have been described in detail (Beil et al. 1997; Brinkmann and Reineke 1992; Gaunt and Evans 1971; Gibson et al. 1974; Haigler and Spain 1989; Haigler et al. 1992; Lehning 1998; Maltseva et al. 1994; Nishio et al. 2001; Pieper et al. 1988; Pollmann et al. 2002; Prucha et al. 1996; Raschke et al. 2001; Sander et al. 1991; Vandenbergh et al. 1981; Verschueren 2001; Wunder 2003; Yadav et al. 1995), but only a few publications on the degradation of 2-chlorotoluene have been presented.
Vandenbergh et al. (1981) described four bacterial strains degrading 2,6-dichlorotoluene, which additionally degraded 2-chlorotoluene in the presence of yeast extract being mandatory. Complete degradation of 2-chlorotoluene was claimed to be encoded on three plasmids. Leahy et al. (2003a) characterised the strain Acinetobacter johnsonii 2-CTOL-1, able to convert 2-chlorotoluene within a mixture of 60 different compounds.
However, in a substrate pattern experiment with 2-chlorotoluene being one of the investigated substrates, the mineralisation potential of 2-chlorotoluene by A. johnsonii 2-CTOL-1 or the other strains presented was not proven (Leahy et al. 2003b).
The degradation of 2-chlorotoluene under anaerobic conditions was demonstrated both for biocoenoses as well as Methylosinus trichosporium as a pure strain. The substance was spiked either in a mixture with other aromatic substrates (Genthner 1999; Halden 1991) or was an intermediate in the biological metabolism of 2,3,6-trichlorotoluene (Ramanand et al. 1993). In the latter case, related reaction kinetics was presented. In these studies none of the authors used 2-chlorotoluene as a single source of carbon and energy.
However, none of the authors showed data about intermediates, chloride balance or substrate conversion rates (with exception of Ramanand et al. 1993) during the degradation of 2-chlorotoluene, which are essential to estimate the total mineralisation of the chlorinated substance. Therefore, up to now, no clear evidence for total degradation of 2-chlorotoluene by native strains has been presented.
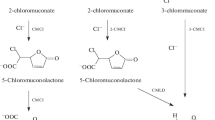
Attempts to construct a genetically engineered strain mineralising 2-chlorotoluene via side chain oxidation to 2-chlorobenzoate, with further degradation by the ortho cleavage pathway (Engesser and Schulte 1989), were not effective. Despite successful combination of genes of the TOD, TOL and chlorocatechol pathways and several attempts to increase the activities of the related enzymes, the initial activity of the monooxygenase was too low to achieve bacterial growth (Gibson et al. 1974, 1968; Haro and de Lorenzo 2001; Lehning 1998; Pieper et al. 1988; Reineke 1998; Rogers and Gibson 1977; Yeh et al. 1977). All attempts to oxidise 2-chlorotoluene by core dioxygenation, yielding 3-chloro-4-methylcatechol or 4-chloro-3-methylcatechol with concomitant cleavage of these catechols by chlorocatechol 1,2-dioxygenase, only led to excretion of dead-end products therefore representing a dead-end pathway (Pollmann et al. 2001, 2002, 2003, 2005). Lehning (1998) postulated that the degradation of 2-chlorotoluene via 4-chloro-3-methylcatechol, 3-chloro-2-methylmuconic acid, 5-methyldienlactone and 2-methyldienlactone is the only feasible pathway. Based on this approach, Pollmann et al. (2001, 2002, 2003, 2005) were able to construct a strain Escherichia coli pSTE44 capable to mineralise 2-chlorotoluene. However, selectivity and degradation rates were too low to allow bacterial growth.
Cleavage of 3-chloro-4-methylcatechol by chlorocatechol-2,3-dioxygenase seems to be unlikely because of suicide inactivation effects (Gibson et al. 1968). A productive meta cleavage of 4-chloro-3-methylcatechol was shown by Higson and Focht (1992) during 3-chloro-2-methylbenzoate degradation. Unfortunately, 2-chlorotoluene as a substrate was not tested.
Despite many attempts for isolation or construction of a strain mineralising 2-chlorotoluene, till now, no such strain could be demonstrated able to mineralise and use this substrate for growth. Thus, the aim of this study was to isolate a native strain, capable to completely degrade 2-chlorotoluene and to describe intermediates as well as some key enzymes involved in this degradation pathway.
Materials and methods
Strain and cultivation
Samples of wood soil, compost, river sediment, agricultural sites as well as river water and sewage sludge were used for isolation of bacteria, which were adequate to mineralise 2-chlorotoluene. Enrichment cultures were grown in a liquid mineral medium (MM) containing (in grams per litre) Na2HPO4, 2.79; KH2PO4, 1.00; (NH4)2SO4, 1.00; MgSO4·H2O, 0.20; Ca(NO3)2·7H2O, 0.01; ammonium ferric citrate C6H8O7·Fe·H4N, 0.01; trace minerals solution, 1.00 mL [consisting of (in grams per litre) H3BO3, 0.30; CoCl2·6H2O, 0.20; ZnSO4·7H2O, 0.10; Na2MoO4·2H2O, 0.03; MnCl2·4 H2O, 0.03; NiCl2·6H2O, 0.02; CuCl2·2H2O, 0.01] and the carbon source (5 mmol L−1). The pH of the MM was maintained at 7.1. Phosphate buffer, nutrient solution and calcium solution were autoclaved at 121°C for 30 min in separate flasks. Bacteria and carbon source were added after cooling the mixed solution. Liquid cultures were prepared by growing in conical flasks on a rotary shaker with 150 rpm at 30°C. Enrichment cultures were fed with 5 mmol L−1 of 2-chlorotoluene as carbon source every third day, and 25 mL of culture was transferred into flasks with new 100 mL MM after 14 days. After repeating the procedure for four times, 100 μL of sample solution was spread on solid MM and incubated with 2-chlorotoluene vapour in an incubation chamber adding 50 μL 2-chlorotoluene per litre of chamber volume each third day for 3 weeks. Initially, colonies grew slowly. Single colonies were stroke with a loop on new plates, and the procedure was repeated four times observing an increase in growth rate. Finally, single colonies were transferred into liquid MM containing 2-chlorotoluene with the same procedure described above. In case of bacterial growth accompanied by chloride accumulation in the liquid phase, the cultures were used for further research.
To prevent the induction of non-2-chlorotoluene-specific enzymes during experimental series for verification of the side chain oxidation activity, chloramphenicol (CAP) was added in these series. The final concentration of the bacteriostatic antibiotic was 100 mg L−1. The strain was deposited in the DSMZ under the accession number DSM 45596T. All chemicals used were of analytical grade and of the highest purity.
Sequencing and analysis of 16S ribosomal DNA
The 16S rRNA gene was PCR amplified using the 27F (E. coli numbering 8-27) and 1492R (E. coli numbering 1492-4510) primers together with Taq DNA polymerase (biomaster GmbH, Windeck, Germany). Then, the PCR product was purified using the GenElute™ PCR Clean-Up Kit (Sigma-Aldrich Chemie GmbH, Taufkirchen, Germany). Further preparations, automated DNA sequencing reactions and analysis using the GenBank database and Ribosomal Database Project were performed by the GATC Biotech AG (Constance, Germany). The gene sequence was deposited in GenBank under accession number JF490021.
Halogenide balances
For balancing of fluoride, chloride or bromide set free during mineralisation of the corresponding 2-halotoluenes, cultures of OCT 10 pre-grown on 2-chlorotoluene were used. Cells were harvested during exponential growth phase by centrifugation (15,000×g, 10 min, 4°C). Bacterial pellets were resuspended in fresh MM, and optical density was regulated to values of 1–2 at 546 nm. Cultures with 50 mL of this suspension and 50 μL of 2-chlorotoluene (supplied in evaporation caps) were cultivated on rotary shakers with 150 rpm at 30°C. After 48 h additional 25 μL of 2-chlorotoluene was added. Samples for measurement of optical density and halide concentration were regularly taken out of the flasks. Latter samples were centrifuged, and the supernatant was conveniently diluted for analyses.
Chromatographic analyses
HPLC analyses were conducted in a Thermo Separation Products SpectraSERIES provided with a UV/VIS detector. A ProntoSIL™ SC-04 Eurobond C18 column (125 × 4 mm i.d., 5 μm; BISCHOFF Chromatography, Leonberg, Germany) was employed. The consistence of the solvent was H2O:CH3OH:H3PO4 (85 w/v%) = 74.9%:25%:0.1%. The flow rate was maintained at 1 mL min−1. Peak detection was at 210 and 270 nm.
Halogenide balances were performed using a 761 Compact Ion Chromatograph (Metrohm, Filderstadt, Germany). Halogenide ions were separated using a Metrosep ASupp-4 column at a flow rate of 1 mL min−1 of eluent (1 mmol L−1 Na2CO3, 4 mmol L−1 NaHCO3) and 50 mmol L−1 H2SO4 as suppressor regeneration solution and deionised water as suppressor rinse solution.
GC-MS analyses were carried out using an Agilent GC 5973 instrument equipped with a MS 6890 detector and a 30 m × 0.25 mm VF-Xms column (Varian). A splitless injection was performed with the injector at 250°C, the transfer line at 280°C and the detector at 230°C using the following programme: 80°C (1 min) to 180°C (1 min) at 7°C min−1, to 240°C at 12°C min−1 and finally to 300°C (8 min) at 20°C min−1. The column flow rate was 1 mL min−1.
Purification of substituted catechols
Five hundred millilitres of exponential grown cells of OCT 10 were harvested by centrifugation and resuspended in fresh MM (100 mL in 250-mL cultivation flask). Fifty microlitres of 2-chlorotoluene and 50 μL of 2-chlorobenzyltrifluoride were added, and the cultures were cultivated for further 3 days. Supernatants of metabolite-rich cultures were collected and extracted with an equivalent volume of diethyl ether. After separation of the organic phase, the aqueous phase was saturated with sodium chloride, acidified to pH 2.0 and extraction was repeated for three times. All organic phases were collected, dried with Na2SO4 and evaporated to a residue of about 500 μL. The residue was brown in colour with oily consistence. Man-made separation columns (7 cm × 5 mm i.d.) with silica gel (60 Å pore size, 70–230 mesh) were purged with 5–10 mL of hexane as preconditioning step. After launching the residue, the following solvents were added as long as 5 mL of each effluent was collected at the bottom of the column: hexane, 90 vol.% hexane:10 vol.% dichloromethane, 50 vol.% hexane:50 vol.% dichloromethane, dichloromethane, acetone and methanol. The bacterial produced catechols were detected within the dichloromethane as well as acetone fraction, while the corresponding yellow-coloured benzoquinones were collected within the methanol fraction.
The fractions containing substituted catechols were combined, concentrated with a nitrogen flow to at least 1 mL residue and afterwards 1–2 mL of hexane was added. While the sample was stored at −18°C overnight, an oily phase containing the catechols appeared. Using a nitrogen flow, the remaining acetone and dichloromethane were evaporated and additional 2–3 mL of hexane was added. After a second night at −18°C, the hexane phase was separated and the oily phase was solubilised in 1 mL dichloromethane. This sample was relaunched on a silica gel column again. The purified 4-chloro-3-methylcatechol was quantitatively detected in the acetone phase and was used for GC-MS analyses. For additional 1H-NMR analyses, the acetone was completely evaporated and the oily phase of 4-chloro-3-methylcatechol was solubilised in CD2Cl2.
Results
Identification and characterization of strain OCT 10
Strain OCT 10 was isolated from an agricultural site near Stuttgart-Büsnau in Germany. The strain degrades and grows well on 2-chlorotoluene, o-xylene, benzyl alcohol as well as benzoate. In contrast, poor growth was observed in case of 2-bromotoluene and p-xylene (Table 1).
For identification of strain OCT 10, its 16S rRNA gene was amplified and the PCR product was sequenced with internal primers. Based on a comparison with the GenBank and Ribosomal Database Project databases, the 16S rRNA sequence exhibited a high level of 99.9% identity with Rhodococcus wratislaviensis NCIMB 13082 (identity of 1,336 of 1,341 bases, 5 bases were not identified). However, the substrate patterns of both strains showed significant differences (Table 1). Hence, Rhodococcus sp. OCT 10 was deposited as a type strain in the DSMZ under the accession number DSM 45596T.
The strain is an aerobic, non-motile, gram-positive bacterium and is well cultivatable at 30°C and pH 7.1. The microscopic morphology of cells depends on incubation time and growth conditions. It changes from distinct y-shaped cells with true branching to bacillary forms in case of cultivation in liquid nutrient broth or Luria broth (LB) to distinct cocci forms in case of cultivation on 2-chlorotoluene-containing solid media. On rich media, the cells tend to coalesce revealing longer rods frequently exhibiting rudimentary branching. Transmission electron microscopy (TEM) images, showing typical agglomerates of Rhodococcus sp. OCT 10 after growth on LB medium, are given in Fig. 1.
Colony morphology shows a distinct filamentous and highly branched structure showing a pale pink to salmon pigmentation (Fig. 2; growth on MM with 2-chlorotoluene). Incubation for several weeks or with easily degradable substrates enhances intensity.
Chloride balance during mineralisation of 2-chlorotoluene
During mineralisation of 12.6 mmol L−1 2-chlorotoluene by OCT 10, chloride accumulated within the liquid phase and the optical density increased by 2.39 units. In contrast to an expected chloride concentration of 446.8 mg Cl− L−1 in case of total mineralisation, a chloride concentration of only 425.8 mg Cl− L−1 was detected. Thus, the recovery rate in chloride was 95.3%. After 78 h 2-chlorotoluene was neither detectable in the liquid phase nor in the gas phase. Finally, the missing 4.7% of chloride may be attributed to substrate evaporation losses during sampling. Therefore, the high recovery rate of chloride proved the complete mineralisation of 2-chlorotoluene by OCT 10.
Degradation of 2-halotoluenes
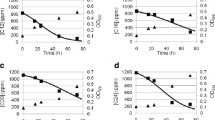
In contrast to 2-chlorotoluene, 2-fluorotoluene and 2-bromotoluene are industrial ‘low-volume’ products. Both are used as intermediates for manufacturing of pharmaceutical and agrochemical intermediates and products. For both compounds no microbial degradation studies exist. Thus, the degradability of both substances by OCT 10 was tested. The time-dependent release of the corresponding halogenide ions as well as the increase in optical density (λ = 546 nm) are shown in Fig. 3. In contrast to 2-chlorotoluene (95.3% Cl−), the amount of halogenide ions released were not stoichiometrical in case of 2-fluorotoluene and 2-bromotoluene with recovery rates of 48.9% F− or 71.4% Br− (start concentrations: 9.1 mmol L−1 2-fluorotoluene, 12.6 mmol L−1 2-chlorotoluene, 8.3 mmol L−1 2-bromotoluene, respectively).
Increase in optical density (OD) at a wavelength λ = 546 nm and halide recovery rate during degradation of 2-fluorotoluene (empty triangle), 2-chlorotoluene (empty circle) or 2-bromotoluene (empty square) by Rhodococcus sp. OCT 10. Initial concentrations of 9.1 mmol L−1 2-fluorotoluene, 12.6 mmol L−1 2-chlorotoluene or 8.3 mmol L−1 2-bromotoluene as well as an initial optical density of 1.6 at 546 nm were adjusted
The corresponding increases in the optical density were 0.52, 2.39 and 1.90 for 2-fluorotoluene, 2-chlorotoluene and 2-bromotoluene, starting at an optical density of 1.6. Therefore, a specific increase in optical density per millimoles released halogenide ion of 0.12 per mmol F−, 0.20 per mmol Cl− and 0.32 per mmol Br−, respectively, was observed. 2-Fluorotoluene is no inducer for the relevant enzymes and can only be transformed and mineralised in a cometabolic way (data not shown).
Side chain oxidation of 2-halotoluenes
Although the experimental data presented above showed complete mineralisation of 2-chlorotoluene, no further information about the degradation pathway was obtained. One possible degradation alternative is the formation and mineralisation of 2-chlorobenzoate by side chain oxidation (Engesser and Schulte 1989). In this case, 2-chlorobenzyl alcohol, 2-chlorobenzaldehyde and 2-chlorobenzoate should be converted by induced enzymes of the 2-chlorotoluene degradation pathway. To avoid induction of ‘non-2-chlorotoluene-specific’ enzymes, CAP was added to the cultures.
But, neither transformation of the halogenated test substances of a possible side chain oxidation nor chloride release was observed over 56 h, irrespective of the addition of CAP. Supplying benzoate with and without CAP, the principal capability of strain OCT 10 to quickly induce enzymes for the degradation of aromatics could be shown, as substrate transformation and cell growth only occurred under non-repressive conditions. Thus, an oxidation of 2-chlorotoluene by side chain oxidation of the methyl group forming 2-chlorobenzyl alcohol, 2-chlorobenzaldehyde or 2-chlorobenzoate could be therefore excluded.
Identification of 4-chloro-3-methylcatechol
Excluding side chain oxidation, 2-chlorotoluene had to be oxidised by direct core oxygenation. In this case four alternatives of substituted catechols can occur theoretically (4-chloro-3-methylcatechol, 5-chloro-4-methylcatechol, 3-chloro-4-methylcatechol and, after possible reductive dehalogenation, 3-methylcatechol). During growth on 2-chlorotoluene, OCT 10 did not show accumulation of any of these catechols in the liquid phase. Consequently, a mixture of 2-chlorotoluene and its structurally related analogue, 2-chlorobenzyltrifluoride, was used as a substrate. By turnover of this substrate mixture, the colour of the culture turned brown and accumulation of catechols was observed. Purifying the supernatant in the way described, an oily, brown-coloured product was obtained. Gas chromatography–mass spectrometric analysis indicated the main product to be a chloromethylcatechol. The molecular ion (M) is consistent with the exact molecular mass of m/z = 158. The M:M + 2 ratio of 32.4:100 is consistent with a single Cl atom. The major fragment at m/z = 123 represents the loss of Cl. The fragment at m/z = 112 is the result of deletion of OH and COH groups. The fragment m/z = 105 is the result of Cl and H2O elimination. Fragment m/z = 139 is produced by removal of H2O and a single proton of the methyl group. For both fragments, the elimination of water is only possible in case of ortho position of both OH groups. The aromatic nature of the molecule is indicated by the ion with m/z = 77. The separation of the methyl substituent only occurs in case of low-mass fragments (m/z = 66→m/z = 51 or m/z = 65→m/z = 50). The related fragmentation spectra are shown in Fig. 4.
Nuclear magnetic resonance signals for the compound's methyl group appeared at δ 2.2725 ppm; its hydroxyl proton signals appeared as a broad feature centred at δ 5.6 ppm. The compound's aromatic protons showed a doublet of doublets at δ 6.6549, 6.6720, 6.7879 and 6.8048 ppm. These doublet effects as well as a strong coupling constant of 8.45 or 8.55 Hz, respectively, confirm the ortho position of both protons. High resolution of δ 6.6549 ppm and δ 6.6720 ppm signals showed additional doublet of signals with a coupling constant of 0.592 Hz and proved a long-range coupling of protons of the methyl group and the ring proton at para position (position 6 in Fig. 4). However, no long-range coupling exists for a proton located in meta position to the protons of the methyl group (δ 6.7879 ppm and δ 6.8048 ppm; position 5 in Fig. 4). These protons of the methyl group are therefore shielded from the ring protons by the Cl and OH groups, demonstrating the molecular structure of 4-chloro-3-methylcatechol.
Mass fragmentation spectra as well as 1H-NMR results are highly similar to the results presented by Higson and Focht (1992) for the degradation intermediate of 3-chloro-2-methylbenzoic acid in Pseudomonas cepacia MB2. The relative fragment intensities of 4-chloro-3-methylcatechol of Higson and Focht as well as of the catechol found in this study are shown in Table 2.
Metabolism of 4-chloro-3-methylcatechol
4-Chloro-3-methylcatechol is commercially not available, and biological transformation reactions did not result in substantial accumulation of this metabolite until now. Therefore, the transformation of other commercially available catechol derivatives was investigated. In transformation experiments, a rapid turnover of these catechols supplied to whole cells as well as cell extracts occurred; obviously, meta cleavage took place as the supernatants were yellow in colour. For example, the transformation of 3-methylcatechol showed a transient increase in absorbance at 387 nm. This proved the assumption of a catechol 2,3-dioxygenase as a key enzyme of the degradation pathway of 2-chlorotoluene.
During mineralisation of 2-chlorotoluene by OCT 10, a transient light yellow coloration of the supernatant (absorption maximum at 390.5 nm) was observed. Based on the data described by Higson and Focht (1992), the meta cleavage product of 4-chloro-3-methylcatechol is postulated to be 5-chloro-2-hydroxy-6-oxo-hepta-2,4-dienoic acid. Further research will be undertaken to clarify the degradation pathway and chloride elimination in more detail.
Discussion
Despite its simple molecular structure, 2-chlorotoluene is hardly biodegradable (Drotleff et al. 1992; Schraa et al. 1987). As shown above, neither native nor genetically engineered bacterial strains were clearly stated for mineralising this substance and using it as single growth substance. In contrast, a few research groups presented data for bacterial degradation of 2-chlorotoluene by biocoenoses as well as single strains (Vandenbergh et al. 1981; Leahy et al. 2003a, b; Genthner 1999; Halden 1991; Ramanand et al. 1993). However, mineralisation of 2-chlorotoluene by these strains was not unequivocally demonstrated. Key data like chloride balances, substrate balance, description of intermediates or any proof of the substance being used as a sole source of carbon and energy were not given. Furthermore, with exception of Ramanand et al. (1993), information about the degradation pathway of this substance was neither presented nor suggested. In addition, the strains isolated by Vandenbergh et al. (1981), after being re-examined by the authors of the present study, could not be verified to degrade 2-chlorotoluene as sole source of carbon and energy.
While initial dehalogenation forming 3-methylcatechol was never observed under aerobic conditions, side chain oxidation of 2-chlorotoluene to form 2-chlorobenzoate is a further promising pathway alternative. However, studies with genetically engineered strains, where genes of the TOD, TOL and chlorocatechol pathway were combined, showed too low transformation rates to permit bacterial growth (Haro and de Lorenzo 2001; Lehning 1998).
Lehning (1998) and Pollmann et al. (2002) therefore described an initial core dioxygenation and subsequent mineralisation via 4-chloro-3-methylcatechol, 3-chloro-2-methylmuconic acid and 5-methyldienlactone, constituting the only feasible degradation pathway of 2-chlorotoluene. The ortho cleavage pathway was favoured over the meta cleavage pathway to prohibit possible suicide inactivation effects in case of the latter pathway (Pollmann et al. 2002). Nevertheless, a productive meta cleavage of 4-chloro-3-methylcatechol was shown by Higson and Focht (1992) during 3-chloro-2-methylbenzoate degradation. This finding was extraordinary due to the well-known incapability of the meta pathway to handle chloro-substituted metabolites. However, 2-chlorotoluene as a substrate was not tested.
The strain Rhodococcus sp. OCT 10 isolated from an agricultural site was able to mineralise 2-chlorotoluene. During this process 95.3% of the theoretical chloride content of the substance was liberated. The missing 4.7% of chloride or substance, respectively, were attributed to evaporation losses. Neither halogenated nor non-halogenated intermediates accumulated permanently. Thus, strain OCT 10 completely mineralises 2-chlorotoluene.
Next to 2-chlorotoluene, the strain was also able to mineralise 2-bromotoluene and to transform 2-fluorotoluene in a cometabolic way. Even though the specific increase in optical density per millimole of substrate supplied was nearly 60% higher in case of 2-bromotoluene related to 2-chlorotoluene, the latter is a better inducer for enzymes and a better growth substrate and showed a 24% higher halogenide balance than the former one. With a 50% higher starting concentration and a logK OW ≈ 3.5 for 2-chlorotoluene instead of logK OW ≈ 2.9 in case of 2-bromotoluene, the higher solubility and therefore higher membrane toxicity of 2-chlorotoluene was probably responsible for the lower specific biomass yield (Duldhardt 2008; Mackay et al. 2006).
The degradation of 2-chlorotoluene by this strain was not initiated by side chain oxidation forming 2-chlorobenzoate as an intermediate. In fact, 2-chlorotoluene was transformed by an initial core oxygenation and the corresponding catechol (4-chloro-3-methylcatechol) was identified by GC-MS and 1H-NMR analyses. Other catechols, i.e. 3-chloro-4-methylcatechol, were not detected.
Cultivating strain OCT 10 on 2-chlorotoluene, a transient yellow shade with an absorption maximum at 390.5 nm occurred in the supernatant during exponential growth of the culture, indicating the presence of the meta cleavage pathway. Catechol 2,3-dioxygenase activity with high activity against chloro- and methyl-substituted catechols was verified by transformation of commercially available catechols with whole cells of strain OCT 10. Ortho cleavage activity was neither detected with whole cells nor with cell extracts. However, due to the lack of 4-chloro-3-methylcatechol as a substrate, only small amounts of the cleavage product accumulated within the supernatant. Thus, identification was not possible. Higson and Focht (1992) though presented data of 5-chloro-2-hydroxy-6-oxo-hepta-2,4-dienoic acid as the meta cleavage product of 4-chloro-3-methylcatechol. Amongst others, they presented the absorption maximum of this intermediate at 391 nm. Hence, the conclusion may be drawn that the yellow colour was caused by 5-chloro-2-hydroxy-6-oxo-hepta-2,4-dienoic acid in case of strain OCT 10 too. The verification of this postulate as well as the syntheses of chloromethylcatechols as substrates for transformation procedures and as standards for identification will be one part of the funding project being submitted.
References
Beratergremium für umweltrelevante Altstoffe–BUA (1989) BUA-Stoffbericht 38–Chlortoluole (Methylchlorbenzole). Gesellschaft Deutscher Chemiker GDCh (ed) VCH, Weilheim, Deutschland
Antonious GF (2004) Trifluralin residues in runoff and infiltration water from tomato production. Bull Environ Contam Toxicol 72(5):962–969. doi:10.1007/s00128-004-0337-9
Beil S, Happe B, Timmis KN, Pieper DH (1997) Genetic and biochemical characterization of the broad spectrum chlorobenzene dioxygenase from Burkholderia sp. PS12. Dechlorination of 1,2,4,5-tetrachlorobenzene. Eur J Biochem 247:190–199. doi:10.1111/j.1432-1033.1997.00190.x
Bohnet M, Ullmann F (2002) Ullmann's encyclopedia of industrial chemistry, vol 8, 6th edn. Wiley-VCH, Weinheim
Brinkmann U, Reineke W (1992) Degradation of chlorotoluenes by in vivo constructed hybrid strains: problems of enzyme specificity, induction and prevention of meta-pathway. FEMS Microbiol Lett 96(1):81–88. doi:10.1111/j.1574-6968.1992.tb05397.x
Buhamra SS (1998) The analysis of VOCs survey data from residences in Kuwait. Environmetrics 9(3):245–253. doi:10.1002/(SICI)1099-095X(199805/06)9:3
Ciba Specialty Chemicals Corporate Remediation (1999) Draft in-situ biopilot report Ciba-Geigy Site, Toms River. EPA US-Environmental Protection Agency, New Jersey
Drotleff J, Fluthwedel A, Pohle H, Spilok K (1992) Handbuch Chlorchemie II–Ausgewählte Produktlinien. Umweltbundesamt Text 42/92, Berlin, Germany
Duldhardt I (2008) Untersuchung des Einflusses organischer Lösungsmittel und umweltrelevanter Schadstoffe auf das Wachstum und die zelluläre Fettsäurezusammensetzung von Thauera aromatica, Geobacter sulfurreducens und Desulfococcus multivorans. Dissertation, Universität Greifswald, Deutschland
Engesser KH, Schulte P (1989) Degradation of 2-bromo-, 2-chloro- and 2-fluorobenzoate by Pseudomonas putida CLB250. FEMS Microbiol Lett 60:143–148
Gaunt JK, Evans WC (1971) Metabolism of 4-chloro-2-methylphenoxyacetate by a soil pseudomonad. Ring-fission, lactonizing and delactonizing enzymes. Biochem J 122(4):533–542
Genthner BRS (1999) Preliminary characterization of four 2-chlorobenzoate-degrading anaerobic bacterial consortia. Biodegradation 10(1):27–33. doi:10.1023/A:1008348123672
Gibson DT, Koch JR, Schuld CL, Kallio RE (1968) Oxidative degradation of aromatic hydrocarbons by microorganisms. II. Metabolism of halogenated aromatic hydrocarbons. Biochemistry 7(11):3795–3802. doi:10.1021/bi00851a003
Gibson DT, Mahadavan V, Davey JF (1974) Bacterial metabolism of para- and meta-xylene: oxidation of the aromatic ring. J Bacteriol 119(3):930–936
Haigler BE, Spain JC (1989) Degradation of p-chlorotoluene by a mutant of Pseudomonas sp. strain JS6. Appl Environ Microbiol 55(2):372–379
Haigler BE, Pettigrew CA, Spain JC (1992) Biodegradation of mixtures of substituted benzenes by Pseudomonas sp. strain JS150. Appl Environ Microbiol 58(7):2237–2244
Halden K (1991) Methanotrophs for renovation of polluted aquifers. Chem Eng Res Des 69(3):181–183
Haro MA, de Lorenzo V (2001) Metabolic engineering of bacteria for environmental applications: construction of Pseudomonas strains for biodegradation of 2-chlorotoluene. J Biotechnol 85(2):103–113. doi:10.1016/S0168-1656(00)00367-9
Heikes DL, Jensen SR, Fleming-Jones ME (1995) Purge and trap extraction with GC-MS determination of volatile organic compounds in table-ready foods. J Agric Food Chem 43(11):2869–2875. doi:10.1021/jf00059a018
Higson FK, Focht DD (1992) Utilization of 3-chloro-2-methylbenzoic acid by Pseudomonas cepacia MB2 through the meta fission pathway. Appl Environ Microbiol 58(8):2501–2504
Leahy JG, Tracy KD, Eley MH (2003a) Degradation of volatile hydrocarbons from steam-classified solid waste by a mixture of aromatic hydrocarbon-degrading bacteria. Biotechnol Lett 25(6):479–483. doi:10.1023/A:1022604112906
Leahy JG, Tracy KD, Eley MH (2003b) Degradation of mixtures of aromatic and chloroaliphatic hydrocarbons by aromatic hydrocarbon-degrading bacteria. FEMS Microbiol Ecol 43:271–276. doi:10.1111/j.1574-6941.2003.tb01067.x
Lehning A (1998) Untersuchungen zum Metabolismus von Chlortoluolen: Konstruktion Chlortoluol und Chlorbenzylalkohol verwertender Mikroorganismen. Dissertation, TU Braunschweig
Mackay D, Shiu WY, Ma KC, Lee SC (2006) Handbook of physical-chemical properties and environmental fate for organic chemicals, vol 1, Introduction and hydrocarbons. CRC Press, Boca Raton
Maltseva OV, Solyanikova IP, Golovleva LA, Schlömann M, Knackmuss HJ (1994) Dienlacton hydrolase from Rhodococcus erythropolis 1CP: purification and properties. Arch Microbiol 162(5):368–374. doi:10.1007/BF00263786
Martí I, Lloret R, Martín-Alonso J, Ventura F (2005) Determination of chlorinated toluenes in raw and treated water samples from the Llobregat river by closed loop stripping analysis and gas chromatrography-mass spectrometry detection. J Chromatogr A 1077(1):68–73. doi:10.1016/j.chroma.2005.04.051
McCulloch B (1992) Process for purification of ortho-chlorotoluene. US Patent 5,143,685
Nikolaou AD, Golfinopoulos G, Kostopoulou MN, Kolokythas GA, Lekkas TD (2002) Determination of volatile organic compounds in surface waters and treated wastewater in Greece. Water Res 36(11):2883–2890. doi:10.1016/S0043-1354(01)00497-3
Nishio T, Patel A, Wang Y, Lau PCK (2001) Biotransformations catalyzed by cloned p-cymene monooxygenase from Pseudomonas putida F1. Appl Microbiol Biotechnol 55(3):321–325. doi:10.1007/s002530000584
O'Brien A, Reiser RG, Gylling H (1997) Spatial variability of volatile organic compounds in streams on Long Island, NY, and in New Jersey. National Water-Quality Assessment (NAWQA) Program. US-Department of the Interior, Washington
Pieper DH, Reineke W, Engesser KH, Knackmuss HJ (1988) Metabolism of 2,4-diochlorophenoxyacetic acid, 4-chloro-2-methylphenoxyacetic acid and 2-methylphenoxyacetic acid by Alcaligenes eutrophus JMP134. Arch Microbiol 150(1):95–102. doi:10.1007/BF00409724
Pollmann K, Beil S, Pieper DH (2001) Tranformation of chlorinated benzenes and toluenes by TecA tetrachlorobenzene dioxygenase and TecB chlorobenzene dihydrodiol dehydrogenase of Ralstonia sp. strain PS12. Appl Environ Microbiol 67(9):4057–4063. doi:10.1128/AEM.67.9.4057-4063.2001
Pollmann K, Kaschabek S, Wray V, Reineke W, Pieper DH (2002) Metabolism of dichloromethylcatechols as central intermediates in the degradation of dichlorotoluenes by Ralstonia eutropha sp. strain PS12. J Bacteriol 184(19):5261–5274. doi:10.1128/JB.184.19.5261-5274.2002
Pollmann K, Wray V, Hecht HJ, Pieper DH (2003) Rational engineering of the regioselectivity of TecA tetrachlorobenzene dioxygenase for the transformation of chlorinated toluenes. Microbiology 149(4):903–913. doi:10.1099/mic.0.26054-0
Pollmann K, Wray V, Pieper DH (2005) Chloromethylmuconolactones as critical metabolites in the degradation of chloromethylcatechols: why 2-chlorotoluene is so difficult to degrade. J Bacteriol 187(7):2332–2340. doi:10.1128/JB.187.7.2332-2340.2005
Prucha M, Wray V, Pieper DH (1996) Metabolism of 5-chlorosubstituted muconolactones. Eur J Biochem 237(2):357–366. doi:10.1111/j.1432-1033.1996.00357.x
Ramanand K, Balba MT, Duffy J (1993) Reductive dehalogenation of chlorinated benzenes and toluenes under methanogenic conditions. Appl Environ Microbiol 59(10):3266–3272
Raschke H, Meier M, Burken JG, Hany R, Müller MD, van der Meer JR, Kohler HPE (2001) Biotransformation of various substituted aromatic compounds the chiral dihydrodihydroxy derivatives. Appl Environ Microbiol 67(8):3333–3339. doi:10.1128/AEM.67.8.3333-3339.2001
Reineke W (1998) Development of hybrid strains for the mineralization of chloroaromatics by patchwork assembly. Annu Rev Microbiol 52:287–331. doi:10.1146/annurev.micro.52.1.287
Rogers JE, Gibson DT (1977) Purification and properties of cis-toluene dihydrodiol dehydrogenase from Pseudomonas putida. J Bacteriol 130(3):1117–1124
Sander P, Wittich RM, Fortnagel P, Wilkes H, Francke W (1991) Degradation of 1,2,4-trichloro- and 1,2,4,5-tetrachlorobenzene by Pseudomonas strains. Appl Environ Microbiol 57(5):1430–1440
Schraa G, Bethe BM, van Neerven ARW, van den Tweel WJJ, van der Wende E, Zehnder AJB (1987) Degradation 1,2-dimethylbenzene by Corynebacterium strain C125. Antonie van Leeuwenhoek 53(3):159–170. doi:10.1007/BF00393844
Scorecard (2009) Scorecard—the pollution information site, search term: 2-chlorotoluene, US-Government. http://www.scorecard.org. Accessed 1 Mar 2011
Bundesministerium für Umwelt, Naturschutz und Reaktorsicherheit BMU (2001) 2-Chlorotoluene. In: OECD-SIDS 2-Chlorotoluene. SIDS initial assessment report for 11th SIAM. UNEP–Publications, Orlando, USA
Vandenbergh PA, Olsen RH, Colaruotolo JF (1981) Isolation and genetic characterization of bacteria that degrade chloroaromatic compounds. Appl Environ Microbiol 42(4):737–739
Verschueren K (2001) Handbook of environmental data on organic chemicals, 4th edn. Wiley, New York
Weisel CP (2006) Investigation of indoor air sources of VOC contamination. New Jersey Department of Environmental Protection, USA
Wunder D (2003) 2,4-Dichlorotoluene graphical pathway map. University of Minnesota
Yadav JS, Wallace RE, Reddy CA (1995) Mineralization of mono- and dichlorobenzenes and simultaneous degradation of chloro- and methyl-substituted benzenes by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol 61(2):677–680
Yeh WK, Gibson DT, Liu TN (1977) Toluene dioxygenase: a multicomponent enzyme system. Biochem Biophys Res Commun 78(1):401–411. doi:10.1016/0006-291X(77)91268-2
Acknowledgement
We want to thank Dr. DH Pieper from the Helmholtz Centre for Infection Research, Braunschweig, for his encouraging ideas and continuous help, Dr. J Lalucat from the Institute of Microbiology of the Universidad de las Islas Baleares, Mallorca, for his help in identification of the used strain and finally, Dr. B Kuch (Institute of Sanitary Engineering, Water Quality and Solid Waste Management, University of Stuttgart) and PD Dr. P Fischer (Institute of Organic Chemistry, University of Stuttgart) for the GC-MS and 1H-NMR analyses.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dobslaw, D., Engesser, KH. Degradation of 2-chlorotoluene by Rhodococcus sp. OCT 10. Appl Microbiol Biotechnol 93, 2205–2214 (2012). https://doi.org/10.1007/s00253-011-3543-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3543-5