Abstract
In theory, heat production rates are very well suited for analysing and controlling bioprocesses on different scales from a few nanolitres up to many cubic metres. Any bioconversion is accompanied by a production (exothermic) or consumption (endothermic) of heat. The heat is tightly connected with the stoichiometry of the bioprocess via the law of Hess, and its rate is connected to the kinetics of the process. Heat signals provide real-time information of bioprocesses. The combination of heat measurements with respirometry is theoretically suited for the quantification of the coupling between catabolic and anabolic reactions. Heat measurements have also practical advantages. Unlike most other biochemical sensors, thermal transducers can be mounted in a protected way that prevents fouling, thereby minimizing response drifts. Finally, calorimetry works in optically opaque solutions and does not require labelling or reactants. It is surprising to see that despite all these advantages, calorimetry has rarely been applied to monitor and control bioprocesses with intact cells in the laboratory, industrial bioreactors or ecosystems. This review article analyses the reasons for this omission, discusses the additional information calorimetry can provide in comparison with respirometry and presents miniaturization as a potential way to overcome some inherent weaknesses of conventional calorimetry. It will be discussed for which sample types and scientific question miniaturized calorimeter can be advantageously applied. A few examples from different fields of microbiological and biotechnological research will illustrate the potentials and limitations of chip calorimetry. Finally, the future of chip calorimetry is addressed in an outlook.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Conventional calorimeters offer high resolution and precision in measuring heat and heat production rates, provided that the mass of the sample is not too small (Winter and Höhne 2003). Furthermore, such instruments were optimized for sensitivity for one century, with the consequence that they not only are highly sophisticated but also very delicate devices primarily designed for laboratory usage. Hence, the conditions inside calorimeter measuring chambers are optimal for calorimetric measurements, but often inadequate for growth and product formation by microorganisms. Moreover, high-throughput (e.g. multi-well plate) analytical equipment, which is established in biotechnology or medical research, lacks calorimetric analogues. Finally, conventional calorimeters are often too expensive and inflexible to monitor ecosystems or bioreactors.
Two main loopholes profiting from the potential of calorimetry without suffering from their weaknesses are recently under investigation. First, for large bioreactors, enthalpy balances of the entire system can be established (von Stockar and Marison 1991). The advantage of this solution using the reactor as the calorimeter is that with any upscaling, the ratio of the heat-producing volume to the heat-exchanging surface becomes more favourable, and thus the accuracy increases with the volume. Such a type of calorimetry is advantageously chosen for the litre to cubic metre scale. The typical sensitivity ranges from 0.005 to a few watts per litre (Garcia-Payo et al. 2002; van Kleeff et al. 1996; Voisard et al. 2002; Türker 2003; Schubert et al. 2007; Biener et al. 2010; Mahadevan et al. 2010). Besides the requirement of large sample sizes and the typically low sensitivity, this type of calorimeter is not suited for high sample throughput or parallel measurements. This approach that has been referred to as “megacalorimetery” or fermentation calorimetry is not the subject of the following review.
Miniaturization is the second strategy to overcome the weaknesses of conventional calorimeters. All calorimetric functions (e.g. well-defined heat conductance to the heat sink, temperature sensors, heating resistors and sample containers) can be integrated on an electronic silicon chip. The resulting type of calorimeter is usually called a chip calorimeter, nano-calorimeter or miniaturized calorimeter. In the following, we will use the term chip calorimeter for the entire calorimeter and designate its core as calorimeter chip. The crucial feature of the calorimeter chip is a thin membrane of several tens of nanometers up to a few micrometres’ thickness which serves as a sample holder. Thin film calibration heaters and temperature sensors for heat and heat flow measurement are integrated therein. The extremely low heat capacity and high thermal resistance of the chip membrane are essential preconditions to investigate ultra-fast processes and to detect heat dissipation from small samples. Flow-through channels can be attached to calorimeter chips qualifying the system for measuring many samples at high frequency. Due to their extraordinarily small size, the arrangement of several calorimeter chips in an array is possible. The production in well-established microelectromechanical systems technology allows in principle cheap mass production. Baier et al. (2005), Lerchner et al. (2008a) and Xu et al. (2008) provide more details about the assembly and production of miniaturized calorimeters. Chip calorimeters are advantageously applied to small samples (nanolitres to microlitres) with a volume-specific heat production rate of a few milliwatts per litre until watts per litre. The detection limit of the heat production rate is typically in the nanowatt range.
Three decades ago, the potential of a miniaturization of calorimetry was recognized and the development started. One of the first integrated thin film thermopiles using polysilicon and gold was designed and used as an infrared detector (Lahiji and Wise 1982). The determination of heat capacities and heats of phase transitions of thin polymeric films, nanoclusters and nanoscaled single crystals calls for extremely high-temperature scanning rates (Efremov et al. 2000; Kwan et al. 2001). Chip calorimeters are particularly suited for that purpose. The excellent dynamic performance of chip calorimeters can also be used for the determination of heats of the absorption of organic vapours into thin polymer films (Lerchner et al. 2006a). More restricted but quite fruitful became the recent use of chip calorimeters for the study of biochemical reactions and metabolic processes in living matter. In 1988, the Dutch company Xensor Integration began to design a first closed-membrane chip calorimeter for enzymatic measurements of glucose, penicillin and other substances in buffers, bioreactor suspensions or, ultimately, in blood. For enzymatic measurements, the calorimeter chip was coated with the enzyme on its back side (Bataillard et al. 1993; van Herwaarden 2005). Even living cells were applied as biosensors after immobilization on the chip (Bataillard 1993). Today, an increasing number of publications demonstrate the scientific and technical attention to this new technology.
This mini-review attempts to explain why calorimetry is a valuable complement to established methods of off-gas analysis, and it introduces the newly emerging generation of chip calorimeters. The strengths and weaknesses of miniaturization are discussed and exemplified for few applications from different fields of microbial and biotechnological research. Finally, some limitations of the application of chip calorimetry in life science are discussed.
Calorimetry versus off-gas analysis
For many decades, off-gas analysis has been successfully used to monitor bioreactors. When the oxygen uptake rate (OUR, r O2 in mol O2/(L h)) and carbon dioxide evolution rate (CER, r CO2 in mol CO2/(L h)) are calculated from off-gas analysis and used to describe the microbial bioconversion, this is referred to as respirometry. The OUR can be applied to estimate the heat production rate of aerobic cultures. This approach is called indirect calorimetry, and direct calorimetry is often regarded dispensable. The question arises, however, whether a combination of respirometry and real calorimetry can deliver additional metabolic information.
In 1917, Thornton plotted the combustion enthalpy of multiple organic compounds versus the degree of reductance, γ i (Eq. 1), and found a good linear correlation. The slope Δ CH e depending on the applied database was −(113 ± 6) kJ/e-mol (Thornton 1917; Cordier et al. 1987).
γ i in Eq. 1 is related to 1 C-mol of the compound i having the composition of CHX1OX2NX3. In this concept, all carbon compounds are arranged from γ i = 0 (CO2, the most oxidized compound with the lowest energy) until γ i = 8 (CH4, the most reduced compound with the highest energy). Deviations in combustion enthalpy, Δ CH i, between the general applicable Thornton rule and any individual chemical species are described by the parameter δ i in Eq. 2.
Multiplying the slope of the Thornton or Cordier plot, Δ CH e −(113 ± 6) kJ/e-mol, by the four electrons accepted by oxygen, a tight correlation between the heat evolved and the OUR in the range of −(452 ± 24) kJ/mol O2 results. Indeed, experimentally determined ratios (r q/r O2) usually attain values between −430 and −480 kJ/mol O2, with an average value of −455 ± 15 kJ/mol O2 (Hansen et al. 2004), and are called the oxycaloric equivalent (Gnaiger and Kemp 1990). Note that any oxidation (in the electron transport phosphorylation or via oxydoreductases) has also to match approximately the oxycaloric equivalent. From this point of view, OUR and heat production rate provide indeed the same information. However, if peroxides or other reactive oxygen species are formed, the oxycaloric equivalent is violated. For example, Oroszi et al. (2011) were confronted with this problem when comparing the photosynthetically heat absorbed with the oxygen evolved. Of more practical importance is that any strong deviation in the oxycaloric equivalent indicates either partially anaerobic metabolism or anaerobic zones in the considered bioreactor or ecosystem. Furthermore, there are biotechnologically important products which deviate significantly from the oxycaloric equivalent. As an example, the ratio of heat produced to oxygen consumed, r q/r O2, provides theoretically a real-time measure for the biomass-related yield coefficient Y X/S (Eq. 3).
δ X, δ S or δ N describe the deviation of the biomass, substrate and ammonia as nitrogen sources from the Thornton rule. The calculated typical δ X and δ S are <10% based on published values (von Stockar et al. 1993). δ N is approximately 2%, taking the data of Vanderzee et al. (1972) and assuming an Δ CH e of −113.75 kJ/e-mol. The effect of these deviations on r q/r O2 is <10%. Indeed, the observed r q/r O2 ratio for complete aerobic growth without product formation ranges between −385 and −495 kJ/mol O2 (Birou et al. 1987). This means that for exploiting the relation in Eq. 3, the OUR as well as the heat production rate have to be measured very accurately.
Contrary to the oxycaloric equivalent, the ratio of the heat released to CER (r q/r CO2) depends more strongly on the degree of reductance, γ S, of the organic compound S undergoing combustion (Eq. 4; Hansen et al. 2004):
If the microorganisms are growing, then the ratio r q/r CO2 provides information on the yield coefficient Y X/S (Eq. 5; Hansen et al. 2004).
Theoretically, the largest influence of Y X/S on r q/r CO2 is given for growth on methane (γ S = 8).
For the application of Eqs. 3 and 5, it is necessary to estimate the relative degree of reductance for biomass γ X. This is facilitated by the fact that the elementary composition of biomass, and thus the degree of reductance, is nearly independent on the bacterial strain or on the growth condition (Stephanopoulos et al. 1998). A good estimate for bacterial biomass is CH1.8O0.5N0.2 with a relative degree of reductance of 4.8 e-mol/C-mol (van Gulik and Heijnen 1995). If also the consumption of ammonia as nitrogen source on r q/r CO2 is considered, Eq. 5 changes slightly to Eq. 6.
Here, x 3 is the number of nitrogen atoms in the hypothetical biomass equation. x 3 equals 0.2 in the case of the van Gulik biomass.
In summary, the calorespirometric ratios measured for living microorganisms, tissues or complex environmental samples (e.g. soil) can deviate from the predicted oxycaloric equivalents and contain information about coupling of catabolic and anabolic processes (Dejean et al. 2001) or anaerobic contributions to the metabolism (Gnaiger and Kemp 1990). In combination with biochemical models, it is possible to even obtain information about the metabolic pathways. For instance, there are reports of calorespirometric values as large as nearly −1,100 kJ/mol O2 (Schön and Wadsö 1988), which have been ascribed to anaerobic lactate formation under anoxic conditions.
Design and operation of chip calorimeters
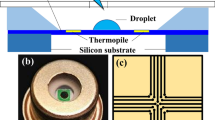
Whereas the accurate off-line gas analysis of bioreactors is the state of the art, heat monitoring is still in its infancy. Chip calorimetry is one way to measure the heat production rate at-line to bioreactors. Figure 1a shows the scheme of a calorimeter chip with an attached flow-through cell. A technical implementation of a calorimeter chip developed by the Freiberg calorimetry group (Lerchner et al. 2008a, c) is presented in Fig. 1b. This is just one example for the principle of chip calorimetry. Many more different designs are described in the literature.
The biocatalysts of interest (i.e. enzymes or microorganisms in either suspended or immobilised states) are located inside of the flow-through cell. In the case of biological samples, steady-state or slowly changing processes are typically taking place. Therefore, a heat flux towards the heat sink is measured rather than the accumulation of heat on the calorimeter chip. The heat is transferred mainly through the chip membrane generating a temperature gradient ΔT, which is converted into a voltage signal by the thermoelectric effect of the thermopile. If the thermal resistance, R th, is well defined, then the output voltage, U TP, is a real-time measure of the heat production rate, P Bio (Eq. 7). The thermopower of the thermopile is given by the Seebeck coefficient α and the number of thermocouples n which are connected in series. Usually, the calorimetric sensitivity, S P, is experimentally determined by Joule (electrical) heating or chemical calibration (Lerchner et al. 2002). Chemical calibration consists in the comparison of the voltage signal with the known heat production rate of a chemical reaction determined by well-defined enthalpy and kinetics.
In theory, the sensitivity can be increased by the choice of material combinations with high Seebeck coefficients, high numbers of thermocouples and high thermal resistance of the heat-conducting pathways. Typical Seebeck coefficients are (all in microvolts per Kelvin; Au–Ni, 22; Ti–Bi, 84; Bi–Sb/Bi, 160; Si–Au, 293, Si–Al, 303). Unfortunately, the choice of the most suited thermocouple is not completely free as it has to be compatible with the applied membrane material. The number of maximum possible thermocouples depends on the geometry of the sample. Samples with a diameter in the micrometre range allow usually a maximum of ten thermocouples, whereas for samples in the millimetre range, a few hundred thermocouples are possible. Tuning the chip calorimeter sensitivity using R th could be mainly done by reducing the membrane thickness. However, there are technological limits and a thin membrane is often less robust. Furthermore, with increasing R th, the uncontrolled heat exchange with the environment gets more importance and the signal is adversely affected. Thus, calorimeter chips with a high-temperature sensitivity and low thermal resistance are preferred.
Materials of the membranes are often epitaxial silicon and silicon oxynitride, but also thin polymer films. The thickness usually ranges from a few nanometres to about 100 μm. The temperature sensors located on the membrane are mostly thermocouples. However, also the temperature dependency of the electrical resistance of thin film thermistors is sometimes utilized.
Sample size and signal-to-noise ratio
Chip calorimeters are preferentially applied to small samples. As recently outlined (Lerchner et al. 2008a), the signal-to-noise ratio (SNR) drops down with decreasing sample size if the heat production rate of the investigated process is slowly changing or in steady state. In this case, the output voltage signal, U TP, is related to the overall heat production rate, P Bio. P Bio depends on the sample volume V, the specific heat production rate of the species (e.g. bacteria, mammalian cell or yeast) q c, and the species concentration c c (Eq. 8).
The signal noise, σ u (standard deviation), is mainly determined by electronic noise and the influence of external temperature fluctuations.
The considered situation is typical for most metabolic processes. However, in the case of fast reactions, e.g. reactions catalyzed by highly active enzymes, only short heat signals are evolved. Here, the heat accumulates on the chip due to the high heat resistance of the membrane and the process becomes mainly adiabatic. Then, the SNR becomes independent of V and a strong reduction of the sample size becomes possible (Lerchner et al. 2008a). Practical consequences for the required sample volume are illustrated in Table 1, which shows the detectable concentrations for a series of microorganisms and mammalian cells assuming a heat flow detection limit of 50 nW and a sample volume of 10 μL. Furthermore, data for the required sample volume for the detection of 106 individuals per millilitres are listed. The values were estimated using data taken from Günther and Bergter (1971), Beezer et al. (1974) and Xu et al. (2008). It is obvious that a chip calorimeter for a sample size of <1 nL and a volume-related detection limit of 18 W L−1 is only applicable to densely packed mammalian cells (Johannessen et al. 2002), whereas in general, microlitre volumes are required for bacterial samples.
Samples in the nanolitre scale have several advantages. They can be directly deposited on the chip calorimetric membrane as lipid droplets. Droplets of a few nanolitres can be merged or mixed within <70 ms using electrostatic forces (Torres et al. 2004). Furthermore, due to the small sample size, several chip calorimeters can be combined to arrays (Verhaegen et al. 2000; Torres et al. 2004; Salemme 2004). Finally, an endothermic evaporation enthalpy of the solvent does not adversely affect the measurement, if the reaction time is short.
Slow reactions with low heat production rates were preferentially analysed in flow-through mode in closed channels (van Herwaarden 2005; Baier et al. 2005). Here, the sample size is a few microlitres. The channels can be glued onto the membrane or micromechanically constructed during the manufacturing of the chip (Zhang and Tadigadapa 2004). The small size of the channel limits the dosage time and reduces the Reynolds number. Thus, the mixing process is slow due to laminarity and the diffusion-controlled mass transfer. Finally, the high surface-to-volume ratio makes the system prone to carryover of contaminations.
The present record in absolute detection limit of chip calorimeters (to our knowledge) is 4.2 nW for a sample volume of 3.5 nL (Lee et al. 2009). However, this value corresponds to a volume-specific detection limit of only 1.2 W/L. Chip calorimeters specially designed for metabolic conversions achieve volume-specific detection limits in the range of a few 10−3 W/L. They are much more sensitive than reaction or fermentation calorimeters (0.005–0.1 W/L), but less sensitive than micro-calorimeters (e.g. approx. 2.5 × 10−5 W/L, σ P = 100 nW, 4 mL, TAMIII; http://www.tainstruments.com). The following example illustrates the sensitivity of micro-calorimeters for easy comparison with the performance of conventionally applied respirometry or concentration measurements. Here, a zero-order kinetic was assumed. The solubility of oxygen in water is approx. 8 mg/L (25°C, 101,325 Pa, air-saturated). Even if this tiny amount of oxygen is consumed after 52.5 days, then this could be calorimetrically detected (assuming −455 kJ/mol O2). The calorimetric detection limit of 2.5 × 10−5 W/L corresponds to the diminutive consumption rate of glucose of 0.03 μM/h or 6 μg/(L h), assuming complete combustion.
Signal evaluation
Figure 2 shows typical signals of a chip calorimeter used in flow-through mode.
Typical chip calorimetric signals obtained with bacteria. a Steady-state heat production rate (SS) after injection and before flush away of the bacterial suspension (*effect of pump actions). b Transient heat production rate of a biofilm after injection of nutrients (PM peak minimum, PI integral of the exothermal peak). c Calorimetric signals obtained from microbial suspensions or biofilms of increasing activity (from a to d). The details are given in Lerchner et al. (2008c)
For separating the heat signal from the baseline, a stopped-flow measurement regime with consecutively alternating injection and waiting periods is applied. The small time constant of the chip calorimeter (about 10 s) allows such a procedure. After baseline stabilization, a bacterial suspension (in the case of growing, planktonic cells) or a substrate solution (in the case of surface-associated growing cells) is injected into the flow line. The signal is affected by both the metabolic heat production rate and the thermal disturbance due to imperfect thermal equilibration. The latter disappears soon after the injection is stopped. The signal drops down to a certain level (SS) after the injection is finished. At that time, the metabolic activity determines the signal. After measuring a stable signal, the bacterial suspension can be flushed away using a metabolically inert solution. Again, the signal goes back to the baseline after the thermal disturbance due to the injection being finished (Fig. 2a). In the case of aerobic metabolism, the oxygen consumption kinetics determines the signal for highly active cells. Here, the signal becomes more dynamic with increasing metabolic activity (see lanes a–d in Fig. 2c). This effect can complicate the signal evaluation. Two solutions are thinkable. First, the oxygen consumption is fitted with a kinetic model and the real heat production rate obtained by extrapolation. Second, the bacterial suspension is amended with an oxygen-enriched medium. However, both strategies have their drawbacks because extrapolation of data above the measurement range is always doubtful and a fast change of oxygen tension may cause metabolic changes of the monitored microorganisms.
Applications of chip calorimetry
In Table 2, an overview of recent applications of the Freiberg chip calorimeter is given. In the following, the chip calorimetric monitoring of the bacterial growth in bioreactors, operated as discontinuous and continuous processes, and the application of the chip calorimeter for the biofilm analysis are outlined in more detail.
Monitoring of discontinuous bioprocesses
The easiest way to follow microbial activities calorimetrically is seen in the connection of a conventional bioreactor with a thermal sensor used at-line (Blomberg et al. 1988). This approach was also used to test activity monitoring by chip calorimetry. Both the rapid sample transfer and the improved technical compatibility with the reactor periphery qualify a chip calorimeter as a diagnostic tool in bioreactor control. Challenges are not the sensitivity required sample size but the robustness against viscosity caused by high biomass density, gas bubbles from the intense aeration of the microbial culture inside the reactor, contamination of the microfluidic components and, most importantly, the need for quick sample transfer in order to avoid oxygen depletion. First results from a batch culture of Escherichia coli growing in a lab-scale bioreactor and monitored by a chip calorimeter were reported by Maskow et al. (2006a). The measured heat signal was compared with the thermokinetically modelled heat signal using non-calorimetric data (i.e. substrate consumption, side product and biomass formation). Results for the aerobic growth of E. coli DH5α on glycerol demonstrate that chip calorimetry is in principle suited for reactor monitoring (Fig. 3). The observed two growth phases result from growth on glycerol and formation of acetate followed by growth on acetate.
Test of the chip calorimeter with E. coli DH5α aerobically grown on glycerol. Triangles represent chip calorimetric measurements. The line represents results of thermokinetic modelling using non-calorimetric data. The details are described in Maskow et al. (2006a)
At-line chip calorimetry can be applied as long as the microbial activity is low and all nutrients are sufficiently available (lanes b and c in Fig. 2c). To avoid situation (d) in Fig. 2c, the sample transfer time to the calorimeter chip has to be minimal.
For that purpose, the Freiberg chip calorimeter was modified and a new sophisticated operation regime was implemented. This led to a considerable increase of its application range with respect to the biomass density. Specifically, the modifications included integrating the heat flow meter with the thermostats, the electronic signal preconditioning components and some of the fluidic components into a compact probe which can be installed very close to the bioreactor. The probe fits into a standard bioreactor port. In this way, the distance between the reactor interior and the sample entry port was reduced to a few centimetres (Fig. 4). The inner diameter of connecting tubes and the heat exchanger were extended from 0.3 to 0.6 mm to tolerate volume flow rates up to 300 μL min−1 and culture broth of high viscosity. The heat exchanger was equipped with an auxiliary heater to improve its efficiency. New fluidic operation modes were implemented for trapping gas bubbles and for online dilution of the absorbed bioreactor broth.
Newly developed chip calorimetric probe (b) designed for a direct connection with any type of bioreactor via a sterilizable standard 25-mm port. The housing of the probe was removed for better insights. In this example, the probe is connected with a pressure bioreactor (a). Details will be published soon by the authors
The housing was designed stable enough for 1,000-kPa overpressure to ensure the applicability to pressurized bioreactors. This chip calorimetric probe was tested by comparing the measured heat production rate with a curve calculated from the OUR (Fig. 5).
Test of the chip calorimetric probe using E. coli K12 aerobically grown on 10 g/L glycerol by comparison with the heat production rates calculated from the oxygen uptake rate (a). b Time until the oxygen of the sample is consumed after leaving the bioreactor. For maximizing this time, the oxygen partial pressure in the bioreactor was controlled to be always higher than 90% air saturation. The oxygen consumption time was calculated from the online monitored oxygen uptake rate (OUR). The details will be published by the authors
A sensitivity of 3 × 10−3 W/L was achieved with the chip calorimetric probe. After 4.2 h, the microbial activity was so high that no stable calorimetric signals were obtained due to fast oxygen depletion (Fig. 5b). The form of the calorimetric signal corresponded to situation (d) in Fig. 2c. Thus, a maximum heat production rate of approx. 0.8 W/L was directly measureable calorimetrically (using steady-state data), whereas the remaining part of the calorimetric results were obtained by extrapolation using the oxygen depletion kinetics as described above. Applying pre-dilution and continuous injection, the measuring range could be extended to above 3 W L−1.
All of the so far described chip calorimeters require a very stable reference temperature, small sample volume or adiabatic systems. In the proposed chip calorimeter, these requirements are met by housing the chip inside a high-precision thermostat (see Fig. 4), with a temperature stability better than 100 μK. In order to overcome such limitations, a group from the Yonsei University Seoul (South Korea) introduced a dual thermopile micro-calorimeter. Consequently, their device was self-compensating to a certain extent so that the typical surrounding adiabatic vacuum or temperature control was no longer required (Kwak et al. 2008).
Monitoring and control of continuous bioprocesses
Growth-associated product formation by microorganisms in continuous processes has various practical advantages in comparison to batch or fed-batch cultivations. Normally, the kinetic profile in continuous culture is determined by the dilution rate. The optimum of the bioprocess is achieved at the dilution rate of maximum productivity which is usually near the maximum dilution rate. However, the maximum dilution rate which corresponds correlates with the maximum specific growth rate of the applied microorganisms could change during continuous bioprocesses due to mutations and phenotypical adaptation (Novick and Szilard 1950; Weikert et al. 1997; Groeneveld et al. 2009). Thus, the online monitoring of the hidden specific maximum growth rate would help operate continuous bioprocesses optimally (Maskow et al. 2006b). Chip calorimetry should be particularly suited for such a task because bacterial samples from the bioreactor can be easily mixed with substrate mixtures to check the metabolic capacity at-line to the bioprocess. Figure 6a–c presents the measurement procedure, the respective thermal trace and the steady-state signal, respectively. The measurement procedure was divided into four parts.
Triggers (a) and heat responses (b) obtained by at-line chip calorimetry of a continuous culture of Heliamphora elongata (D = 0.12 h−1). c Maximum thermal signals with (filled triangles) and without additional substrate (open triangles) as a function of the dilution rate. The dotted line indicates the calculated maximum thermal signals (without additional substrate). Details will be published soon by the authors
First, the thermal signal of the culture broth from the chemostat with additional substrate (250 mg L−1, sample + pulse) was measured. Due to the surplus of the substrate, this signal represents cells working with their maximum metabolic capacity. Second, measuring a metabolically inactive reference solution provided the baseline. Third, the heat signal of the culture broth alone was measured. This signal represents the metabolic activity as it represents the actual stage of the chemostat. Finally, the response to the metabolically inert reference solution as baseline was monitored again. The difference between the signal with the amended substrate and the culture broth alone should represent the still unexploited metabolic capacity. Indeed, plotting both signals in dependency on the dilution rate show a tangency point at the maximum dilution rate (Fig. 6c). These results show that chip calorimetry can help run chemostatic bioprocesses at the dilution rate of maximum productivity.
Application for biofilm analysis
In the past, nearly all calorimetric measurements have addressed planktonic microorganisms, whereas in nature, an estimated 90% of all microorganisms live in biofilms. This surprising disproportion is caused by the demanding interpretation of thermodynamic data from immobilised biomass. Relatively recent data from pioneering calorimetric measurements have not been completely exploited in quantitative terms (von Rège and Sand 1998; Wentzien et al. 1994). Traditional biofilm experiments are usually carried out in flow lines. Therefore, the transfer of this technique to calorimetry requires biofilm cultivation in flow-through calorimeters. It has allowed the detection of biofilm poisoning (von Rège and Sand 1998; Wentzien et al. 1994) and detachment (Peitzsch et al. 2008) within a few minutes after its occurrence. Weaknesses of biofilm investigations with conventional micro-calorimeters have been (1) the costs of the calorimeters; (2) their inadequacy for high-throughput measurements; (3) the occupation of the instruments for weeks of biofilm cultivation; and (4) restrictions regarding the appropriate substratum for biofilm growth. In a conventional micro-calorimeter, the flow-through channel is often made of gold, which is difficult to substitute or modify. Chip calorimetric technique can overcome these weaknesses by employing exchangeable flow chambers. With the achieved detection limit of 20 nW, it is possible to analyse even early-stage biofilms (approx. 3 × 105 cells/cm2; Lerchner et al. 2008b). First investigations show the applicability of this technology for screening the efficiency of antibiotic treatment (Buchholz et al. 2010; Fig. 7).
The experiments are performed as roughly sketched in the following. Biofilms are pre-cultivated on a multitude of exchangeable chambers outside of the calorimeter. Then, a couple of the chambers holding a mature biofilm are mounted sequentially in the calorimeter and monitored. The leftover can be used for reference analytics. Using the microfluidics of the calorimeter, the biofilm is flushed with the nutrient solution and, where necessary, treated with biologically active substances. Later, in-depth analyses with conventional techniques (e.g. CFU counting, confocal laser scanning microscopy, etc.) can follow. The combination of calorimetry with conventional techniques promises new insights into biofilm formation and resistance against biocides, antibiotics or biological treatments.
In light of the increasing attention of biofilms for industrial bioconversion processes (Qureshi et al. 2005), the chip calorimetric monitoring technology is likely to gain more importance.
Conclusion and outlook
Calorimetry is a powerful method for the analysis of biochemical and metabolic processes, in particular if the measured heat production rate is compared with metabolic fluxes or respirometry. Due to their extraordinary sensitivity, conventional micro-calorimetry is applied successfully for the detection of bacterial infections in body fluids (Ripa et al. 1977; Trampuz et al. 2007) and for the screening of the impact of pharmaceuticals, nanoparticles, metal ions (Zhou et al. 2010) and xenobiotics on the metabolism of microorganisms (Mariana et al. 2010), mammalian cells (Braissant et al. 2010; Braissant and Daniels 2011) and small animals (Normant et al. 2007). As outlined above, the advantages of chip calorimeters in comparison with conventional micro-calorimeters (e.g. the small time constant and the low sample requirement) qualify this technology for screening. However, some limitations with respect to the measurement range and high-throughput screening (HTS) abilities have to be considered and overcome.
First, chip calorimeters will never be as sensitive and accurate as conventional micro-calorimeters due to the planar geometry of the heat power detector. Bacterial densities <106 mL−1 are hard to detect in chip calorimetrically. This is important for studies on the efficiency of antibiotic treatment and for the detection of bacterial infections in body fluids. The analysis of the thermodynamics and kinetics of peptide–ligand interactions, which is an important field in biocalorimetry, also requires considerably lower volume-specific detection limits than chip calorimetry can achieve. Here, conventional micro-calorimetry will assert its position. Two ways can be considered to increase the sensitivity of chip calorimetry. In theory, the SNR can be improved with increasing the sample sizes. Indeed, Higuera-Guisset et al. (2005) achieved an extremely low volume-specific detection limit of 2 × 10−4 W/L combining a big calorimetric vessel volume (0.6 mL) with chip calorimetric detection. However, inhomogeneities bias the signal with increasing sample volume (Torra et al. 2001) and external temperature fluctuations get more influence (Lerchner et al. 2006b). Enrichment of bacteria or biomolecules might be a more reasonable way to reduce the detection limits. The Freiberg calorimetry group achieved a reduction in detection limit by at least one order of magnitude by a combination of the biomagnetic separation method, i.e. the binding of bacteria to magnetic beads (Deponte et al. 2004), and chip calorimetry (unpublished results). Using the same technique, the enthalpy of DNA hybridization could be determined (Ahmad et al. 2010). Unfortunately, additional sample preparation steps are required and bead transfer techniques for routine use are still lacking.
Second, oxygen consumption during the sample transfer to the calorimeter chip is limiting the measurable biomass density for aerobic bioprocesses. It is probably impossible to monitor bacterial activities chip calorimetrically when they result in more than 5 W L−1. Thus, the application to high cell density cultures is impossible. For such cases, megacalorimetry or fermentation calorimetry appears to be more promising.
Third, the applicability of chip calorimeters for HTS should be considered. The low thermal time constants appear promising for high sample throughput. However, laminar flow conditions lead to sample dispersion and the high surface-to-volume ratio of the fluidic components provokes contaminations, which often establish as stable biofilms. Such a biofilm requires time-consuming cleaning operations. Therefore, even chip calorimetry is hardly suited to compete with HTS based on multilocation microplates which are then monitored electrochemically or optically. Again, two potential solutions are under consideration. Arrays of calorimeter chips (Verhaegen et al. 2000; Torres et al. 2004; Salemme 2004; Recht et al. 2008) which allow highly parallelized measurements could be one solution of the problem. However, high costs of bigger silicon devices, expensive robotic systems for sample dispensing, evaporation of the solvent of free-standing droplets and cleaning efforts restrict the application of this technique. These problems led to the termination of the development of one array design (i.e. MiDiCal™, Vivactis, BE). An “enthalpy array” of the Palo Alto Group (USA; Torres et al. 2004) is currently still in use, but mainly for the analysis of (fast) enzyme-catalyzed reactions and not for bacterial metabolism, possibly since it is too small (0.5 μL). Recently, a multiwall plate calorimeter prototype has been presented in the Internet (http://www.symcel.se). However, this prototype is not a miniaturized calorimeter as it uses the technology of a conventional heat flow calorimeter.
The other potential solution is to speed up the serial throughput using a single-chip calorimeter. In collaboration with the Institute for Bioprocessing and Analytical Measurement Techniques, Germany, the Freiberg Group is going to integrate the segmented flow technology with chip calorimetry. Aqueous sample segments of a few microlitres are embedded into a carrier flow of a water immiscible liquid (see, e.g. (Köhler et al. 2004). The interface tension between the carrier and sample liquid forces plug flow, and the hydrophobicity of the internal surface of the fluidic walls leads to the formation of a thin film between the sample and wall, which avoids contaminations. It is expected that a sampling frequency of at least 15 per hour can be achieved in this way.
After the wide applicability of chip calorimetry in life sciences has been demonstrated over the last 10 years, it appears time to let a broader scientific community profit from the potential of this new technology. For that purpose, the Freiberg chip calorimeter will be commercialized by a UK company (TTP LabTech Ltd., Melbourn, UK; Lewis et al. 2011).
References
Ahmad LM, Towe B, Wolf A, Mertens F, Lerchner J (2010) Binding event measurement using a chip calorimeter coupled to magnetic beads. Sens. Actuators, B 145:239–245
Baier V, Foedisch R, Ihring A, Kessler E, Lerchner J, Wolf G (2005) Highly sensitive thermopile heat power sensor for micro-fluid calorimetry of biochemical processes. Sens Actuators, B 123–124:354–359
Bataillard P (1993) Calorimetric sensing in bioanalytical chemistry: principles, applications and trends. Trends Anal Chem 12(10):387–394
Bataillard P, Steffgen E, Haemmerli S, Manz A, Widmer HM (1993) An integrated silicon thermopile as biosensor for the thermal monitoring of glucose, urea and penicillin. Biosens Bioelectron 8(2):89–98
Beezer AE, Bettelheim KA, Newell RD, Stevens J (1974) The diagnosis of bacteriuria by flow microcalorimetry, a preliminary report. Sci Tools 21:13–16
Biener R, Steinkämper A, Hofmann J (2010) Calorimetric control for high cell density cultivation of a recombinant Escherichia coli strain. J Biotechnol 146(1–2):45–53
Birou B, Marison IW, Stockar UV (1987) Calorimetric investigation of aerobic fermentations. Biotechnol Bioeng 30(5):650–660
Blomberg A, Larsson C, Gustafsson L (1988) Microcalorimetric monitoring of growth of Saccharomyces cerevisiae: osmotolerance in relation to physiological state. J Bacteriol 170(10):4562
Braissant O, Daniels AU (2011) Closed ampoule isothermal microcalorimetry (IMC) for continuous real-time detection and evaluation of cultured mammalian cell activity and responses. In: Stoddard M (ed) Mammalian cell viability methods. Springer, Heidelberg, pp 191–208
Braissant O, Wirz D, Goepfert B, Daniels AU (2010) Biomedical use of isothermal microcalorimeters. Sensor 10:9369–9383
Buchholz F, Wolf A, Lerchner J, Mertens F, Harms H, Maskow T (2010) Chip calorimetry for fast and reliable evaluation of bactericidal and bacteriostatic treatments of biofilms. Antimicrob Agents Chemother 54(1):312–319
Cordier JL, Butsch BM, Birou B, Stockar U (1987) The relationship between elemental composition and heat of combustion of microbial biomass. Appl Microbiol Biotechnol 25(4):305–312
Dejean L, Beauvoit B, Bunoust O, Fleury C, Guerin B, Rigoulet M (2001) The calorimetric–respirometric ratio is an on-line marker of enthalpy efficiency of yeast cells growing on a non-fermentable carbon source. Biochim Biophys Acta 1503(3):329–340
Deponte S, Steingroewer J, Löser C, Boschke L, Bley T (2004) Biomagnetic separation of Escherichia coli by use of anion-exchange beads: measurement and modeling of the kinetics of cell–bead interactions. Anal Bioanal Chem 379:419–426
Efremov MY, Schiettekatte F, Zhang M, Olson EA, Kwan AT, Berry RS, Allen LH (2000) Discrete periodic melting point observations for nanostructure ensembles. Phys Rev Lett 85(17):3560–3563
Garcia-Payo MC, Ampuero S, Liu JS, Marison IW, von Stockar U (2002) The development and characterization of a high resolution bio-reaction calorimeter for weakly exothermic cultures. Thermochim Acta 391(1–2):25–39
Gnaiger E, Kemp RB (1990) Anaerobic metabolism in aerobic mammalian cells: information from the ratio of calorimetric heat flux and respirometric oxygen flux. Biochim Biophys Acta 1016:328–332
Groeneveld P, Stouthamer AH, Westerhoff HV (2009) Super life—how and why ‘cell selection’ leads to the fastest-growing eukaryote. FEBS J 276(1):254–270
Günther HH, Bergter F (1971) Bestimmung der Trockenmasse von Zellsuspensionen durch Extinktionsmessungen. Z Allg Mikrob 11(3):191–197
Hansen LD, Macfarlane C, McKinnon N, Smith BN, Criddle RS (2004) Use of calorespirometric ratios, heat per CO2 and heat per O2, to quantify metabolic paths and energetics of growing cells. Thermochim Acta 422(1–2):55–61
Higuera-Guisset J, Rodriguez-Viejo J, Chacon M, Munoz FJ, Vigues N, Mas J (2005) Calorimetry of microbial growth using a thermopile based microreactor. Thermochim Acta 427(1):187–191
Johannessen EA, Weaver JMR, Bourova L, Svoboda P, Cobbold PH, Cooper JM (2002) Micromachined nanocalorimetric sensor for ultra-low-volume cell-based assays. Anal Chem 74(9):2190–2197
Köhler JM, Henkel T, Grodrian A, Kirner T, Roth M, Martin K, Metze J (2004) Digital reaction technology by micro segmented flow—components, concepts and applications. Chem Eng J 101(1–3):201–216
Kwak BS, Kim BS, Cho HH, Park JS, Jung HI (2008) Dual thermopile integrated microfluidic calorimeter for biochemical thermodynamics. Microfluidics and Nanofluidics 5(2):255–262
Kwan AT, Efremov MY, Olson EA, Schiettekatte F, Zhang M, Geil PH, Allen LH (2001) Nanoscale calorimetry of isolated polyethylene single crystals. J Polym Sci, Part B: Polym Phys 39(11):1237–1245
Lahiji GR, Wise KD (1982) A batch-fabricated silicon thermopile infrared detector. IEEE Trans Electron Devices 29(1):14–22
Lee W, Fon W, Axelrod BW, Roukes ML (2009) High-sensitivity microfluidic calorimeters for biological and chemical applications. Proc Natl Acad Sci USA 106(36):15225
Lerchner J, Wolf G, Auguet C, Torra V (2002) Accuracy in integrated circuit (IC) calorimeters. Thermochim Acta 382(1–2):65–76
Lerchner J, Kirchner R, Seidel J, Wahlisch D, Wolf G, Konig WA (2006a) Determination of molar heats of absorption of enantiomers into thin chiral coatings by combined IC-calorimetric and microgravimetric (QMB) measurements. II. Thermodynamics of enantio selectivity in modified cyclodextrins. Thermochim Acta 445(2):98–103
Lerchner J, Wolf A, Wolf G, Baier V, Kessler E, Nietzsch M, Krügel M (2006b) A new micro-fluid chip calorimeter for biochemical applications. Thermochim Acta 445(2):144–150
Lerchner J, Maskow T, Wolf G (2008a) Chip calorimetry and its use for biochemical and cell biological investigations. Chem Eng Process 47(6):991–999
Lerchner J, Wolf A, Buchholz F, Mertens F, Neu TR, Harms H, Maskow T (2008b) Miniaturized calorimetry—a new method for real-time biofilm activity analysis. J Microbiol Meth 74(2–3):74–81
Lerchner J, Wolf A, Schneider HJ, Mertens F, Kessler E, Baier V, Funfak A, Nietzsch M, Krügel M (2008c) Nano-calorimetry of small-sized biological samples. Thermochim Acta 477(1–2):48–53
Lewis G, Pardoe R, Bowen W, Lerchner J (2011) A low volume, flow-based approach to biocalorimetry. Paper presented at the 19. Ulm-Freiberger Kalorimetrietage, Freiberg, 16–18 March 2011
Mahadevan S, Dhandapani B, Sivaprakasam S, Mandal AB (2010) Batch kinetic studies on growth of salt tolerant Pseudomonas aeruginosa secreting protease in a biocalorimeter. Biotechnol Bioprocess Eng 15(4):670–675
Mariana F, Buchholz F, Harms H, Yong Z, Yao J, Maskow T (2010) Isothermal titration calorimetry—a new method for the quantification of microbial degradation of trace pollutants. J Microbiol Methods 82(1):42–48
Maskow T, Lerchner J, Peitzsch M, Harms H, Wolf G (2006a) Chip calorimetry for the monitoring of whole cell biotransformation. J Biotechnol 122(4):431–442
Maskow T, Müller S, Losche A, Harms H, Kemp R (2006b) Control of continuous polyhydroxybutyrate synthesis using calorimetry and flow cytometry. Biotechnol Bioeng 93(3):541
Normant M, Dziekonskia M, Drzazgowskib J, Lamprecht I (2007) Metabolic investigations of aquatic organisms with a new twin heat conduction calorimeter. Thermochim Acta 458(1–2):101–106
Novick A, Szilard L (1950) Experiments with the chemostat on spontaneous mutations of bacteria. Proc Natl Acad Sci USA 36(12):708
Oroszi S, Jakob T, Wilhelm C, Harms H, Maskow T (2011) Photosynthetic energy conversion in the diatom Phaeodactylum tricornutum. J Therm Anal Calorim 104:223–231
Peitzsch M, Kiesel B, Harms H, Maskow T (2008) Real time analysis of Escherichia coli biofilms using calorimetry. Chem Eng Process 47(6):1000–1006
Qureshi N, Annous BA, Ezeji TC, Karcher P, Maddox IS (2005) Biofilm reactors for industrial bioconversion processes: employing potential of enhanced reaction rates. Microb Cell Fact 4(1):24
Recht MI, Bruyker DD, Bell AG, Wolkin MV, Peeters E, Anderson GB, Kolatkar AR, Bern MW, Kuhn P, Bruce RH (2008) Enthalpy array analysis of enzymatic and binding reactions. Anal Biochem 377(1):33–39
Ripa KT, Mardh PA, Hovelius B, Ljungholm K (1977) Microcalorimetry as a tool for evaluation of blood culture media. J Clin Microbiol 5(4):393–396
Salemme FR (2004) High-throughput biochemistry heats up. Nat Biotechnol 22(9):1100–1101
Schön A, Wadsö I (1988) The use of microcalorimetry in studies of mammalian cells. J Therm Anal Calorim 33(1):47–54
Schubert T, Breuer U, Harms H, Maskow T (2007) Calorimetric bioprocess monitoring by small modifications to a standard bench-scale bioreactor. J Biotechnol 130(1):24–31
Stephanopoulos GN, Aristidou AA, Nielsen J (1998) Metabolic engineering: principles and methodologies. Academic, San Diego
Thornton W (1917) The relation of oxygen to the heat of combustion of organic compounds. Philos Mag 33:196–203
Torra V, Auguet C, Lerchner J, Marinelli P, Tachoire H (2001) Identification of micro-scale calorimetric devices. I. Establishing the experimental rules for accurate measurements. J Therm Anal Calorim 66(1):255–264
Torres FE, Kuhn P, De Bruyker D, Bell AG, Wolkin MV, Peeters E, Williamson JR, Anderson GB, Schmitz GP, Recht MI, Schweizer S, Scott LG, Ho JH, Elrod SA, Schultz PG, Lerner RA, Bruce RH (2004) Enthalpy arrays. Proc Natl Acad Sci USA 101(26):9517–9522
Trampuz A, Salzmann S, Antheaume J, Daniels AU (2007) Microcalorimetry: a novel method for detection of microbial contamination in platelet products. Transfusion 47(9):1643–1650
Türker M (2003) Measurement of metabolic heat in a production-scale bioreactor by continuous and dynamic calorimetry. Chem Eng Commun 190:573–598
van Gulik WM, Heijnen JJ (1995) A metabolic network stoichiometry analysis of microbial growth and product formation. Biotechnol Bioeng 48(6):681–698
van Herwaarden AW (2005) Overview of calorimeter chips for various applications. Thermochim Acta 432(2):192–201
van Kleeff BHA, Kuenen JG, Heijnen JJ (1996) Heat flux measurements for the fast monitoring of dynamic responses to glucose additions by yeasts that were subjected to different feeding regimes in continuous culture. Biotechnol Prog 12(4):510–518
Vanderzee CE, Mansson M, Wadsö I, Sunner S (1972) Enthalpies of formation of mono- and diammonium succinates and of aqueous ammonia and ammonium ion. J Chem Thermodyn 4(4):541–550
Verhaegen K, Baert K, Simaels J, Van Driessche W (2000) A high-throughput silicon microphysiometer. Sensors and Actuators, A 82(1–3):186–190
Voisard D, Pugeaud P, Kumar AR, Jenny K, Jayaraman K, Marison IW, von Stockar U (2002) Development of a large-scale biocalorimeter to monitor and control bioprocesses. Biotechnol Bioeng 80(2):125–138
von Rège H, Sand W (1998) Evaluation of biocide efficacy by microcalorimetric determination of microbial activity in biofilms. J Microbiol Methods 33(3):227
von Stockar U, Marison IW (1991) Large-scale calorimetry and biotechnology. Thermochim Acta 193:215–242
von Stockar U, Gustafsson L, Larsson C, Marison I, Tissot P, Gnaiger E (1993) Thermodynamic considerations in constructing energy balances for cellular growth. Biochim Biophys Acta 1183(2):221–240
Weikert C, Sauer U, Bailey JE (1997) Use of a glycerol-limited, long-term chemostat for isolation of Escherichia coli mutants with improved physiological properties. Microbiology 143(5):1567
Wentzien S, Sand W, Albertsen A, Steudel R (1994) Thiosulfate and tetrathionate degradation as well as biofilm generation by Thiobacillus intermedius and Thiobacillus versutus studied by microcalorimetry, HPLC, and ion-pair chromatography. Arch Microbiol 161(2):116–125
Winter W, Höhne GWH (2003) Chip-calorimeter for small samples. Thermochim Acta 403(1):43–53
Xu J, Reiserer R, Tellinghuisen J, Wikswo JP, Baudenbacher FJ (2008) A microfabricated nanocalorimeter: design, characterization, and chemical calibration. Anal Chem 80(8):2728–2733
Zhang Y, Tadigadapa S (2004) Calorimetric biosensors with integrated microfluidic channels. Biosens Bioelectron 19(12):1733–1743
Zhou Y, Yao J, He M, Choi MMF, Feng L, Chen H, Wang F, Chen K, Zhuang R, Maskow T (2010) Reduction in toxicity of arsenic(III) to Halobacillus sp. Y35 by kaolin and their related adsorption studies. J Hazard Mater 176(1–3):487–494
Acknowledgement
Financial support of the German Research Council (Deutsche Forschungsgemeinschaft, Le1128/1-1, Ma3746/2-1, Ma3746/2-3) and German Federation of Industrial Research Associations (AiF BMWi, AiF-Nr. 244 ZBG) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maskow, T., Schubert, T., Wolf, A. et al. Potentials and limitations of miniaturized calorimeters for bioprocess monitoring. Appl Microbiol Biotechnol 92, 55–66 (2011). https://doi.org/10.1007/s00253-011-3497-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3497-7