Abstract
Xylooligosaccharides have strong bifidogenic properties and are increasingly used as a prebiotic. Nonetheless, little is known about the degradation of these substrates by bifidobacteria. We characterized two recombinant β-xylosidases, XylB and XylC, with different substrate specificities from Bifidobacterium adolescentis. XylB is a novel β-xylosidase that belongs to the recently introduced glycoside hydrolase family 120. In contrast to most reported β-xylosidases, it shows only weak activity on xylobiose and prefers xylooligosaccharides with a degree of polymerization above two. The remaining xylobiose is efficiently hydrolyzed by the second B. adolescentis β-xylosidase, XylC, a glycoside hydrolase of family 43. Furthermore, XylB releases more xylose from arabinose-substituted xylooligosaccharides than XylC (30% and 20%, respectively). The different specificities of XylB, XylC, and the recently described reducing-end xylose-releasing exo-oligoxylanase RexA show how B. adolescentis can efficiently degrade prebiotic xylooligosaccharides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a rising interest in gut health and its modulation, either by direct administration of healthy bacteria (probiotics) or through supplementation of nondigestible food ingredients that selectively stimulate these bacteria (prebiotics). Typically, the aim is to increase the number of bifidobacteria and/or lactobacilli. In vitro studies show xylooligosaccharides (XOS) are among the strongest bifidogenic prebiotics (Crittenden and Playne 1996; Rycroft et al. 2001), and this bifidogenic effect is also seen in vivo in animals and humans (Campbell et al. 1997; Gobinath et al. 2010; Hsu et al. 2004; Okazaki et al. 1990). In mice and rats, XOS consumption not only stimulates bifidobacterial growth, but also leads to a decreased cecal and fecal pH (Campbell et al. 1997; Hsu et al. 2004; Younes et al. 1995), increases short-chain fatty acids (Younes et al. 1995), reduces sulfite-reducing clostridia (Santos et al. 2006), reduces the formation of precancerous lesions (Hsu et al. 2004), and improves diabetic symptoms in diabetic rats (Gobinath et al. 2010; Imaizumi et al. 1991). Positive effects for humans include a decreased fecal pH (Chung et al. 2007; Okazaki et al. 1990) and a reduction of severe constipation in pregnant women (Tateyama et al. 2005). In 1996, the Japanese Government awarded XOS a “foods for special health use” label (Crittenden and Playne 1996). Around 650 tons of XOS is produced annually in Japan (Taniguchi 2004), and today, several companies are marketing a wide range of XOS-supplemented foods (Vázquez et al. 2000).
Despite the increasing use of XOS as a bifidogenic prebiotic, little is known about the enzymes used by bifidobacteria to degrade these substrates. Only one glycoside hydrolase family 51 (GH51) β-xylosidase from Bifidobacterium breve was purified and characterized (Shin et al. 2003), and recently, a GH8 reducing-end xylose-releasing exo-oligoxylanase from Bifidobacterium adolescentis was characterized after recombinant production (Lagaert et al. 2007). While β-xylosidases (E.C. 3.2.1.37) remove xylose residues from the nonreducing end of XOS to the final product xylose, reducing-end xylose-releasing exo-oligoxylanases (E.C. 3.2.1.156) remove xylose residues from the reducing end to the final products xylose and xylobiose. Hence, B. adolescentis needs complementary enzymes to completely hydrolyze XOS.
To further increase our knowledge on XOS hydrolysis by B. adolescentis, we cloned and characterized two of its β-xylosidases and investigated their substrate specificities. XylC was selected for its homology with other GH43 β-xylosidases, while XylB was chosen due to its genomic location immediately downstream of RexA. As recent reports show arabinoxylan oligosaccharides (AXOS) also may have prebiotic properties (Cloetens et al. 2008, 2010; Van Craeyveld et al. 2008), we also measured their hydrolytic capacity towards arabinoxylan and AXOS.
Materials and methods
Cloning
The XylB and XylC coding sequences were amplified from the genomic DNA of B. adolescentis LMG10502 (also catalogued as ATCC15703, DSM20083, and VTT E-981074) by PCR using a HotStar HiFidelity Polymerase kit (Qiagen, Hilden, Germany). Forward primer 5′-ATGA AGT TTG AAT ACC-3′ and reverse primer 5′-GTT GTT CCA TTC CCAA-3′ (derived from the BAF39080 sequence of B. adolescentis ATCC15703) were used for the amplification of the XylB sequence, while forward primer 5′-ATG AAG ATT TCC AAC-3′ and reverse primer 5′-CTG GTT ATC GGA AAG-3′ (based on BAF39209) were used for XylC. The PCR products were ligated in a pEXP5-CT/TOPO expression vector (Invitrogen, Carlsbad, CA, USA), introducing the coding sequences in frame with the C-terminal His6 sequence. Next, Escherichia coli BL21(DE3)pLysS cells (Invitrogen) were transformed with these constructs and sequences were verified by ABI PRISM Big Dye Terminator chemistry (Applied Biosystems, Foster City, CA, USA).
Bioinformatical analysis
Signal peptide prediction was performed at the SignalP 3.0 Server (Bendtsen et al. 2004; http://www.cbs.dtu.dk/services/SignalP/). Similarity searches were done with the BlastP algorithm (http://www.ncbi.nlm.nih.gov/BLAST/). Protein structure prediction was done with the Phyre server (Kelley and Sternberg 2009; www.sbg.bio.ic.ac.uk/phyre/).
Recombinant expression and purification
Transformants were grown in 25 ml liquid LB growth medium containing 100 μg/ml ampicillin for 16 h at 37°C. These cells were used to inoculate 500 ml LB medium containing 100 μg/ml ampicillin which was shaken at 37°C until the optical density at 600 nm reached 0.6. Recombinant expression was then induced by 1 mM isopropyl-β-d-thiogalactopyranoside. After 4 h incubation at 37°C, the cells were harvested by centrifugation and resuspended in 20 ml lysis buffer (50 mM NaH2PO4, 300 mM NaCl, and 60 mM imidazole, pH 8.0). After three freeze-thaw cycles and a sonication step, cell debris was removed by centrifugation and filtration (0.22 μm).
The supernatant was loaded on a HisTrap™ HP 1-ml column (GE Healthcare, Uppsala, Sweden), using an Äkta FPLC (GE Healthcare). After a washing step with the lysis buffer, the enzyme was eluted by increasing the imidazole concentration to 250 mM. Enzyme purity was estimated by SDS-PAGE (Laemmli 1970), and combined enzyme fractions were dialyzed against 25 mM sodium citrate, pH 6.0. Protein concentrations were determined by absorbance measurements at 280 nm using the theoretical extinction coefficient (138,030 and 148,085 M−1 cm−1 for XylB and XylC, respectively) based on the amino acid sequence (Pace et al. 1995).
Substrates
p-Nitrophenyl-β-d-xylopyranoside (pNP-Xyl) and p-nitrophenyl-α-l-arabinofuranoside (pNP-Ara) were purchased from Sigma-Aldrich (St. Louis, MO). XOS were purchased from Megazyme (Bray, Ireland). Water-extractable arabinoxylans (AX) from wheat [arabinose to xylose ratio (A/X) = 0.49; AX content 99%] and rye (A/X = 0.97; AX content 84%) were obtained as described by Trogh et al. (2004). Two AXOS fractions with an average degree of polymerization (DP) of 3 and A/X of 0.25 (AXOS-3-0.25, AXOS content 72%) and an average DP of 15 and A/X of 0.26 (AXOS-15-0.26, AXOS content 8%) were produced according to Swennen et al. (2006). Xylotetraose linked to o-nitrophenol at the reducing end (oNP-X4) was produced and provided by Dr. Anna Kulminskaya and coworkers (Russian Academy of Science, St. Petersburg, Russia) following the protocols of Eneyskaya et al. (2003).
Enzyme assays
Enzyme activities were measured by adding 15 μl of enzyme solution to 15 μl of 5 mM pNP-substrate. Enzyme concentrations tested for XylB were 1.5–7.8 nM and 3.1–10.4 μM on pNP-Xyl and pNP-Ara, respectively. Enzyme concentrations for XylC were 0.5–1.5 and 4.6–15.4 μM. After 30 min incubation the reaction was stopped with 225 μl of 1% Tris and the absorbance of released p-nitrophenol was measured at 410 nm. The pH and temperature optimum were determined by performing the test at different pH and temperature conditions, respectively. The buffers used were 30 mM citric acid (pH 2.2–7.2), 30 mM HEPES (pH 6.8–8.2), and 30 mM trihydrogen borate (pH 8.5–10.2), adjusted to the different pH's using HCl or NaOH solutions. One unit of activity (U) was defined as the quantity of enzyme leading to the release of 1 μmol of p-nitrophenol at optimal pH and temperature.
To examine if xylose was released from the reducing or nonreducing ends of XOS, hydrolytic products of xylotetraose labeled with oNP at the reducing end (oNP-X4) were analyzed as described by Pollet et al. (2010). In short, oNP-X4 was incubated with enzyme (12.6 nM RexA, 3.2 nM XylB, or 1.5 nM XylC) for several time periods and hydrolytic products were analyzed by reversed phase high-performance liquid chromatography.
Enzyme activity was further assessed by following XOS hydrolysis (20 μM XOS, DP 2–6) as a function of time as described by Lagaert et al. (2007). Because XylB and XylC were not stable at their optimal working temperatures (see Supplementary Figs. 1 and 2) and to ensure enzyme stability for the complete time course of the reaction, incubations were carried out at 40°C, pH 6.0 (25 mM sodium citrate buffer). Enzyme concentrations were chosen to ensure similar activities of XylB (58.6 nM) and XylC (29.4 nM) under the conditions of the assay. At regular time intervals, aliquots were heat inactivated and the hydrolytic products were analyzed by high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD). Standard deviations on these measurements are small (<5%, Lagaert et al. 2007). Progress curves of XOS cleavage were used to determine the k cat/K M of the reactions according to the equation of Matsui et al. (1991).
Enzymatic degradation products of AX, AXOS, and xyloglucan were determined after incubation of 5 mg/ml substrate with enzyme in 25 mM sodium citrate buffer, pH 6.0 for 1 h at 40°C. Excess of enzyme (1.9 μM XylB and 6.4 μM XylC) was used to obtain end hydrolysis products. After heat inactivation for 10 min at 95°C, the amount of released glucose, xylose, and arabinose was measured by gas chromatography of alditol acetates, as described by Courtin et al. (2000).
Results
According to the CAZy database, XylC (BAF39209) belongs to GH43, which includes β-xylosidases, β-1,3-xylosidases, α-l-arabinofuranosidases, arabinanases, xylanases, and galactan 1,3-β-galactanases. XylC and β-xylosidases from Bacillus subtilis (XynB), Selenomonas ruminantium (Xsa) and Geobacillus stearothermophilus (XynB3) have sequence identities of approximately 50%. XylB (BAF39080) lies directly upstream of the gene for the xylose-releasing exo-oligoxylanase (RexA) from B. adolescentis. XylB was recently classified as a member of GH120 and is 48% identical to a β-xylosidase from Thermoanaerobacterium saccharolyticum JW/SL YS485 (Shao et al. 2011). BlastP analysis reveals homologues in several intestinal bacteria, such as Bifidobacterium, Lactobacillus, Clostridium, and Bacteroides species. Protein fold prediction by Phyre suggests XylB is folded as a β-helix, which might indicate it is part of the glycoside hydrolase clan N. Although XylB is annotated in GenBank as a putative outer membrane protein, SignalP suggests both enzymes are nonsecretory.
Recombinant expression yielded 20 mg purified XylB and 50 mg purified XylC per liter cell culture. On SDS-PAGE, they appeared at their theoretical values of 76 and 62 kDa for XylB and XylC, respectively. Purity was estimated to be over 95%. The optimal reaction conditions for pNP-Xyl hydrolysis were 60°C at pH 5 for XylB and 50°C at pH 6–7 for XylC (Fig. 1). XylB as well as XylC were also able to degrade pNP-Ara and showed the same optimal reaction conditions on this substrate, but their activity on pNP-Xyl was higher. At optimal temperature and pH, the specific activity of XylB was 68,973 and 32 mU/mg on pNP-Xyl and pNP-Ara, respectively. For XylC, these values were 164 and 18 mU/mg, respectively. Thus, XylB is around 2,300 times more active on pNP-Xyl than on pNP-Ara whereas this ratio is about 9 for XylC.
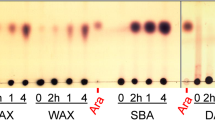
Hydrolysis of XOS (DP 2–6) was followed as a function of time with HPAEC-PAD (Fig. 2a, b). XylC acts as most known β-xylosidases and constitutively hydrolyzes a xylose residue from XOS until only xylose remains (Fig. 2b). XylB also cleaves one xylose at a time from XOS, but it shows only very weak activity on xylobiose (Fig. 2a). While XylB can degrade all xylotetraose in 55 min, 90% of xylobiose remains under the same conditions. The low activity of XylB on xylobiose is also apparent from the specificity constants k cat/K M calculated for the different XOS (Table 1). During hydrolysis of XOS, no oligomers with a higher degree of polymerization were produced, showing that both enzymes lack transglycosylation activity under these conditions.
To examine if xylose is released from the reducing or nonreducing end of the substrates, xylotetraose labeled with oNP at the reducing end (oNP-X4) was incubated with the enzymes. For both enzymes, oNP-X3 was formed during the initial stages of hydrolysis, demonstrating activity at the nonreducing end and, thus, showing XylB and XylC are β-xylosidases (data not shown). With increasing hydrolysis times, the substrate was completely hydrolyzed to o-nitrophenol by XylB as well as XylC. The reducing-end xylose-releasing exo-oligoxylanase from B. adolescentis was not able to hydrolyze oNP-X4.
In addition to hydrolysis of synthetic substrates and small XOS, degradation of more complex substrates was investigated by measuring end hydrolysis products of AXOS, AX, and xyloglucan. Although XylB and XylC hydrolyze both pNP-Xyl and pNP-Ara, only xylose residues were cleaved from AXOS and no free arabinose was detected (Table 2). During incubation of xyloglucan and rye AX (A/X = 0.97) with XylB or XylC, no xylose, arabinose, or glucose was liberated, while only threshold amounts of xylose were liberated from wheat AX (A/X = 0.49). XylB released more xylose from AXOS than XylC.
Discussion
In this study, two β-xylosidases from B. adolescentis were cloned and characterized. XylC belongs to GH43, while XylB is only the second characterized enzyme from GH120. Both enzymes hydrolyze pNP-Xyl and pNP-Ara, although activity on pNP-Xyl is higher. When the enzymes are incubated with natural substrates, they are only able to release xylose. XylC shows the activity of a typical β-xylosidase by converting XOS to xylose. In contrast, XylB has a remarkably low activity on xylobiose and the final products of XOS hydrolysis by this enzyme are xylose and xylobiose. In general, β-xylosidases readily degrade xylobiose (Knob et al. 2010) and often show a higher activity on xylobiose compared to other XOS (Katapodis et al. 2006; Kumar and Ramón 1996; Matsuo et al. 1987; Rasmussen et al. 2006; Saha 2003; Shao and Wiegel 1992; Takenishi et al. 1973). In some cases, xylobiose is less efficiently hydrolyzed than XOS with a larger chain length (John et al. 1979; Rizzatti et al. 2001; Saxena et al. 1995; Yan et al. 2008), but the observed differences are in general much smaller than those observed with XylB. Slow and incomplete degradation of xylobiose, however, is a typical feature of GH30 β-xylosidases (Adelsberger et al. 2004; Lee and Zeikus 1993; Smaali et al. 2006; Wagschal et al. 2005, 2008). The lack of transglycosylation activity of XylB on XOS contrasts with the characteristics of family 30 enzymes (Lee and Zeikus 1993; Muzard et al. 2009; Smaali et al. 2006). Future characterization studies will have to show if the low activity on xylobiose of XylB is typical for relatives of this new GH, as xylobiose hydrolysis was not described in detail for the GH120 β-xylosidase from T. saccharolyticum (Shao et al. 2011).
Another noticeable difference between the two β-xylosidases is their ability to cleave xylose from more complex substrates such as AX and AXOS. As these enzymes are not able to cleave arabinose substitutions, they can only hydrolyze xylose from the nonreducing end of the xylan backbone until they are hindered by substituted residues. Hence, little activity is expected on AX, which has only a low amount of nonreducing substrate ends. This is confirmed by our results, which show no detectable activity on AX with a high degree of substitution (A/X = 0.97). On AX with a lower degree of substitution (A/X = 0.49) and thus a higher probability of more successive unsubstituted xylose residues at the nonreducing end, low amounts of xylose are indeed released. On AXOS, which has a high amount of nonreducing ends, both enzymes show a much higher release of xylose. Up to 12% of xylose residues are released from AXOS with an average DP of 15. From AXOS with an average DP of 3, and thus with more nonreducing ends, higher amounts are released, up to 30%. From both AXOS fractions, XylB is able to produce more xylose and, thus, seems to be less hampered by arabinose substitutions than XylC.
B. adolescentis possesses three known enzymes to degrade prebiotic XOS: one reducing-end xylose-releasing exo-oligoxylanase (RexA, Lagaert et al. 2007) and two β-xylosidases (XylB and XylC) with different substrate specificities (Table 3). This allows the degradation of XOS from both the reducing and nonreducing end at the same time. As the final hydrolysis products of both RexA and XylB are xylose and xylobiose, XylC is needed for the efficient conversion of xylobiose to xylose. The higher release of xylose from AXOS by XylB compared to XylC may be useful for the degradation of potentially prebiotic AXOS. In this case, the joint action of XylB, XylC, RexA, and the recently described arabinofuranosidases of B. adolescentis would lead to the complete degradation of these substrates (Lagaert et al. 2010; Van Laere et al. 1999). Further research on expression levels of these enzymes on different XOS and AXOS could increase our knowledge on the hydrolysis of prebiotic substrates by B. adolescentis and place these results in a bacterial physiology and metabolism context.
XylB, XylC, and RexA all lack a signal peptide as indicated by SignalP, suggesting they occur intracellularly. This would imply that B. adolescentis imports XOS before hydrolyzing them. This is in agreement with several in vitro studies with other subcultures of the same strain used in this study, which show that B. adolescentis is able to grow on XOS, but only poorly on xylose (Crittenden et al. 2002; Moura et al. 2007; Pastell et al. 2009; Wang et al. 2010). Furthermore, two studies which followed the carbohydrate composition in the medium noticed that during the growth of B. adolescentis on XOS, extracellular xylose levels remained steady (Moura et al. 2007; Wang et al. 2010). This shows that B. adolescentis has a membrane transporter for XOS, but not for free xylose. Importing XOS before hydrolysis may offer a competitive advantage against cross-feeding by other microbes in the gut (Crittenden et al. 2002; Mäkeläinen et al. 2009).
Structural data on XylB and XylC should provide more insight into why the former shows a much weaker activity on xylobiose, but is less hampered by arabinose substitutions on the xylose backbone. A XylB structure, combined with the characterization of homologues, would also provide more information on the features of the recently introduced GH120.
References
Adelsberger H, Hertel C, Glawischnig E, Zverlov VV, Schwarz WH (2004) Enzyme system of Clostridium stercorarium for hydrolysis of arabinoxylan: reconstitution of the in vivo system from recombinant enzymes. Microbiology 150:2257–2266
Bendtsen JD, Nielsen H, von Heijne G, Brunak S (2004) Improved prediction of signal peptides: SignalP 3.0. J Mol Biol 340:783–795
Campbell JM, Fahey J, Wolf BW (1997) Selected indigestible oligosaccharides affect large bowel mass, cecal and fecal short-chain fatty acids, pH and microflora in rats. J Nutr 127:130–136
Chung YC, Hsu CK, Ko CY, Chan YC (2007) Dietary intake of xylooligosaccharides improves the intestinal microbiota, fecal moisture, and pH value in the elderly. Nutr Res 27:756–761
Cloetens L, De Preter V, Swennen K, Broekaert WF, Courtin CM, Delcour JA, Rutgeerts P, Verbeke K (2008) Dose-response effect of arabinoxylooligosaccharides on gastrointestinal motility and on colonic bacterial metabolism in healthy volunteers. J Am Coll Nutr 27:512–518
Cloetens L, Broekaert WF, Delaedt Y, Ollevier F, Courtin CM, Delcour JA, Rutgeerts P, Verbeke K (2010) Tolerance of arabinoxylan-oligosaccharides and their prebiotic activity in healthy subjects: a randomised, placebo-controlled cross-over study. Br J Nutr 103:703–713
Courtin CM, Van den Broeck H, Delcour JA (2000) Determination of reducing end sugar residues in oligo-and polysaccharides by gas-liquid chromatography. J Chromatogr A 866:97–104
Crittenden RG, Playne MJ (1996) Production, properties and applications of food-grade oligosaccharides. Trends Food Sci Technol 7:353–361
Crittenden R, Karppinen S, Ojanen S, Tenkanen M, Fagerström R, Mättö J, Saarela M, Mattila-Sandholm T, Poutanen K (2002) In vitro fermentation of cereal dietary fibre carbohydrates by probiotic and intestinal bacteria. J Sci Food Agric 82:781–789
Eneyskaya EV, Brumer H, Backinowsky LV, Ivanen DR, Kulminskaya AA, Shabalin KA, Neustroev KN (2003) Enzymatic synthesis of β-xylanase substrates: transglycosylation reactions of the β-xylosidase from Aspergillus sp. Carbohydr Res 338:313–325
Gobinath D, Madhu AN, Prashant G, Srinivasan K, Prapulla SG (2010) Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br J Nutr 104:40–47
Hsu CK, Liao JW, Chung YC, Hsieh CP, Chan YC (2004) Xylooligosaccharides and fructooligosaccharides affect the intestinal microbiota and precancerous colonic lesion development in rats. J Nutr 134:1523–1528
Imaizumi K, Nakatsu Y, Sato M, Sedarnawati Y, Sugano M (1991) Effects of xylooligosaccharides on blood glucose, serum and liver lipids and cecum short-chain fatty acids in diabetic rats. Agric Biol Chem 1:199–205
John M, Schmidt B, Schmidt J (1979) Purification and some properties of five endo-1,4-β-D-xylanases and β-d-xylosidase produced by a strain of Aspergillus niger. Biochem Cell Biol 57:125–134
Katapodis P, Nerinckx W, Claeyssens M, Christakopoulos P (2006) Purification and characterization of a thermostable intracellular β-xylosidase from the thermophilic fungus Sporotrichum thermophile. Process Biochem 41:2402–2409
Kelley LA, Sternberg MJE (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4:363–371
Knob A, Terrasan C, Carmona E (2010) β-Xylosidases from filamentous fungi: an overview. World J Microbiol Biotechnol 26:389–407
Kumar S, Ramón D (1996) Purification and regulation of the synthesis of a β-xylosidase from Aspergillus nidulans. FEMS Microbiol Lett 135:287–293
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lagaert S, Van Campenhout S, Pollet A, Bourgois TM, Delcour JA, Courtin CM, Volckaert G (2007) Recombinant expression and characterization of a reducing-end xylose-releasing exo-oligoxylanase from Bifidobacterium adolescentis. Appl Environ Microbiol 73:5374–5377
Lagaert S, Pollet A, Delcour JA, Lavigne R, Courtin CM, Volckaert G (2010) Substrate specificity of three recombinant α-l-arabinofuranosidases from Bifidobacterium adolescentis and their divergent action on arabinoxylan and arabinoxylan oligosaccharides. Biochem Biophys Res Commun 402:644–650
Lee YE, Zeikus JG (1993) Genetic organization, sequence and biochemical characterization of recombinant β-xylosidase from Thermoanaerobacterium saccharolyticum strain B6A-RI. J Gen Microbiol 139:1235–1243
Mäkeläinen H, Juntunen M, Hasselwander O (2009) Prebiotic potential of xylo-oligosaccharides. In: Charalampopoulos D, Rastall RA (eds) Prebiotics and probiotics science and technology. Springer, New York, pp 245–258
Matsui I, Ishikawa K, Matsui E, Miyairi S, Fukui S, Honda K (1991) Subsite structure of Saccharomycopsis α-amylase secreted from Saccharomyces cerevisiae. J Biochem 109:566–569
Matsuo M, Fujie A, Win M, Yasui T (1987) Four types of β-xylosidases from Penicillium wortmanni IFO 7237. Agric Biol Chem 51:2367–2379
Moura P, Barata R, Carvalheiro F, Girio FM, Loureiro-Dias MC, Esteves MP (2007) In vitro fermentation of xylo-oligosaccharides from corn cobs autohydrolysis by Bifidobacterium and Lactobacillus strains. Lebensm Wiss Technol 40:963–972
Muzard M, Aubry N, Plantier-Royon R, O'Donohue M, Rémond C (2009) Evaluation of the transglycosylation activities of a GH 39 β-d-xylosidase for the synthesis of xylose-based glycosides. J Mol Catal B Enzym 58:1–5
Okazaki M, Fujikawa S, Matsumoto N (1990) Effect of xylo-oligosaccharide on the growth of bifidobacteria. Bifidobact Microflora 9:77–86
Pace CN, Vajdos F, Fee L, Grimsley G, Gray T (1995) How to measure and predict the molar absorption coefficient of a protein. Protein Sci 4:2411–2423
Pastell H, Westermann P, Meyer AS, Tuomainen P, Tenkanen M (2009) In vitro fermentation of arabinoxylan-derived carbohydrates by bifidobacteria and mixed fecal microbiota. J Agric Food Chem 57:8598–8606
Pollet A, Lagaert S, Eneyskaya E, Kulminskaya A, Delcour JA, Courtin CM (2010) Mutagenesis and subsite mapping underpin the importance for substrate specificity of the aglycon subsites of glycoside hydrolase family 11 xylanases. Biochim Biophys Acta 1804:977–985
Rasmussen LE, Sørensen HR, Vind J, Viksø-Nielsen A (2006) Mode of action and properties of the β-xylosidases from Talaromyces emersonii and Trichoderma reesei. Biotechnol Bioeng 94:869–876
Rizzatti ACS, Jorge JA, Terenzi HF, Rechia CGV, Polizeli MLTM (2001) Purification and properties of a thermostable extracellular β-xylosidase produced by a thermotolerant Aspergillus phoenicis. J Ind Microbiol Biotechnol 26:156–160
Rycroft CE, Jones MR, Gibson GR, Rastall RA (2001) A comparative in vitro evaluation of the fermentation properties of prebiotic oligosaccharides. J Appl Microbiol 91:878–887
Saha BC (2003) Purification and properties of an extracellular β-xylosidase from a newly isolated Fusarium proliferatum. Bioresour Technol 90:33–38
Santos A, San Mauro M, Díaz DM (2006) Prebiotics and their long-term influence on the microbial populations of the mouse bowel. Food Microbiol 23:498–503
Saxena S, Fierobe HP, Gaudin C, Guerlesquin F, Belaich JP (1995) Biochemical properties of a β-xylosidase from Clostridium cellulolyticum. Appl Environ Microbiol 61:3509–3512
Shao W, Wiegel J (1992) Purification and characterization of a thermostable β-xylosidase from Thermoanaerobacter ethanolicus. J Bacteriol 174:5848–5853
Shao W, Xue Y, Wu A, Kataeva I, Pei J, Wu H, Wiegel J (2011) Characterization of a novel β-xylosidase, XylC, from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl Environ Microbiol 77:719–726
Shin HY, Lee JH, Lee JY, Han YO, Han MJ, Kim DH (2003) Purification and characterization of ginsenoside Ra-hydrolyzing β-d-xylosidase from Bifidobacterium breve K-110, a human intestinal anaerobic bacterium. Biol Pharm Bull 26:1170–1173
Smaali I, Rémond C, O'Donohue M (2006) Expression in Escherichia coli and characterization of β-xylosidases GH39 and GH-43 from Bacillus halodurans C-125. Appl Microbiol Biotechnol 73:582–590
Swennen K, Courtin CM, Lindemans GC, Delcour JA (2006) Large-scale production and characterisation of wheat bran arabinoxylooligosaccharides. J Sci Food Agric 86:1722–1731
Takenishi S, Tsujisaka Y, Fukumoto J (1973) Studies on hemicellulases. IV. Purification and properties of the β-xylosidase produced by Aspergillus niger van Tieghem. J Biochem (Tokyo) 73:335
Taniguchi H (2004) Carbohydrate research and industry in Japan and the Japanese Society of Applied Glycoscience. Starch 56:1–5
Tateyama I, Hashii K, Johno I, Iino T, Hirai K, Suwa Y, Kiso Y (2005) Effect of xylooligosaccharides intake on severe constipation in pregnant women. J Nutr Sci Vitaminol (Tokyo) 51:445–448
Trogh I, Courtin CM, Delcour JA (2004) Isolation and characterization of water-extractable arabinoxylan from hull-less barley flours. Cereal Chem 81:576–581
Van Craeyveld V, Swennen K, Dornez E, Van de Wiele T, Marzorati M, Verstraete W, Delaedt Y, Onagbesan O, Decuypere E, Buyse J, De Ketelaere B, Broekaert WF, Delcour JA, Courtin CM (2008) Structurally different wheat-derived arabinoxylooligosaccharides have different prebiotic and fermentation properties in rats. J Nutr 138:2348–2355
Van Laere KMJ, Voragen CHL, Kroef T, van den Broek LAM, Beldman G, Voragen AGJ (1999) Purification and mode of action of two different arabinoxylan arabinofuranohydrolases from Bifidobacterium adolescentis DSM 20083. Appl Microbiol Biotechnol 51:606–613
Vázquez MJ, Alonso JL, Domínguez H, Parajó JC (2000) Xylooligosaccharides: manufacture and applications. Trends Food Sci Technol 11:387–393
Wagschal K, Franqui-Espiet D, Lee CC, Robertson GH, Wong DWS (2005) Enzyme-coupled assay for β-xylosidase hydrolysis of natural substrates. Appl Environ Microbiol 71:5318–5323
Wagschal K, Franqui-Espiet D, Lee C, Robertson G, Wong D (2008) Cloning, expression and characterization of a glycoside hydrolase family 39 xylosidase from Bacillus halodurans C-125. Appl Biochem Biotechnol 146:69–78
Wang J, Sun B, Cao Y, Wang C (2010) In vitro fermentation of xylooligosaccharides from wheat bran insoluble dietary fiber by Bifidobacteria. Carbohydr Polym 82:419–423
Yan QJ, Wang L, Jiang ZQ, Yang SQ, Zhu HF, Li LT (2008) A xylose-tolerant β-xylosidase from Paecilomyces thermophila: characterization and its co-action with the endogenous xylanase. Bioresour Technol 99:5402–5410
Younes H, Garleb K, Behr S, Remesy C, Demigne C (1995) Fermentable fibers or oligosaccharides reduce urinary nitrogen excretion by increasing urea disposal in the rat cecum. J Nutr 125:1010–1016
Acknowledgments
We thank C. Grootaert (Laboratory for Microbial Ecology and Technology, Ghent University, Belgium) for providing a genomic DNA sample of B. adolescentis. We gratefully acknowledge the financial support from the “Instituut voor de aanmoediging van Innovatie door Wetenschap en Technologie in Vlaanderen” (I.W.T., SBO IMPAXOS project funding) and the Research Fund K.U. Leuven (project IDO/03/005).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lagaert, S., Pollet, A., Delcour, J.A. et al. Characterization of two β-xylosidases from Bifidobacterium adolescentis and their contribution to the hydrolysis of prebiotic xylooligosaccharides. Appl Microbiol Biotechnol 92, 1179–1185 (2011). https://doi.org/10.1007/s00253-011-3396-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3396-y





