Abstract
Lignans are ubiquitous plant polyphenols, which have relevant health properties being the major phytoestrogens occurring in Western diets. Secoisolariciresinol (SECO) is the major dietary lignan mostly found in plants as secoisolariciresinol diglucoside (SDG). To exert biological activity, SDG requires being deglycosylated to SECO and transformed to enterodiol (ED) and enterolactone (EL) by the intestinal microbes. The involvement of bifidobacteria in the transformation of lignans glucosides has been investigated for the first time in this study. Twenty-eight strains were assayed for SDG and SECO activation. They all failed to transform SECO into reduced metabolites, excluding any role in ED and EL production. Ten Bifidobacterium cultures partially hydrolyzed SDG, giving both SECO and the monoglucoside with yields < 25%. When the cell-free extracts were assayed in SDG transformation, seven additional strains were active in the hydrolysis. Cellobiose induced β-glucosidase activity and caused the enhancement of both the rate of SDG hydrolysis and the final yield of SECO only in the strains capable of SDG bioconversion. The highest SDG conversion to SECO was achieved by Bifidobacterium pseudocatenulatum WC 401, which exhibited 75% yield in cellobiose-based medium after 48 h. These results indicate that SDG hydrolysis is not a common feature in Bifidobacterium genus, but selected probiotic strains can be combined to β-glucoside-based prebiotics to enhance the release of SECO, thus improving its bioavailability for absorption by colonic mucosa and/or the biotransformation to ED and EL by other intestinal microorganisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The human colon harbors a complex and dense anaerobic microbiota, with up to 1011 microorganisms per gram of intestinal content, mostly represented by bacteria (Zoetendal et al. 2008). Although more than 50 bacterial phyla have been described, it is dominated by Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria (Eckburg et al. 2005). Within these phyla, the number of different bacterial species and strains is extremely high, accounting for several thousands of diverse microorganisms (Rajilić-Stojanović et al. 2007). The gut microbiota produces enzymes that the host lacks, which are involved in the breakdown of complex molecules, and benefits the host through nutrient digestion and energy recovery (Wong et al. 2007; Venema 2010). Furthermore, microbial enzymes transform a wide spectrum of non-nutritional dietary molecules into bioactive compounds, such as polyphenols, which exert a number of health benefits (Manach et al. 2004; Clavel et al. 2006a; Rossi et al. 2010).
Lignans are polyphenols involved in plant cell wall formation and ubiquitously occur in many plants, although at low levels. They are found especially in flaxseeds, berries, rye, and a wide range of seeds, fruits, and vegetables occurring abundantly in Western diets (Giada Mde 2010; Manach et al. 2004; Smeds et al. 2007). Likewise isoflavones and coumestans, lignans have a similarity in structure to the human female hormone estradiol, being regarded as phytoestrogens. They mimic the action of estrogens on target organs, exerting many health benefits against hormone-dependent diseases (Adlercreutz 2007, Mueller et al. 2004; Moutsatsou 2007; Swedenborg et al. 2009) as assessed by several human and animal studies (Adolphe et al. 2010).
Anti-oxidative, anti-proliferative, anti-estrogenic, and anti-angiogenic properties make secoisolariciresinol diglucoside (SDG) metabolites protective against breast (Saarinen et al. 2007; Adams and Chen 2009), colon (Bommareddy et al. 2006), and prostate cancer. Furthermore, the ability of enterolactone (EL) to inhibit aromatase (Brooks and Thompson 2005) may be beneficial in estrogen-responsive breast cancers. Inhibition of the enzyme 5α-reductase helps to relieve lower urinary tract symptoms in patients with benign prostatic hyperplasia (Zhang et al. 2008a). The anti-oxidative activity of SDG and its metabolites (Kitts et al. 1999) reduces oxidative stress and inflammation and provides protection against cardiovascular diseases (Peterson et al. 2010; Prasad 1999, 2009). Also, diabetes and metabolic syndrome take advantage by the consumption of faxseed lignans (Prasad 2001; Zhang et al. 2008b). Additionally, SDG metabolites induce phase 2 proteins (Wang et al. 1998), which promote the scavenging of oxidants, decrease the probability of oxidant formation, and reduce oxidative stress (Juurlink 2001).
Lignans are ubiquitous in plants, being the major phytoestrogens occurring in plant-based foods and diets. Secoisolariciresinol (SECO) is the major dietary lignan, being contained in berries, legumes, cereals, and seeds, particularly in flaxseed (Milder et al. 2005; Smeds et al. 2007). In plant tissues, SECO is mostly found as SDG, which generally is ester-linked with 3-hydroxy-3-methylglutaric acid and other phenolic compounds (e.g., p-coumaric and/or ferulic acid glycosides) in the form of SDG oligomers (Ford et al. 2001; Yuan et al. 2008).
Lignan-containing foods have been demonstrated to provide the abovementioned health-beneficial effects. However, SDG and the other lignans are not active in the forms as they occur in plants. Their beneficial effects greatly depend on the bioconversion into aglycones and particularly into enterolignans enterodiol (ED) and EL, which exert estrogen-dependent and -independent activity (Rowland et al. 2003; Adlercreutz 2007). This transformation is carried out within the colon by the resident microbiota (Woting et al. 2010). Deglycosylation by microbial β-glucosidases (β-glu, EC 3.2.1.21) is the first step of SDG transformation (Fig. 1). One of two glucose moieties is first removed from SDG to produce secoisolariciresinol monoglucoside (SMG), which is further deglycosylated to yield aglycone SECO (Yuan et al. 2008). Finally, SECO undergoes microbial demethylation and dehydroxylation to yield ED and EL (Clavel et al. 2006b) (Fig. 1).
The conversion of SDG into ED and EL results from the interaction between dominant and subdominant intestinal anaerobes (Clavel et al. 2006a, b; Woting et al. 2010). SDG hydrolysis can be carried out by some strains of the genera Bacteroides and Clostridium (Clavel et al. 2006a, b; Eeckhaut et al. 2008), but several other groups of bacteria are known to possess β-glu activity, including bifidobacteria (Hawkesworth et al. 1971; Donkor and Shah 2008; Raimondi et al. 2009). Nevertheless, the role of bifidobacteria in the transformation of SDG toward ED and EL production is still unexplored, and strains belonging to these genera have never been reported among the bacteria which can perform steps of this bioconversion (Clavel et al. 2006a, b).
Bifidobacteria are natural colonizers of the gut, strictly gaining energy through the fermentation of carbohydrates. They acidify the large intestine, restricting putrefactive and potentially pathogenic bacteria, produce vitamins and amino acids, stimulate the immune response, repress the conversion of primary bile salts, exert anti-inflammatory activity, and reduce risk of colon cancer (Rossi and Amaretti 2010; Williams 2010). Because of these beneficial health effects, they are generally considered probiotics and are increasingly being used in functional foods and pharmaceutical products. Commensal bifidobacteria exert a number of beneficial health-related effects through a variety of different molecular mechanisms and are increasingly being used in functional foods and pharmaceutical products, being generally regarded as probiotics. Probiotic bacteria have been extensively investigated for their ability to activate soybean isoflavones, but lignans transformation by these bacteria has never been explored. The present study seeks to fill this gap in order to gain further insight into the function of bifidobacteria in SDG activation.
Materials and methods
Chemicals
All chemicals were purchased from Sigma-Aldrich (Steinheim, Germany) unless otherwise stated. Methanol solutions of 50 mM secoisolariciresinol, 5 mM enterodiol, and 5 mM enterodiol were used in appropriate amounts to prepare the HPLC calibration curves and for biotransformation experiments. Secoisolariciresinol monoglucoside (90% purity) was prepared, separated, and purified as described by Li et al. (2008). Water solutions of 50 mM secoisolariciresinol diglucoside were used in appropriate amounts for analytical purposes (99% purity, Chromadex, Irvine, CA, USA) and for biotransformation experiments (technical grade, Chemas GmbH, Regenstauf, Germany).
Bacterial strains and culture conditions
Twenty-eight strains of Bifidobacterium were assayed for the ability to transform secoisolariciresinol diglucoside. The strains were taken from our strain collection or were obtained from ATCC (Table 1). They were subcultured at 37 °C in MRS broth containing 0.5 g/l l-cysteine HCl (hereinafter called MRS-glu) in an anaerobic cabinet (Anaerobic System, Forma Scientific Co., Marietta, OH, USA) under a N2 85%, CO2 10%, H2 5% atmosphere. The capability of bifidobacteria to grow on cellobiose as the only carbon source was tested in a modified MRS broth (MRS-cel) containing 20 g/L cellobiose as carbon source in place of 20 g/L glucose. The cultures were always propagated at least five times in MRS-cel before quantifying growth by measuring the optical density at 600 nm (OD600).
Bioconversion of SDG and SECO with growing cells
Sixteen-hour cultures grown in MRS-glu or MRS-cel were inoculated (10% v/v) into 5 ml of the same medium supplemented with 500 μM SDG or 500 μM SECO and incubated at 37 °C in anaerobiosis for 8 days. As controls, 5 ml of culture without SDG or SECO and 5 ml of non-inoculated medium containing 500 μM SDG or 500 μM SECO were incubated in anaerobiosis. Bacterial growth was monitored following the OD600.
Bioconversion of SDG with cell extracts
The bioconversion of SDG was carried out with cell-free extracts of 16-h cultures grown in MRS-glu or MRS-cel. The biomass was centrifuged (6,000 × g for 10 min at 4 °C), washed twice with 50 mM sodium phosphate buffer (pH 6.5), and resuspended in the same buffer to 30 units of OD600. A 4-ml cell suspension was subjected to mechanical disruption through one passage at 40.0 kPsi in One Shot Cell Disrupter (Constant Systems) and centrifuged (13,000 × g for 15 min at 4 °C) to remove whole cells and debris. A total of 250 μl of fresh-made cell-free extract was supplemented with 5 μl of 50 mM SDG and incubated at 37 °C. The reactions were stopped after 24 h with the addition of 250 μl of 15% (w/v) trichloroacetic acid.
Assay for β-glucosidase activity
β--Glucosidase (β-glu) was assayed in permeabilized cells, non-permeabilized cells, and cell-free extracts. Sixteen-hour cultures were centrifuged at 6,000 × g for 5 min at 4 °C. Biomass was washed twice with 0.1 M phosphate buffer (pH 7), suspended in the same buffer to 1.4 units of OD600, and used for the assay. Permeabilized cells were prepared by mixing 1 ml of suspension with 0.2 ml of CHCl3 and vortexing for 10 s at room temperature.
β-Glu was assayed with p-nitrophenyl-β-d-glucopyranoside (pNPG) (Amaretti et al. 2006). One milliliter of appropriately diluted sample was mixed with 0.2 ml of 4 mg/ml pNPG and incubated at 37 °C for 10 min. The reaction was stopped by adding 0.5 ml of 1 M Na2CO3. The sample was centrifuged (13,000 × g for 5 min) to remove the debris and absorbance was read at 420 nm. One unit of β-glu was defined as the amount of enzyme required to release 1 μmol of nitrophenol per minute under the assay conditions. To enable the comparison of intact and permeabilized cells, their activity was referred as units per milligram of dry weight. The specific activity of cell-free extracts was defined as units per milligram of proteins. Protein concentration was determined with copper/Folin phenol reagent assay kit (Sigma-Aldrich).
HPLC analysis
SDG, SMG, SECO, EL, and ED were analyzed by HPLC according to the method of Struijs et al. (2009), though slightly modified. The culture was centrifuged (13,000 × g for 5 min at 4 °C), the supernatant was filtered (cellulose acetate filter, 0.22 μm; Albet Filalbet, Barcelona, Spain), and 1 μl was injected into a HPLC device (Agilent 1100, Agilent Technologies Inc., Santa Clara, CA, USA) equipped with a variable wavelength detector and ZORBAX Eclipse XDB-C18 column (rapid resolution, 1.8 μm particle size, 4.6 × 50 mm, Agilent). The mobile phase was composed of 0.1% (v/v) acetic acid in water (solvent A) and 0.1% acetic acid in acetonitrile (solvent B). The flow rate was 0.7 ml/min. The following gradient of solvent B was applied: 0–25 min, linear from 10% to 30%; 25–30 min, linear to 50%; 30–40 min, isocratic on 50%; 40–42 min, linear to 100%; 42–47 min; 47–50 min, linear to 10%; 50–60 min, isocratic on 10%. The analytes SDG, SMG, SECO, EL, and ED were identified at 280 nm by retention time (11.8, 14.5, 18.0, 22.1, and 29.0 min, respectively) and were quantified by external standard method. Linearity was demonstrated from 10 to 1,000 μM for all the lignans analyzed (R 2 > 0.996). The limits of detection (LOD) of SDG, SMG, SECO, ED, and EL were 31, 14, 19, 11, and 3 μM, respectively. LOD was calculated as 3·(Sy/x)/b, where Sy/x represents the residual standard deviation and b is the slope of the linear calibration.
Statistical analysis
Values are means from three separate experiments plus/minus standard deviation. Statistical analysis was done using GraphPad Prism 4.0 (Graphpad Software, San Diego, CA, USA). Comparisons were evaluated using one-way ANOVA followed by Tukey post hoc comparisons. Differences were considered as statistically significant for P < 0.05.
Results
Bioconversion of SDG and SECO in glucose-based medium
Twenty-eight strains of Bifidobacterium were assayed for the ability to hydrolyze SDG into SMG and/or SECO and for the ability to transform SECO into the enterolignans ED and EL. The strains were cultured for 8 days in MRS-glu containing 500 μM SDG or SECO. In uninoculated cultures, the conversion of SDG and SECO did not occur in this span of time. The presence of lignans did not affect (P > 0.05) the extent of biomass growth (data not shown).
Eighteen out of 28 strains were incapable of any removal of glucose moieties from SDG. Only ten strains (Bifidobacterium bifidum WC 418, Bifidobacterium breve WC 421, Bifidobacterium catenulatum ATCC 27539, Bifidobacterium longum subsp. infantis ATCC 15697, B. longum subsp. longum WC 436, B. longum subsp. longum WC 439, Bifidobacterium pseudocatenulatum WC 401, B. pseudocatenulatum WC 402, B. pseudocatenulatum WC 403, and B. pseudocatenulatum WC 407) hydrolyzed SDG at some extent, with every strain yielding both SMG and SECO in similar amounts (P > 0.05). However, most of SDG always remained unaltered and the conversion yield ranged from 21% to 24% for the most effective strains (Table 1), B. pseudocatenulatum WC 401, B. pseudocatenulatum WC 403, B. pseudocatenulatum WC 407, and B. catenulatum ATCC 27539. HPLC chromatograms revealed that bifidobacteria did not produce any other lignan or lignan-derived molecule. Consistently, all of the 28 strains failed to transform SECO.
SDG hydrolysis in cellobiose-based medium
We hypothesized that the cultivation of bifidobacteria in cellobiose-based medium may improve β-glucosidases activity and enhance the removal of glucose from SDG. Then, bifidobacteria were screened for their ability to grow on the disaccharide cellobiose, consisting of two glucose molecules linked by a β(1 → 4) bond. Seven strains grew abundantly (OD600 ≥ 2.0) in MRS-cel and were assayed for SDG hydrolysis in this medium (Table 1). Bifidobacterium animalis WC 409, B. animalis WC 410, and B. breve WC 422 were unable to hydrolyze SDG in both MRS-glu and MRS-cel. B. pseudocatenulatum WC 407 performed the hydrolysis in MRS-cel and MRS-glu with similar yields (P > 0.05). B. breve WC 421, B. catenulatum ATCC 27539, and B. pseudocatenulatum WC 401 exhibited a higher conversion yield in MRS-cel than in MRS-glu (P < 0.05).
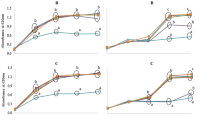
The kinetics of SDG biotransformation by B. pseudocatenulatum WC 401, B. pseudocatenulatum WC 407, B. catenulatum ATCC 27539, and B. breve WC 421 were compared in MRS-glu and MRS-cel (Fig. 2). All of these strains performed bioconversion faster in MRS-cel than in MRS-glu since the biotransformation reached its maximum extent within 2 days on cellobiose and 6–8 days on glucose. Hydrolysis was also more efficient in MRS-cel since it yielded SECO as the major hydrolysis product at the expense of the intermediate SMG (Fig. 2). Also in cultures growing on cellobiose, further biotransformations of SECO into ED, EL, or reduced intermediate products did not occur.
Time course of SDG (circle), SMG (square), and SECO (triangle) in batch cultures of B. pseudocatenulatum WC 401, B. pseudocatenulatum WC 407, B. catenulatum ATCC 27539, and B. breve WC 421 in glucose- and cellobiose-based media containing 500 μM SDG. Lignans are expressed as the percentage of the initial SDG concentration. Fermentations were carried out in triplicate. Error bars represent the standard deviation
β-Glucosidase activity and location
In order to determine the location of β-glucosidase (β-glu, EC 3.2.1.21), the specific activity was measured in intact and permeabilized cells, harvested from 16-h MRS-glu and MRS-cel (Fig. 3). For most of the strains presenting some β-glu, the activity was mostly intracellular.
Specific activity of β-glucosidase in permeabilized (dark bar) and intact cells (light bar) of bifidobacteria cultured in glucose- and cellulose-based MRS. Values are means ± standard deviation (n = 3). Stars indicate mean values with a statistically significant difference (P < 0.05) compared to glucose. Eighteen strains with activity < 0.03 U/mg in both glucose and cellobiose were omitted
With the exception of B. animalis ATCC 27536, B. animalis WC 411, B. animalis subsp. lactis WC 412, B. pseudocatenulatum WC 402, and B. pseudocatenulatum WC 403 (Fig. 4), the strains that were unable to utilize cellobiose gave cell-free extracts lacking β-glu activity. Most of the strains fermenting cellobiose presented β-glu also in MRS-glu. However, the activity was induced by cellobiose, being significantly higher in MRS-cel than in MRS-glu (P < 0.05). In MRS-cel, B. catenulatum ATCC 27539 exhibited the highest β-glu activity (0.91 U/mg) and a greater increase compared to MRS-glu (< 0.2 U/mg). A remarkably high β-glu activity on cellobiose was observed also for B. pseudocatenulatum WC 401, B. pseudocatenulatum WC 407, B. animalis WC 409, and B. animalis WC 410 (Fig. 3).
Specific activity of β-glucosidase in the cell-free extracts of bifidobacteria cultured in glucose- and cellulose-based MRS. Values are means ± standard deviation (n = 3). Stars indicate mean values with a statistically significant difference (P < 0.05) compared to glucose. Eighteen strains with activity < 0.03 U/mg in both glucose and cellobiose were omitted
Hydrolysis of SDG by cell-free extracts
The cell-free extracts, obtained from early stationary cultures in MRS-glu or MRS-cel, were assayed for their ability to hydrolyze SDG. Evidence of growth within the cell-free samples was excluded by microscopic observation at the end of incubation. All of the strains which were able to hydrolyze SDG during growth in MRS-glu or MRS-cel gave active cell-free extracts (Table 1). The extracts of B. breve WC 422, B. breve WC 424, and B. animalis strains, the MRS-glu or MRS-cel cultures of which were unable to hydrolyze SDG, released SECO from SDG (Table 1). In agreement with the pattern of β-glu activity, the cell-free extracts of the strains grown on cellobiose hydrolyzed SDG at a higher extent than the corresponding glucose-grown ones. In particular, the glucose moieties of SDG were quantitatively removed by the extracts of B. pseudocatenulatum WC 401, B. pseudocatenulatum WC 407, B. catenulatum ATCC 27539, and B. breve WC 422 grown on cellobiose, and only SECO was produced.
Discussion
Bifidobacteria have received special attention for their long history of safe use in probiotic products and fermented functional foods, and it would be very interesting if they could exert a role in the bioactivation of lignans in order to improve phytoestrogen bioavailability in Western diets. Like most phytoestrogens, lignans occur in the diet as glyco-conjugates. They are hydrolyzed by the colonic microbiota into their aglycones, which may undergo further microbial transformations before being absorbed across intestinal epithelial cells (Manach et al. 2004). An important target for the development of novel probiotics is the selection of strains that combine the intrinsic beneficial properties with specific health-related activities, such as the activation of phytochemicals. In this perspective, this study aimed to identify Bifidobacterium strains capable of activating the lignans occurring in fiber-rich foods.
Colonic bacteria belonging to the genus Bifidobacterium are able to hydrolyze the glucosides of soy isoflavones, exerting a role in improving their bioavailability (Tsangalis et al. 2002; Marotti et al. 2007; Donkor and Shah 2008; Raimondi et al. 2009; Marazza et al. 2009). Unlike the glucoconjugate soy isoflavones, the lignan SDG resulted to be quite resistant to hydrolysis. The data herein reported demonstrate that bifidobacteria hardly hydrolyze SDG into the aglycone SECO and do not carry out demethylation and C4-dehydroxylation in phenylpropanoid monomers, reactions required for the transformation of lignans to the more estrogenic compounds EL and ED. Most of the strains of the taxonomically related B. catenulatum and B. pseudocatenulatum were capable of hydrolyzing SDG, while this feature was infrequent among the species B. bifidum, B. breve, B. longum subsp. infantis, and B. longum subsp. longum.
β-Glucosidases (β-glu, EC 3.2.1.21) are glycosyl hydrolases which act upon the terminal non-reducing β-d-glucosyl residues of glucose-based oligosaccharides (e.g., the disaccharide cellobiose) or glucose-substituted molecules. The analysis of Bifidobacterium genome and nucleotide sequences for predictable β-glu suggests that only B. bifidum lacks any gene, consistent with the observation that the tested strains were unable to grow on cellobiose and to hydrolyze SDG or pNPG. On the contrary, B. pseudocatenulatum, B. longum subsp. longum, B. longum subsp. infantis, B. animalis subsp. lactis, and B. breve possess two to three genes encoding β-glu with cytoplasmic or membrane location (http://www.ncbi.nlm.nih.gov/gene). The ability of bifidobacteria to grow on cellobiose was investigated, and the β-glu activity and SDG hydrolysis of glucose- and cellobiose-based cultures were compared. Seven out of 28 Bifidobacterium strains fermented cellobiose but behaved diversely with respect to the capacity of hydrolyzing SDG. Four of them hydrolyzed SDG while three strains were unable to perform this reaction in both glucose- and cellobiose-based media.
A positive relationship among the presence of β-glu activity, the capability to ferment cellobiose, and the ability to hydrolyze SDG was observed. In agreement with annotated β-glucosidases, which are predicted to be intracellular or membrane-associated, β-glu activity was found in the cell extracts. Cellobiose induced β-glu activity and, in the strains capable of SDG hydrolysis, caused the enhancement of both the rate of SDG bioconversion and the final yield of SECO. The highest conversion of SDG to SECO was exhibited by strains belonging to the species B. pseudocatenulatum, B. catenulatum, and B. breve, cultured in cellobiose-based medium.
The β-glucosidase values herein presented are in agreement with previous studies reporting that B. adolescentis and B. pseudocatenulatum generally showed the highest activity, B. catenulatum, B. animalis, and B. breve exhibited intermediate values, and B. bifidum, B. infantis, and B. longum displayed no detectable activity (Desjardins et al. 1989; Tsangalis et al. 2003; Marotti et al. 2007; Dabek et al. 2008). In the present study, B. longum subsp. infantis, B. longum subsp. longum, and B. bifidum were generally unable to perform the hydrolysis of SDG, failed to grow on cellobiose, and did not present β-glu activity. The B. animalis strains presented some β-glu activity, but only two of them were able to ferment cellobiose and none hydrolyzed SDG in both glucose- and cellobiose-based media. Conversely, the cell-free extracts of B. animalis exhibited a hydrolytic activity against SDG. A similar behavior was observed for B. breve WC 422, the extract of which hydrolyzed SDG efficiently while the corresponding cultures did not. Hence, membrane transporters are likely involved in making the substrate available to cytosolic β-glucosidase. These results suggest that, in bifidobacteria, the inability to hydrolyze SDG may be due to the absence of β-glu or to the lack of a transport system enabling SDG to enter the cell and encounter cytosolic enzyme.
The analysis of nucleotide sequences revealed that bifidobacteria can possess up to three genes encoding cell-associated β-glu and a number of other glycosyl-hydrolytic enzymes. Thus, SDG hydrolysis can be due to the activity of diverse cytosolic β-glucosidases, exhibiting a different affinity for cellobiose and the β-glucosides of dietary polyphenols. However, other glycosyl hydrolases may be involved in the aspecific hydrolysis of lignans gluco-conjugates. Furthermore, although β-glu are intracellular, it cannot be excluded that they are released with cell lysis, causing the hydrolysis in the supernatant as well. Thus, the analysis of genome sequences for predictable metabolic reactions cannot be sufficient for estimating whether Bifidobacterium strains are capable to hydrolyze lignans gluco-conjugates.
An extensive investigation of the involvement of bifidobacteria in the hydrolysis of lignans glucosides has been accomplished for the first time in the present study. These overall results show that bifidobacteria exert a role in the hydrolysis of lignan glycoconjugates. Nonetheless, any involvement in the reactions required for the transformation into enterolactone and enterodiol can be excluded. The positive relationship between β-glu activity and SDG hydrolysis suggests the formulation of novel symbiotic preparations that can both positively affect the gut microbiota balance and speed up the release of lignan aglycones, improving the bioavailability for further biotransformations to EL and ED by other intestinal microorganisms. These symbiotics can be based on selected probiotic bifidobacteria able to quickly perform SDG hydrolysis and β-glucoside-based prebiotics. This application represents a novel challenge for the use of probiotics that can be exploited for the bioactivation of dietary polyphenols, modulating their biological activity.
References
Adams LS, Chen S (2009) Phytochemicals for breast cancer prevention by targeting aromatase. Front Biosci 14:3846–3863
Adlercreutz H (2007) Lignans and human health. Crit Rev Clin Lab Sci 44:483–525
Adolphe JL, Whiting SJ, Juurlink BH, Thorpe LU, Alcorn J (2010) Health effects with consumption of the flax lignan secoisolariciresinol diglucoside. Br J Nutr 103:929–938
Amaretti A, Tamburini E, Bernardi T, Pompei A, Zanoni S, Vaccari G, Matteuzzi D, Rossi M (2006) Substrate preference of Bifidobacterium adolescentis MB 239: compared growth on single and mixed carbohydrates. Appl Microbiol Biotechnol 73:654–662
Bommareddy A, Arasada BL, Mathees DP, Dwivedi C (2006) Chemopreventive effects of dietary flaxseed on colon tumor development. Nutr Cancer 54:216–222
Brooks JD, Thompson LU (2005) Mammalian lignans and genistein decrease the activities of aromatase and 17β-hydroxysteroid dehydrogenase in MCF-7 cells. J Steroid Biochem Mol Biol 94:461–467
Clavel T, Doré J, Blaut M (2006a) Bioavailability of lignans in human subjects. Nutr Res Rev 19:187–196
Clavel T, Henderson G, Engst W, Doré J, Blaut M (2006b) Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol Ecol 55:471–478
Dabek M, McCrae SI, Stevens VJ, Duncan SH, Louis P (2008) Distribution of β-glucosidase and β-glucuronidase activity and of β-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol Ecol 66:487–495
Desjardins ML, Roy D, Goulet J (1989) Growth of bifidobacteria and their enzyme profiles. J Dairy Sci 73:299–307
Donkor ON, Shah NP (2008) Production of beta-glucosidase and hydrolysis of isoflavone phytoestrogens by Lactobacillus acidophilus, Bifidobacterium lactis, and Lactobacillus casei in soymilk. J Food Sci 73:M15–M20
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA (2005) Diversity of the human intestinal microbial flora. Science 308:1635–1638
Eeckhaut E, Struijs K, Possemiers S, Vincken JP, Keukeleire DD, Verstraete W (2008) Metabolism of the lignan macromolecule into enterolignans in the gastrointestinal lumen as determined in the simulator of the human intestinal microbial ecosystem. J Agric Food Chem 56:4806–4812
Ford JD, Huang KS, Wang HB, Davin LB, Lewis NG (2001) Biosynthetic pathway to the cancer chemopreventive secoisolariciresinol diglucoside-hydroxymethyl glutaryl ester-linked lignan oligomers in flax (Linum usitatissimum) seed. J Nat Prod 64:1388–1397
Giada Mde L (2010) Food applications for flaxseed and its components: products and processing. Recent Pat Food Nutr Agric 2:181–186
Hawkesworth G, Drasar BS, Hill MJ (1971) Intestinal bacteria and the hydrolysis of glycosidic bonds. J Med Microbiol 4:451–459
Juurlink BHJ (2001) Therapeutic potential of dietary phase 2 enzyme inducers in ameliorating diseases that have an underlying inflammatory component. Can J Physiol Pharmacol 79:266–282
Kitts DD, Yuan YV, Wijewickreme AN, Thompson LU (1999) Antioxidant activity of the flaxseed lignan secoisolariciresinol diglycoside and its mammalian lignan metabolites enterodiol and enterolactone. Mol Cell Biochem 202:91–100
Li X, Yuan JP, Xu SP, Wang JH, Liu X (2008) Separation and determination of secoisolariciresinol diglucoside oligomers and their hydrolysates in the flaxseed extract by high-performance liquid chromatography. J Chromatogr A 1185:223–232
Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L (2004) Polyphenols: food sources and bioavailability. Am J Clin Nutr 79:727–747
Marazza JA, Garro MS, de Giori GS (2009) Aglycone production by Lactobacillus rhamnosus CRL981 during soymilk fermentation. Food Microbiol 26:333–339
Marotti I, Bonetti A, Biavati B, Catizone P, Dinelli G (2007) Biotransformation of common bean (Phaseolus vulgaris L.) flavonoid glycosides by Bifidobacterium species from human intestinal origin. J Agric Food Chem 55:3913–3919
Milder IE, Arts IC, van de Putte B, Venema DP, Hollman PC (2005) Lignan contents of Dutch plant foods: a database including lariciresinol, pinoresinol, secoisolariciresinol and matairesinol. Br J Nutr 93:393–402
Moutsatsou P (2007) The spectrum of phytoestrogens in nature: our knowledge is expanding. Hormones (Athens) 6:173–193
Mueller SO, Simon S, Chae K, Metzler M, Korach KS (2004) Phytoestrogens and their human metabolites show distinct agonistic and antagonistic properties on estrogen receptor alpha (ERalpha) and ERbeta in human cells. Toxicol Sci 80:14–25
Peterson J, Dwyer J, Adlercreutz H, Scalbert A, Jacques P, McCullough ML (2010) Dietary lignans: physiology and potential for cardiovascular disease risk reduction. Nutr Rev 68:571–603
Prasad K (1999) Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation 99:1355–1362
Prasad K (2001) Secoisolariciresinol diglucoside from flaxseed delays the development of type 2 diabetes in Zucker rat. J Lab Clin Med 138:32–39
Prasad K (2009) Flaxseed and cardiovascular health. J Cardiovasc Pharmacol 54:369–377
Raimondi S, Roncaglia L, De Lucia M, Amaretti A, Leonardi A, Pagnoni UM, Rossi M (2009) Bioconversion of soy isoflavones daidzin and daidzein by Bifidobacterium strains. Appl Microbiol Biotechnol 81:943–950
Rajilić-Stojanović M, Smidt H, de Vos WM (2007) Diversity of the human gastrointestinal tract microbiota revisited. Environ Microbiol 9:2125–2136
Rossi M, Amaretti A (2010) Probiotics properties of bifidobacteria. In: Mayo B, van Sinderen D (eds) Bifidobacteria—genomics and molecular aspects. Caister Academic, Norfolk, pp 97–123
Rossi M, Amaretti A, Roncaglia L, Leonardi A, Raimondi S (2010) Dietary isoflavones and intestinal microbiota: metabolism and transformation into bioactive compounds. In: Thomson MJ (ed) Isoflavones: biosynthesis, occurrence and health effects. Nova Science, Hauppauge, pp 137–161
Rowland I, Faughnan M, Honey L, Wähälä K, Williamson G, Cassidy A (2003) Bioavailability of phyto-oestrogens. Br J Nutr 89:S45–S58
Saarinen NM, Wärri A, Airio M, Smeds A, Mäkelä S (2007) Role of dietary lignans in the reduction of breast cancer risk. Mol Nutr Food Res 51:857–866
Smeds AI, Eklund PC, Sjöholm RE, Willför SM, Nishibe S, Deyama T, Holmbom BR (2007) Quantification of a broad spectrum of lignans in cereals, oilseeds, and nuts. J Agric Food Chem 55:1337–1346
Struijs K, Vincken JP, Gruppen H (2009) Bacterial conversion of secoisolariciresinol and anhydrosecoisolariciresinol. J Appl Microbiol 107:308–317
Swedenborg E, Power KA, Cai W, Pongratz I, Rüegg J (2009) Regulation of estrogen receptor beta activity and implications in health and disease. Cell Mol Life Sci 66:3873–3894
Tsangalis D, Ashton JF, McGill AEJ, Shah NP (2002) Enzymatic transformation of isoflavone phytoestrogens in soymilk by β-glucosidase-producing bifidobacteria. J Food Sci 67:3104–3113
Tsangalis D, Ashton JF, McGill AEJ, Shah NP (2003) Biotransformation of isoflavones by bifidobacteria in fermented soymilk supplemented with d-glucose and l-cysteine. J Food Sci 68:623–631
Venema K (2010) Role of gut microbiota in the control of energy and carbohydrate metabolism. Curr Opin Clin Nutr Metab Care 13:432–438
Wang W, Liu LQ, Higuchi CM, Chen H (1998) Induction of NADPH:quinone reductase by dietary phytoestrogens in colonic Colo205 cells. Biochem Pharmacol 56:189–195
Williams NT (2010) Probiotics. Am J Health-Syst Pharm 67:449–458
Wong JM, de Souza R, Kendall CW, Emam A, Jenkins DJ (2007) Colonic health: fermentation and short chain fatty acids. J Clin Gastroenterol 40:235–243
Woting A, Clavel T, Loh G, Blaut M (2010) Bacterial transformation of dietary lignans in gnotobiotic rats. FEMS Microbiol Ecol 72:507–514
Yuan JP, Li X, Xu SP, Wang JH, Liu X (2008) Hydrolysis kinetics of secoisolariciresinol diglucoside oligomers from flaxseed. J Agric Food Chem 56:10041–10047
Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ (2008a) Effects of dietary flaxseed lignan extract on symptoms of benign prostatic hyperplasia. J Med Food 11:207–214
Zhang W, Wang X, Liu Y, Tian H, Flickinger B, Empie MW, Sun SZ (2008b) Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr 99:1301–1309
Zoetendal EG, Rajilic-Stojanovic M, de Vos WM (2008) High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut 57:1605–1615
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Roncaglia, L., Amaretti, A., Raimondi, S. et al. Role of bifidobacteria in the activation of the lignan secoisolariciresinol diglucoside. Appl Microbiol Biotechnol 92, 159–168 (2011). https://doi.org/10.1007/s00253-011-3338-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3338-8