Abstract
Hypericum perforatum is a well-known medicinal plant which contains a wide variety of metabolites, including xanthones, which have a wide range of biological properties, including antifungal activity. In the present study, we evaluated the capability of roots regenerated from calli of H. perforatum subsp. angustifolium to produce xanthones. Root biomass was positively correlated with the indole-3-butyric acid concentration, whereas a concentration of 1 mg l−1 was the most suitable for the development of roots. High auxin concentrations also inhibited xanthone accumulation. Xanthones were produced in large amounts, with a very stable trend throughout the culture period. When the roots were treated with chitosan, the xanthone content dramatically increased, peaking after 7 days. Chitosan also induced a release of these metabolites into the culture. The maximum accumulation (14.26 ± 0.62 mg g−1 dry weight [DW]) and release (2.64 ± 0.13 mg g−1 DW) of xanthones were recorded 7 days after treatment. The most represented xanthones were isolated, purified, and spectroscopically characterized. Antifungal activity of the total root extracts was tested against a broad panel of human fungal pathogen strains (30 Candida species, 12 Cryptococcus neoformans, and 16 dermatophytes); this activity significantly increased when using chitosan. Extracts obtained after 7 days of chitosan treatment showed high antifungal activity (mean minimum inhibitory concentration of 83.4, 39.1, and 114 μg ml−1 against Candida spp., C. neoformans, and dermatophytes, respectively). Our results suggest that root cultures can be considered as a potential tool for large-scale production of extracts with stable quantities of xanthones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The pharmacological properties of Hypericum perforatum are well known. It contains a wide variety of metabolites with documented biological activities, including phenolic acids, flavonoids, naphthodianthrones, phloroglucinols, and xanthones (Nahrstedt and Butterweck 2010). In a preliminary study on naturally grown plants of H. perforatum subsp. angustifolium (unpublished), we found that xanthones were mainly produced in the roots, though in very low quantities, whereas flavonoids, naphthodianthrones, and phloroglucinols were distributed in the aerial parts of the plant.
Xanthones have a wide range of biological and pharmacological properties, such as monoamine oxidase inhibition, and antioxidant, antimicrobial, cytotoxic, and hepatoprotective activity (Fotie and Bohle 2006). The antifungal activity of many xanthones has also been well documented (Lavault et al. 2005; Wang et al. 2005; Pinto et al. 2011). This reflects the renewed interest in plant extracts with antifungal activities, which has been due to the increasing number of immunecompromised persons (e.g., organ transplant recipients, chemotherapy patients, and persons with AIDS), and the consequent sharp increase in the frequency of fungal infections (Pfaller and Diekema 2010). This in turn has led to an increase in the prescription of antifungal drugs and the emergence of drug resistance. In light of these circumstances and considering that there is only a limited arsenal of antifungal drugs and agents, new therapeutic approaches must be developed (Rapp 2004; Pfaller et al. 2010). Although the antifungal activity of xanthones from a number of Hypericum species against human pathogenic fungi has been investigated (Fenner et al. 2005; Radulovic et al. 2007), little is known about the possible application of xanthones from H. perforatum roots.
The plant kingdom has historically been a major source of bioactive compounds for medicine, food additives, pigments, insecticides, cosmetics, and fine chemicals. However, the recovery of bioactive compounds from natural sources is often problematic, in that the extract may contain only very small quantities or its composition may vary with the season or the environment. To obtain qualitatively and quantitatively standardized extracts, plant biotechnology can represent a valuable alternative.
In the present study, we evaluated the capability of regenerated roots from calli of H. perforatum subsp. angustifolium to produce xanthones, which has never been investigated. To increase xanthone production, we used chitosan, an effective biotic elicitor for improving xanthone biosynthesis in cell cultures of H. perforatum subsp. angustifolium (Tocci et al. 2010). We also tested the antifungal activity of total extracts and of the most representative xanthones against a broad panel of human fungal pathogens.
Materials and methods
Plant material and tissue culture
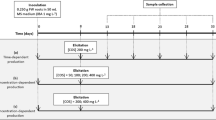
Seeds of H. perforatum subsp. angustifolium were surface sterilized in accordance with Zobayed et al. (2004) and placed on Murashige and Skoog (MS) (1962) medium (Duchefa Biochemie, Haarlem, The Netherlands) supplemented with 3% (w/v) sucrose and solidified with agar. Seed cultures were grown under a photoperiod of 16/8 h (light/dark) at 26°C (photon lux density, 70 μmol m−2 s−1). To induce the regeneration of adventitious roots, leaf and stem explants were excised from 2-month-old seedlings and placed on solid MS medium supplemented with 5 mg l−1, 2,4-dichlorophenoxyacetic acid (Duchefa Biochemie, Haarlem, The Netherlands), 1 mg l−1 kinetin (Duchefa Biochemie, Haarlem, The Netherlands), and 3% (w/v) sucrose; the cultures were maintained under continuous darkness. After 28 days of culture, the callus were transferred to hormone-free (HF) agarized MS medium to induce the formation of roots. Regenerated roots, 2–3 cm in length, were isolated from the callus (after 30 days) and subcultured in the same medium. To induce branching (lateral root formation) and to increase biomass, the roots (after 30 days) were cultured in MS medium supplemented with different concentrations of indole-3-butyric acid (IBA) (0, 0.5, 1, 1.5, and 2 mg l−1). Liquid cultures were established after 30 days, by inoculating 0.02 g dry weight (DW) of roots in 250 ml Erlenmeyer flasks containing 100 ml half-strength liquid MS medium supplemented with 1 mg l−1 IBA. The flasks were shaken at 100 rpm at 25 ± 1°C and maintained in the dark. Subcultures in liquid medium were performed every 23 days.
Elicitation
Xanthones were elicited using chitosan (medium molecular weight; Sigma-Aldrich, Milan, Italy) at a final concentration of 200 mg l−1, which was added on the eighth day of culture. Root samples were harvested by filtration on the 11th, 15th, 18th, and 23rd days of culture.
Determination of root biomass
The biomass was determined by recording the DW of the roots. The roots were separated from the medium by passing them through a stainless steel sieve and washed with distilled water to remove medium contaminants. They were then dried in an oven at 40°C for 72 h, and the DW was measured. The growth index was calculated as follows:
HPLC analysis
Ground dried roots (4–6 months in culture) were extracted three times with methanol at room temperature. The culture media were extracted with ethyl acetate using a separating funnel. The final volume of the extracts was dried with a rotavapor and subjected to high-performance liquid chromatography (HPLC). The HPLC analysis of the extracts was performed using an apparatus consisting of a pump (Waters 1525 Binary HPLC Pump), equipped with a UV detector (Waters 2487 Dual λ Detector) and a reverse-phase C18 column (4.6 × 150 mm; 5 μm; Waters). The extract composition was determined by linear gradient elution, using a modified version of the method of Dias et al. (1999). The mobile phase was a gradient prepared from 100:0.1 (v/v) water–phosphoric acid (component A) and methanol (component B) (Carlo Erba, Milan, Italy); the gradient program was from 10% to 50% B in 13 min, held for 10 min, and then from 50% to 95% B in 42 min. The injection volume was 20 μl, and the mobile-phase flow rate was 1.0 ml min−1. The compounds were identified at 260 and 320 nm by the external standard method, using, as reference, xanthones that had been previously purified and spectroscopically characterized in our laboratory (1,3,6,7-tetrahydroxyxanthone, 1,3,5,6-tetrahydroxyxanthone, toxyloxanthone B, paxanthone, and cadensin G). 1,7-Dihydroxyxanthone and 5-methoxy-2-deprenylrheediaxanthone B were purified from in vitro regenerated roots of H. perforatum subsp. angustifolium. Xanthones were quantified as paxanthone equivalents at 260 nm.
Purification and 1H NMR analysis of xanthones
The isolation and purification of 1,7-dihydroxyxanthone and 5-methoxy-2-deprenylrheediaxanthone B were carried out by preparative thin-layer chromatography (silica gel, hexane/ethyl acetate 2:1) (Carlo Erba, Milan, Italy). The nuclear magnetic resonance (NMR) spectroscopic data were recorded at room temperature on an Avance 400 MHz NMR spectrometer. The NMR chemical shifts (δ) are expressed as parts per million relative to the residual proton chemical shifts of the deuterated solvent (acetone-D6) set relative to external tetramethylsilane.
1,7-Dihydroxyxanthone (euxanthone): 1H NMR (400 MHz, AcD6) δ 12.72 (1H, s, OH–C1), 7.72 (1H, t, J = 8.0 Hz, H-3), 7.63 (1H, d, J = 2.8 Hz, H-8), 7.54 (1H, d, J = 9.2 Hz, H-5), 7.45 (1H, dd, J = 9.2 and 2.8 Hz, H-6), 7.02 (1H, d, J = 8.0 Hz, H-4), 6.78 (1H, d, J = 8 Hz, H-2).
5-Methoxy-2-deprenylrheediaxanthone: B 1H NMR (400 MHz, AcD6) δ 13.40 (OH-1, s), 7.85 (H-8, d, J = 8.7 Hz), 7.03 (H-7, d, J = 8.7 Hz), 6.18 (H-2, s), 4.60 (H-2′, q, J = 6.6 Hz), 4.02 (OMe, s), 1.65 (4′-CH3, s), 1.43 (3′-CH3, d, J = 6.6 Hz), 1.35 (5′-CH3, s).
Organisms
For the antifungal evaluation, strains obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA), from the German Collection of Microorganisms (DSMZ, Braunschweig, Germany), and from the Pharmaceutical Microbiology Culture Collection (PMC, Department of Public Health and Infectious Diseases, Sapienza, Rome, Italy) were tested. The strains obtained from the ATCC were Candida albicans ATCC90028, C. albicans ATCC90029, C. albicans ATCC10261, C. albicans ATCC10231, C. albicans ATCC3153, C. albicans ATCC2091, C. albicans ATCC76615, C. albicans ATCC24433 (tested as reference strain), Candida parapsilosis ATCC22019 (tested as quality control strain), and Trichophyton mentagrophytes ATCC9972 (tested as reference strain). The strains obtained from the DSMZ were C. parapsilosis DSM11224, C. parapsilosis DSM5784, Candida krusei DSM6128, Candida tropicalis DSM11953, Candida glabrata DSM11226, Cryptococcus neoformans DSM11959, C. neoformans DSM6972, T. mentagrophytes DSM4870, Trichophyton rubrum DSM4167, Microsporum canis DSM10708, and Microsporum gypseum DSM 3824. The strains obtained from PMC were C. albicans (PMC 1011, PMC 1015, PMC 1023, PMC 1071, PMC 1075, PMC 1083, PMC 1088, PMC 1097), C. parapsilosis (PMC 0703, PMC 0711), C. tropicalis PMC 0908, C. krusei (PMC 0613, PMC 0625, PMC 0639), C. glabrata (PMC 0805, PMC 0821, PMC 0843), C. neoformans (PMC 2103, PMC 2107, PMC 2111, PMC 2123, PMC 2136, PMC 2138, PMC 2142, PMC 2157, PMC 2169, PMC 2185), T. mentagrophytes (PMC 6503, PMC 6509, PMC 6515, PMC 6527, PMC 6531, PMC 6552), T. rubrum PMC 6612, M. gypseum (PMC 7303, PMC 7331, PMC 7342), and M. canis PMC 7426. All the strains were stored at −70°C in 10–15% glycerol solution (CLSI 2008a, b). Yeasts were grown on sabouraud dextrose agar (Sigma-Aldrich, St. Louis, MO, USA) plate for 24–48 h at 35°C, in accordance with the procedures of the Clinical and Laboratory Standards Institute (CLSI) (CLSI 2008a). Dermatophytes were grown on potato dextrose agar (Sigma-Aldrich, St. Louis, MO, USA) at 30°C for 4–5 days or until good conidial growth was obtained (CLSI 2008b).
Antifungal susceptibility testing
In vitro antifungal susceptibility was evaluated using the total extracts from untreated and treated roots obtained on the 15th day of culture and from isolated and purified xanthones. The tests were conducted in accordance with the CLSI M27-A3 and CLSI M38-A2 broth microdilution methods (CLSI 2008a, b). Yeast and dermatophyte inocula were prepared as described in the CLSI protocols. The antifungal references amphotericin B and fluconazole (Sigma-Aldrich, St. Louis, MO, USA) were also tested (ranges, 0.031 to 16 μg ml−1 for amphotericin B and 0.0078 to 250 μg ml−1 for fluconazole). The final concentrations ranged from 1,000 to 1 μg ml−1 for total extracts and from 64 to 0.125 μg ml−1 for single xanthones. Microdilution trays containing 100 μl of serial twofold dilutions of each substance in RPMI 1640 medium (Sigma-Aldrich, St. Louis, MO, USA) were inoculated with an organism suspension adjusted to attain a final inoculum concentration of 1.0 × 103–1.5 × 103 cells ml−1 for yeasts and 0.4 × 104–5 × 104 CFU ml−1 for dermatophytes. The panels were incubated at 35°C and observed for the presence of growth at 48 h (Candida spp.) and 72 h (C. neoformans and dermatophytes). Quality control was performed by testing the CLSI-recommended strain C. parapsilosis ATCC 22019 (CLSI 2008c). The minimum inhibitory concentration (MIC) was defined as, for yeasts, the lowest concentration that showed ≥50% growth inhibition (MIC50) compared with the growth control and, for dermatophytes, the lowest concentration that showed ≥80% reduction in growth (MIC80) with respect to the growth control. However, when testing strains against amphotericin B, the MIC was defined as the lowest concentration that prevented any discernible growth (MIC100). The MIC100 was also evaluated for all of the substances. All of the experiments were performed in triplicate on three consecutive test days.
Cell viability assay
Human leukemic monocyte lymphoma cells (U937) were obtained from the ATCC (CRL1593.2, Rockville, MD, USA). The cells (2 × 104 cells well−1) were seeded into 96-well plates containing 100 μl of supplemented RPMI 1640 (Invitrogen, San Diego, CA, USA) without phenol red, supplemented with 10% fetal bovine serum (Invitrogen, San Diego, CA, USA), l-glutamine (0.3 mg ml−1), penicillin (100 U ml−1), and streptomycin (100 μg ml−1) (EuroClone, Celbio, Milan, Italy), and they were cultured at 37°C in 5% CO2. Total extracts, dissolved in dimethyl sulfoxide (DMSO), from untreated and treated roots obtained on the 15th day of culture (with concentrations ranging from 64 to 1,000 μg ml−1) were added to the wells. Each concentration and control was assayed in four replicates with at least five concentrations. The cells were cultured at 37°C and 5% CO2 for 24 h, and cell viability was determined using an MTT assay (Sigma-Aldrich, St. Louis, MO, USA) (Mosmann 1983). MTT solution was added to each well in an amount equal to 10% of the culture volume, and the plates were incubated for 3–4 h at 37°C in 5% CO2. MTT solvent (Sigma-Aldrich, St. Louis, MO, USA) was successively added to dissolve the intracellular crystal. The plates were then incubated at 37°C in 5% CO2 for 1 h, and the optical density of each well was measured spectrophotometrically at 570 nm. The cytotoxicity of the root extracts was calculated as the percentage reduction in viable cells with respect to the control culture (cells treated with DMSO only). The 50% cytotoxic concentration (CC50) was evaluated as the drug concentration required to reduce human cell viability by 50% compared to the drug-free control.
Statistical analysis
The arithmetic mean and standard deviation (SD) were calculated. The significance of the differences between the means was tested by a two-tailed Student’s t test. P < 0.05 was considered to be significant; P < 0.001 was considered to be highly significant.
Results
Induction and development of adventitious roots
The seeds of H. perforatum subsp. angustifolium germinated within 2 days. On the eighth day of culture, cotyledons were evident. Leaf and stem explants from 2-month-old seedlings were inoculated in a callogenic medium. After 28 days of culture, the callus, which was cream-colored and friable, was transferred to HF medium to induce root regeneration. Twenty days later, regenerated roots were visible. When roots reached a length of 2–3 cm, they were separated from the callus and subcultured in the same medium. Their growth in length was initially very slow, but they grew rapidly after about 30 days of culture. The roots showed regular morphology and structure.
The effect of IBA on biomass growth and xanthone production
To achieve the most suitable culture conditions, the roots were cultivated in MS liquid medium supplemented with different concentrations of IBA (0, 0.5, 1, 1.5, and 2 mg l−1). Root biomass increase was monitored for 23 days based on the growth curve (Fig. 3a). In this system, the log phase ended on the 18th day of culture and no additional increase in biomass was observed until the 23rd day. Concerning biomass increase, the highest growth indexes (8.5 and 9.5) were registered for roots cultivated in the presence of the highest IBA concentrations (Fig. 1) (1.5 and 2 mg l−1), yet in these conditions the roots showed callus formation. In the medium supplemented with 1 mg l−1 IBA, the roots showed a growth index of 6.5, which corresponded to a 7.5 increase in biomass with respect to the initial inoculum. Even if the biomass increase with IBA 1.5 and 2 mg l−1 was significantly higher (P < 0.05) than with IBA 1 mg l−1, this last medium has been chosen because it allowed the formation of morphologically unaltered roots. This medium was also found to have been the most suitable to induce a significantly higher (P < 0.05) xanthone accumulation (Fig. 2) (3.26 ± 0.09 mg g−1 DW).
Effect of chitosan treatment on root biomass growth
The growth curve of root biomass was recorded during a culture cycle of 23 days (Fig. 3a). Chitosan, which was added during the exponential phase (eighth day of culture), resulted in a cessation in growth and a decrease in biomass.
a Growth curves of untreated and chitosan-treated root cultures, b accumulation, and c release of detected xanthones by root cultures of H. perforatum subsp. angustifolium throughout the growth period. Results are means (±SD) of three independent replicates. The straight arrow in a indicates the day of chitosan treatment
Xanthone production in untreated and chitosan-treated roots
The HPLC analysis showed that chitosan treatment greatly, though only transiently, enhanced xanthone production. Xanthone accumulation in untreated roots did not vary dramatically during the culture period (Fig. 3b), with a mean of 4.5 ± 1.5 mg g−1 DW. The major xanthones accumulated in the untreated roots were 1,7-dihydroxyxanthone, 5-methoxy-2-deprenylrheediaxanthone B, and paxanthone. 1,7-Dihydroxyxanthone was detected from the beginning of the culture period until the 15th day, whereas afterwards it was not detected (Table 1). The xanthone profile in treated root extracts was qualitatively similar to the one in untreated root extracts. In treated roots, total xanthone accumulation peaked 7 days after treatment (which corresponded to the 15th day of culture) at 14.26 ± 0.62 mg g−1 DW, which is significantly higher (P < 0.05) than the accumulation in the control on the same day of culture. Afterwards, total xanthone accumulation rapidly decreased, until reaching 3.01 ± 0.09 mg g−1 DW on the 23rd day of culture, which is similar to that for untreated roots (3.26 ± 0.09 mg g−1 DW) (Fig. 3b). Moreover, the peak accumulation of 1,7-dihydroxyxanthone in treated roots (7 days after treatment) was 10 times higher than the accumulation in untreated roots.
Xanthone release in the culture medium
The HPLC analysis of the culture media of treated and untreated roots showed qualitatively similar chemical profiles for xanthones, with 1,7-dihydroxyxanthone being the main compound in all of the extracts (Table 2). Total xanthone release was significantly enhanced by chitosan (P < 0.05), peaking on the 15th day of culture (7 days after treatment) at 2.64 ± 0.13 mg g−1 DW (21.12 mg l−1 medium); 1.7 ± 0.1 mg g−1 DW (13.6 mg l−1 medium) of the total xanthone release was represented by 1,7-dihydroxyxanthone.
Antifungal activity
The antifungal activity of the extracts obtained from untreated and treated roots at the 15th day of culture was evaluated against strains of Candida spp., C. neoformans, and dermatophytes. Chitosan increased the antifungal activity against all of the strains tested. In particular, for the treated roots, the MIC range against Candida spp. strains was 16–250 μg ml−1, compared to 64–1,000 μg ml−1 for untreated roots (Table 3). Regarding the extracts from treated roots, when comparing strains of Candida non-albicans species with C. albicans, the MIC range was, respectively, 16–250 and 64–250 μg ml−1 (Table S1 of the Electronic supplementary material). For strains of C. neoformans, the MIC range was 16–125 μg ml−1 for treated root extracts and 64–250 μg ml−1 for untreated root extracts. For dermatophyte strains, the MIC range was 64–125 and 125–500 μg ml−1 for treated root and untreated root extracts, respectively (Tables 4 and 5) (additional data are given in Tables S2 and S3 of the Electronic supplementary material). The difference in MIC between treated and untreated roots was highly significant (two-tailed Student’s t test: P < 0.001) for Candida and Cryptococcus strains (Tables 3 and 4) and significant (P < 0.05) for dermatophytes (Table 5). Total growth inhibition (MIC100) for all tested strains was obtained with concentrations ranging from about 125 to 1,000 μg ml−1. The main xanthones isolated and purified from treated root extracts (1,7-dihydroxyxanthone, cadensin G, paxanthone, and 5-methoxy-2-deprenylrheediaxanthone B) were also investigated for their antifungal activity; the MIC ranged from 4 to >64 μg ml−1 (Tables 3, 4, and 5) (additional data are given in Tables S1, S2, and S3 of the Electronic supplementary material). 1,3,5,6-Tetrahydroxyxanthone and 1,3,6,7-tetrahydroxyxanthone have not been tested because none of the solvents used allowed their separation due to their structural similarity.
Cytotoxic activity
The cytotoxicity of extracts obtained from untreated and treated roots at the 15th day of culture was evaluated against mammalian cells (U937). The CC50 was calculated. For both untreated and treated root extracts, the CC50 was >1,000 μg ml−1.
Discussion
In a comparative study of H. perforatum subspecies, using in-field grown plants, the accumulation of secondary metabolites was greater for H. perforatum subsp. angustifolium, compared to the other subspecies (Males et al. 2006). Xanthones with interesting biological activities have been found in H. perforatum total plant extracts (Kitanov and Blinova 1987; Hölzl et al. 1989; Nahrstedt and Butterweck 1997). In an in vitro study of H. perforatum, Pasqua et al. (2003) demonstrated that xanthones were accumulated in the roots regenerated from plantlets or formed by callus. These results were confirmed in an unpublished study on H. perforatum subsp. angustifolium in which we found that, in naturally grown plants, xanthones were mainly accumulated in the roots, yet in very low quantities, ranging from 200 to 500 μg g−1 DW (unpublished). We also observed that xanthone accumulation greatly depended on the season, the soil composition, and the developmental stage of the plant. For these reasons, it is very difficult to recover an exploitable and standardized extract containing xanthones from natural sources.
To overcome these problems, we focused on in vitro regenerated root cultures as a biotechnological system for obtaining standardized extracts containing xanthones. The large-scale cultivation of untransformed roots for producing important phytochemicals has been achieved for medicinal plants such as Nothapodytes foetida (Fulzele et al. 2002), Panax notoginseng (Gao et al. 2005), and Echinacea purpurea (Wu et al. 2007). Although root cultures of H. perforatum have also been established by other authors (Cui et al. 2010; Goel et al. 2009), the capacity of these cultures to produce xanthones has never been explored.
As reported by many authors (Pasqua et al. 2003; Zobayed et al. 2004; Franklin and Dias 2006), H. perforatum has a high capacity for regeneration, and in our study, root regeneration from calli was very easily achieved. The roots were cultivated with IBA, based on our preliminary results and on the results of studies on H. perforatum root cultures conducted by Cui et al. (2010) and Goel et al. (2009). According to these studies, IBA and indole-3-acetic acid were more effective for lateral root induction and root growth, whereas 1-naphthaleneacetic acid was not effective and induced callused roots. Moreover, we chose to use IBA because it is more stable than IAA; in fact, many reports stress that IAA is unstable in culture media at room temperature. Root biomass was positively correlated with IBA concentration (Fig. 1), though a concentration of 1 mg l−1 was the most suitable to obtain morphologically unaltered roots.
The highest concentrations of IBA inhibited xanthone accumulation (Fig. 2), confirming reports that high auxin levels are often deleterious to secondary metabolite accumulation in other species (Dörnenburg and Knorr 1995; Chan et al. 2005).
In our study, xanthones were produced in large amounts, with a very stable trend throughout the culture period. To stimulate xanthone accumulation, chitosan, a polymer that is able to stimulate the biosynthesis of xanthones in cell cultures of H. perforatum subsp. angustifolium (Tocci et al. 2010), was used. Chitosan acts as an external stimulus on plant cells, which is recognized by receptors localized on the plasma membrane, leading to the induction of a defense response (oxidative burst and phytoalexin production) without penetrating the cell (Kaku et al. 2006). Chitosan treatment resulted in a cessation in growth and a decrease in biomass. Moreover, after elicitation, the xanthone content in roots dramatically increased, peaking after 7 days. This increase supports the hypothesis that xanthones act as a phytoalexin, playing a role in the chemical defense of the plant against fungal pathogens (Franklin et al. 2009). Fifteen days after treatment with chitosan, the xanthone content decreased, and the level at the end of the culture period, day 23, was similar to that of the untreated control. Moreover, after chitosan treatment, we observed an increased accumulation of these metabolites in the culture medium (Fig. 3c), which constitutes an important advantage for a future scale-up of the production process for the easy recovery of bioactive substances, without destroying plant material.
Our results suggest that root cultures can be considered as a potential tool for large-scale xanthone production, in that accumulation is much higher than that found in the roots of naturally grown plants and root cultures possess suitable characteristics for a successful scale-up: high biomass growth, stable production of secondary metabolites, sensitivity to external stimuli, and metabolite release in the culture medium.
Regarding the antifungal activity of the extracts, an encouraging level was observed for all of the fungal strains tested: Candida spp., the main agent responsible for nosocomial fungal infections (Pfaller and Diekema 2010); C. neoformans, a common life-threatening human fungal pathogen (Mitchell and Perfect 1995); and dermatophytes, which are responsible for fungal skin infections (Seebacher et al. 2008). This activity significantly increased when using chitosan, and high activity was found for extracts obtained after 7 days of treatment spp., C. neoformans, and dermatophytes, respectively). The higher antifungal activity found for extracts from treated roots might be explained by the greater accumulation of xanthones in these roots. Further studies are needed to determine whether the observed activity is the consequence of an increase in xanthones or other unidentified compounds produced in response to elicitation. The increased amount of xanthones and the corresponding improved antifungal activity of the extracts suggest that xanthones could be the principal constituents responsible for the antifungal activity. Metabolomic analyses are currently being performed to better clarify the extract composition and to perform purification of xanthone-rich fractions to be tested for antifungal activity.
In conclusion, the system described herein is an efficient means of obtaining root biomass, which is not subject to pedoclimatic variations or antropic contaminants and which allows extracts with stable quantities of xanthones and good antifungal activity to be obtained. The cytotoxicity tests were performed to demonstrate that the root extracts are not toxic for animal cells and to continue studies for an eventual applicative use in human disease. Our preliminary cytotoxicity analyses show a low cytotoxicity of extracts on mammalian cells, though further studies need to be performed.
References
Chan LK, Dewi PR, Boey PL (2005) Effect of plant growth regulators on regeneration of plantlets from bud cultures of Cymbopogon nardus L. and the detection of essential oils from the in vitro plantlets. J Plant Biol 48:142–145. doi:https://doi.org/10.1007/BF03030574
CLSI (2008a) Reference method for broth dilution antifungal susceptibility testing of yeasts; approved standard—third edition. CLSI document M27A3. Clinical and Laboratory Standards Institute, Wayne
CLSI (2008b) Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; approved standard—second edition. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne
CLSI (2008c) Reference method for broth dilution antifungal susceptibility testing of yeasts; third informational supplement. CLSI document M27-S3. Clinical and Laboratory Standards Institute, Wayne
Cui XH, Chakrabarty D, Lee AJ, Paek KY (2010) Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour Technol 101:4708–4716. doi:https://doi.org/10.1016/j.biortech.2010.01.115
Dias ACP, Seabra RM, Andrade PB, Fernandes-Ferreira M (1999) The development and evaluation of an HPLC-DAD method for the analysis of the phenolic fractions from in vivo and in vitro biomass of Hypericum species. J Liq Chromatogr Relat Technol 22:215–227. doi:https://doi.org/10.1081/JLC-100101655
Dörnenburg H, Knorr D (1995) Strategies for the improvement of secondary metabolite production in plant cell cultures. Enzym Microb Tech 17:674–684. doi:https://doi.org/10.1016/0141-0229(94)00108-4
Fenner R, Sortino M, Kuze Rates SM, Dall’Agnol R, Ferraz A, Bernardi AP, Albring D, Nör C, von Poser G, Schapoval E, Zacchino S (2005) Antifungal activity of some Brazilian Hypericum species. Phytomedicine 12:236–240. doi:https://doi.org/10.1016/j.phymed.2003.11.004
Fotie J, Bohle DS (2006) Pharmacological and biological activities of xanthones. Anti-Infective Agents Med Chem 5:15–31
Franklin G, Dias ACP (2006) Organogenesis and embryogenesis in several Hypericum perforatum genotypes. In vitro Cell Dev Biol 42:324–330
Franklin G, Conceição LFR, Kombrink E, Dias ACP (2009) Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 70:60–68. doi:https://doi.org/10.1016/j.phytochem.2008.10.016
Fulzele DP, Satdive RK, Pol BB (2002) Untrasformed root cultures of Nothapodytes foetida and production of campothecin. Plant Cell Tiss Organ Cult 68:285–288. doi:https://doi.org/10.1023/A:1015657927304
Gao X, Zhu C, Jia W, Gao W, Qiu M, Zhang Y, Xiao P (2005) Induction and characterization of adventitious roots directly from the explants of Panax notoginseng. Biotechnol Lett 27:1771–1775. doi:https://doi.org/10.1007/s10529-005-3553-4
Goel MK, Kulreja AK, Bishut NS (2009) In vitro manipulations in St. John’s wort (Hypericum perforatum L.) for incessant and scale up micropropagation using adventitious roots in liquid medium and assessment of clonal fidelity using RAPD analysis. Plant Cell Tiss Organ Cult 96:1–9. doi:https://doi.org/10.1007/s11240-008-9453-2
Hölzl J, Demisch L, Gollnik B (1989) Investigation about antidepressive and mood-changing effects of Hypericum perforatum. Planta Med 55:643
Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, Minami E, Shibuya N (2006) Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci 103:11086–11091. doi:https://doi.org/10.1073/pnas.0508882103
Kitanov GM, Blinova KF (1987) Modern state of the chemical study of species of the genus Hypericum. Chem Nat Compd 23:151–166. doi:https://doi.org/10.1007/BF00598748
Lavault M, Landreau A, Larcher G, Bouchara JP, Pagniez F, Le Pape P, Richomme P (2005) Antileishmania and antifungal activities of xantholides isolated from Xanthium macrocarpum. Fitoterapia 76:363–366
Males Z, Brantner AH, Sovic K, Pilepic KH, Plazibat M (2006) Comparative phytochemical and antimicrobial investigations of Hypericum perforatum L. subsp. perforatum and Hypericum perforatum subsp.angustifolium (D.C.) Gaudin. Acta Pharm 56:359–367
Mitchell TG, Perfect JR (1995) Cryptococcosis in the era of AIDS--100 years after the discovery of Cryptococcus neoformans. Clin Microbiol Rev 8:515–548
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assay. J Immunol Methods 65:55–63. doi:https://doi.org/10.1016/0022-1759(83)90303-4
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:https://doi.org/10.1111/j.1399-3054.1962.tb08052.x
Nahrstedt A, Butterweck V (1997) Biologically active and other chemical constituents of the herb of Hypericum perforatum L. Pharmacopsychiatry 30:129–134. doi:https://doi.org/10.1055/s-2007-979533
Nahrstedt A, Butterweck V (2010) Lessons learned from herbal medicinal products: the example of St. John’s Wort (perpendicular). J Nat Prod 28:1015–1021
Pasqua G, Avato P, Monacelli B, Santamaria AR, Argentieri MP (2003) Metabolites in cell suspension cultures, calli, and in vitro regenerated organs of Hypericum perforatum cv. Topas Plant Sci 165:977–982. doi:https://doi.org/10.1016/S0168-9452(03)00275-9
Pfaller MA, Diekema DJ (2010) Epidemiology of invasive mycoses in North America. Cri Rev Microbiol 36:1–53. doi:https://doi.org/10.3109/10408410903241444
Pfaller MA, Diekema DJ, Gibbs DL, Newell VA, Ellis D, Tullio V, Rodloff A, Fu W, Ling TA, The Global Antifungal Surveillance Group (2010) Results from the ARTEMIS DISK global antifungal surveillance study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Clin Microbiol 48:1366–1377. doi:https://doi.org/10.1128/JCM.43.12.5848-5859.2005
Pinto E, Afonso C, Duarte S, Vale-Silva L, Costa E, Sousa E, Pinto M (2011) Antifungal activity of xanthones: evaluation of their effect on ergosterol biosynthesis by high-performance liquid chromatography. Chem Biol Drug Des. doi:https://doi.org/10.1111/j.1747-0285.2010.01072.x
Radulovic N, Stankov-Jovanovic V, Stojanovic G, Smelcerovic A, Spiteller M, Asakawa Y (2007) Screening of in vitro antimicrobial and antioxidant activity of nine Hypericum species from the Balkans. Food Chem 103:15–21. doi:https://doi.org/10.1016/j.foodchem.2006.05.062
Rapp RP (2004) Changing strategies for the management of invasive fungal infections. Pharmacotherapy 24:4–28. doi:https://doi.org/10.1592/phco.24.3.31S.33152
Seebacher C, Bouchara JP, Mignon B (2008) Updates on the epidemiology of dermatophyte infections. Mycopathology 166:335–352. doi:https://doi.org/10.1007/s11046-008-9100-9
Tocci N, Ferrari F, Santamaria AR, Valletta A, Rovardi I, Pasqua G (2010) Chitosan enhances xanthone production in Hypericum perforatum subsp. angustifolium cell cultures. Nat Prod Res 24:286–293. doi:https://doi.org/10.1080/14786410903006353
Wang H, Hou AJ, Zhu GF, Chen DF, Sun HD (2005) Cytotoxic and antifungal isoprenylated xanthones and flavonoids from Cudrania fruticosa. Planta Med 71:273–274. doi:https://doi.org/10.1055/s-2005-837829
Wu CH, Murthy HN, Hahn EJ, Paek KY (2007) Large-scale cultivation of adventitious roots of Echinacea purpurea in airlift bioreactors for the production of cichoric acid, chlorogenic acid and caftaric acid. Biotechnol Lett 29:1179–1182. doi:https://doi.org/10.1007/s10529-007-9399-1
Zobayed SMA, Murch SJ, Rupasinghe HPV, Saxena PK (2004) In vitro production and chemical characterization of St. John’s wort (Hypericum perforatum L. cv ‘New Stem’). Plant Sci 166:333–340. doi:https://doi.org/10.1016/j.plantsci.2003.10.005
Acknowledgements
This work was partially supported by the Italian Ministry of Instruction, Universities and Research (Special Project “Fund for Investment on Basic research”—Reti FIRB RBPR05NWWC_006).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors Noemi Tocci and Giovanna Simonetti contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Supplemental to Table 3: Antifungal activity of total extracts from untreated and chitosan-treated roots of H. perforatum subsp. angustifolium obtained on the 15th day of culture and isolated and of purified xanthones against 30 Candida strains (16 C. albicans, 4 C. parapsilosis, 2 C. tropicalis, 4 C. krusei, and 4 C. glabrata) and C. parapsilosis ATCC22019 (quality control strain). MIC arithmetic mean of minimum inhibitory concentration, MIC100 lowest drug concentration that prevented any discernible growth with respect to the untreated control; data represent ranges of three separate experiments in triplicate. (DOC 71.0 kb)
Table S2
Supplemental to Table 4: Antifungal activity of total extracts from untreated and chitosan-treated roots of H. perforatum subsp. angustifolium obtained on the 15th day of culture and isolated and of purified xanthones against 12 strains of C. neoformans. MIC arithmetic mean of minimum inhibitory concentration, MIC 100 lowest drug concentration that prevented any discernible growth with respect to the untreated control; data represent ranges of three separate experiments in triplicate. (DOC 42.5 kb)
Table S3
Supplemental to Table 5: Antifungal activity of total extracts from untreated and chitosan-treated roots of H. perforatum subsp. angustifolium obtained on the 15th day of culture and isolated and of purified xanthones against 16 dermatophytes (8 T. mentagrophytes, 2 T. rubrum, 4 M. gypseum, and 2 M. canis); data represent ranges of three separate experiments in triplicate. (DOC 48.5 kb)
Rights and permissions
About this article
Cite this article
Tocci, N., Simonetti, G., D’Auria, F.D. et al. Root cultures of Hypericum perforatum subsp. angustifolium elicited with chitosan and production of xanthone-rich extracts with antifungal activity. Appl Microbiol Biotechnol 91, 977–987 (2011). https://doi.org/10.1007/s00253-011-3303-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3303-6