Abstract
The production of the anticoagulant drug heparin from non-animal sources has a number of advantages over the current commercial production of heparin. These advantages include better source material availability, improved quality control, and reduced concerns about animal virus or prion impurities. A bioengineered heparin would have to be chemically and biologically equivalent to be substituted for animal-sourced heparin as a pharmaceutical. In an effort to produce bioengineered heparin that more closely resembles pharmaceutical heparin, we have investigated a key step in the process involving the N-deacetylation of heparosan. The extent of N-deacetylation directly affects the N-acetyl/N-sulfo ratio in bioengineered heparin and also impacts its molecular weight. Previous studies have demonstrated that the presence and quantity of N-acetylglucosamine in the nascent glycosaminoglycan chain, serving as the substrate for the subsequent enzymatic modifications (C5 epimerization and O-sulfonation), can impact the action of these enzymes and, thus, the content and distribution of iduronic acid and O-sulfo groups. In this study, we control the N-deacetylation of heparosan to produce a bioengineered heparin with an N-acetyl/N-sulfo ratio and molecular weight that is similar to animal-sourced pharmaceutical heparin. The structural composition and anticoagulant activity of the resultant bioengineered heparin was extensively characterized and compared to pharmaceutical heparin obtained from porcine intestinal mucosa.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heparin is one of the most widely used anticoagulant drugs (Linhardt 1991; Dahlback 2000). Its anticoagulant activity is exploited in circumstances where the normal propensity for blood to clot must be overcome. Surgical procedures often require heparin, as do extra-corporeal therapies, such as heart–lung oxygenation and kidney dialysis. Heparin is also coated on the surface of experimental or medical devices such as test tubes and rental dialysis machines to form an anticoagulant surface. Heparin also controls the clotting of blood in diseased vessels, and is therefore used to treat deep vein thrombosis and acute coronary syndrome (Linhardt 1991; Agnelli et al. 1998).
Heparin is a glycosaminoglycan composed of a major trisulfated disaccharide repeating unit and a number of additional undersulfated disaccharide units (Linhardt et al. 1988; Loganathan et al. 1990). These additional disaccharide units make heparin’s structure complex, yet also comprise the antithrombin pentasaccharide binding site that is critical for heparin’s anticoagulant activity (Loganathan et al. 1990). Heparin exerts its anticoagulation activity primarily by binding to antithrombin at a specific pentasaccharide sequence (Loganathan et al. 1990; Lindahl 2000; Casu and Lindahl 2001). The binding of heparin to antithrombin causes a conformational change of the serine protease inhibitor, antithrombin, which results in its conformational activation and antithrombin-mediated inhibition of thrombin, factor Xa, and other blood coagulation cascade proteases (Linhardt 2003; Munoz and Linhardt 2004).
Currently, United States Pharmacopeia (USP) heparin is primarily extracted from porcine intestine. The USP heparin preparation process consists of several steps to extract raw heparin from porcine intestine, which is usually performed at or near the slaughterhouse [not under current good manufacturing practice (cGMP) conditions] (Okuyama et al. 1975). The raw heparin material then goes through final purification steps to yield USP heparin in pharmaceutical companies under cGMP (Gerard and Pierre 1961; Vidic 1981). The current production process for pharmaceutical heparin for qualification as USP heparin has its concomitant drawbacks: (1) its animal source origin imposes the risk of animal virus and prion impurities; (2) process steps without cGMP regulation further increases the risk of product contamination or adulteration; and (3) the amount of heparin produced is limited by the amount of porcine intestines available, which is the major reason that the USA relies on foreign countries to supply heparin raw material. A heparin contamination crisis occurred in early 2008, which was marked by the increase in serious adverse events associated with heparin therapy and affecting thousands of patients (Kishimoto et al. 2008; Liu et al. 2009). The contaminant was later identified as oversulfated chondroitin sulfate (Guerrini et al. 2008; Liu et al. 2009). While it remains unclear how oversulfated chondroitin sulfate was introduced into the heparin products, it appears that an adulteration took place during the preparation or consolidation of raw heparin prior to the cGMP process.
Efforts have been made to replace the problematic production process for animal sourced heparin. A number of publications have proposed using a combination of fermentation, chemical, and/or enzymatic reactions to make heparin analogs (Casu et al. 1994; Linhardt and Toida 1997; Kuberan et al. 2003a, b; Chen et al. 2005; Lindahl et al. 2005; Chen et al. 2007; Zhang et al. 2008; Liu et al. 2009). In 2009, our labs initiated a research consortium to develop a commercially viable process to produce kilogram quantities of bioengineered (non-animal sourced) heparin within a period of 5 years. This bioengineered heparin must be chemically and biologically equivalent to pharmaceutical porcine heparin. This would result in an industrially scalable process to produce a generic version of heparin.
Porcine intestinal heparin typically contains 10–15 trisulfated disaccharides in a single heparin chain, and one to two disulfated disaccharides per chain (Loganathan et al. 1990). Porcine intestinal heparin differs from bovine lung heparin (no longer in widespread use because of bovine spongiform encephalopathy) in that it has an antithrombin pentasaccharide binding site that primarily contains an N-acetylglucosamine residue (Loganathan et al. 1990; Linhardt and Gunay 1999; Linhardt 2003).
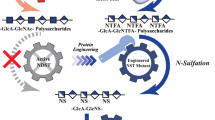
Bioengineered heparin preparation starts with the fermentation of Escherichia coli K5 to afford heparosan polysaccharide for subsequent chemoenzymatic modification (Zhang et al. 2008). The fermentation process has been extensively studied and controlled to yield ideal heparosan material as the precursor for preparing bioengineered heparin, and the fermentation process has been optimized to increase the yield and lower the cost (Wang et al. 2010). The next step in the bioengineered heparin process is the chemical N-deacetylation and N-sulfonation step (Fig. 1). To enable the final bioengineered heparin resemble the structure of the USP porcine heparin, the ratio of N-acetylglucosamine and N-sulfo-glucosamine must match those in porcine heparin. This requires fine control of the chemical N-deacetylation reaction to preserve the appropriate proportion of N-acetyl groups. This is a critical step for several reasons: (1) the N-deacetylation reaction directly affects the N-acetyl content of the bioengineered heparin produced; (2) chemical N-deacetylation uses aqueous sodium hydroxide that reduces the molecular weight of heparosan through a limited depolymerization reaction, affording a smaller polysaccharide chain comparable in size to heparin; and (3) the N-sulfo, N-acetyl heparosan afforded following N-sulfonation serves as the new backbone for subsequent enzymatic modifications, and the positioning of the N-sulfo and N-acetyl groups directly impacts the activity of the enzymes in affording the desired final structure of heparin (Kuberan et al. 2003a, b; Chen et al. 2005). In this study, the N-deacetylation of heparosan with aqueous sodium hydroxide was examined to control the N-acetyl content to match pharmaceutical heparin. The molecular weight of the resulting N-sulfo, N-acetyl heparosan was also controlled to obtain the desired molecular size and polydispersity required for the preparation of a bioengineered heparin with the same molecular weight properties as pharmaceutical heparin.
Materials and methods
Preparation of E. coli K5 heparosan
E. coli K5 heparosan was produced by E. coli K5 strain fermentation and purified from the culture supernatant as described previously (Wang et al. 2010).
N-deacetylation/N-sulfonation of K5 heparosan
K5 polysaccharide (100 mg) was dissolved in 25 ml of 2 M NaOH, incubated for 5 h at 60 °C, 5-ml aliquots were removed every hour, cooled to room temperature, and adjusted to pH 7 with HCl. Each time point was then warmed to 45–50 °C, sodium carbonate (60 mg) and trimethylamine–sulfur trioxide complex (60 mg) were added in a single step, and the mixture was incubated for 12 h. An equal portion of sodium carbonate and trimethylamine–sulfur trioxide was again added after 12 h and the selective N-sulfonation was continued for an additional 12 h at 45–50 °C. The solutions were then brought to room temperature, dialyzed overnight against distilled water using a 3,500-Da molecular weight cut-off (MWCO) cellulose membrane. The dialysate was lyophilized to obtain salt-free, N-sulfo, N-acetyl heparosan polysaccharide (Kuberan et al. 2003a).
Expression of heparan sulfate biosynthetic enzymes and heparin lyases
The catalytic domains of human C5-epimerase, hamster 2-O-sulfotransferase, hamster 6-O-sulfotransferase-1, mouse 6-O-sulfotransferase-3, and mouse 3-O-sulfotransferase-1 were recombinantly expressed in E. coli and purified as described previously (Chen et al. 2007; Zhang et al. 2008). Heparin lyases I, II, and III were cloned from the genomic DNA of Flavobacterium heparinum. The expression of the recombinant heparin lyases was also carried out in E. coli (Zhang et al. 2008).
Preparation of bioengineered heparin from N-sulfo, N-acetyl heparosan by enzymatic modification
Three enzymatic modification steps were used to convert N-sulfo, N-acetyl heparosan to a bioengineered anticoagulant heparin: (1) C5-epimerization/2-O-sulfonation (with an equi-unit mixture of C5-epimerase and 2-O-sulfotransferase); (2) 6-O-sulfonation (with an equi-unit mixture of 6-O-sulfotransferase-1 and -3); and (3) 3-O-sulfonation (with 3-O-sulfotransferase-1). Each step was performed in buffer in the presence of a PAPs regeneration system (Chen et al. 2005; Zhang et al. 2008).
NMR analysis of N-sulfo, N-acetyl heparosan and heparin
All 1H NMR were conducted on a Brüker 600 MHz NMR spectrometer. The samples were prepared in 5-mm standard NMR tubes. Acquisition of the spectra was carried out using TOPSPIN 2.0 software. All the spectra were acquired at the temperature of 298 K. A recycle delay time of 10 s was used. The acquired 1H NMR spectra were processed with Mnova NMR software for phase and baseline correction. The peaks in commercial heparin and bioengineered heparin for integration were chosen as described previously (Guerrini et al. 2001). The peak areas were calculated with the “manual integration” or “line fitting” function of Mnova NMR software.
Polyacrylamide gel electrophoresis molecular weight determination of commercial heparin and bioengineered heparin
A 12% polyacrylamide gel of dimensions 0.75 mm × 6.8 cm × 8.6 cm was used in heparin molecular weight analyses. Heparin samples (5 μg) were loaded onto gels and then subjected to electrophoresis (200 V for 25 min) and stained with Alcian blue for 0.5 h, and then destained in water. Gels were scanned and the resulting digital images were analyzed using UN-SCANIT computer software following the manufacturer’s user guide and number average molecular weight (MN), weight average molecular weight (MW), and polydispersity index (PDI) were calculated as previously described (Ly et al. 2011). Bovine lung heparin oligosaccharide ladder was used as standards for calculation of molecular weight.
Size exclusion chromatography of K5 heparosan and N-deacetylated heparosan for molecular weight determination
Size exclusion chromatography was performed using a TSK-GEL G3000PWxl size exclusion column with a sample injection volume of 20 μl and a flow rate of 0.8 ml/min on an apparatus composed of a Shimadzu LC-10Ai pump, a Shimadzu CBM-20A controller, and a Shimadzu RID-10A refractive index detector. The buffer consisted of 0.2 M Na2SO4. The column was maintained at 60 °C with an Eppendorf column heater during the chromatography. The size exclusion chromatograms were recorded with the LCsolution version 1.25 software and analyzed with its “GPC Postrun” function to calculate MN, MW, and PDI. Dextran of different molecular weights was used as calibrants in preparing the standard curve.
Disaccharide composition analysis using liquid chromatography–mass spectrometry
Heparinase II was added into the heparin samples and incubated at 37 °C for 24 h. The products were recovered by centrifugal filtration with a YM-10 10K MWCO spin column. The filtrates were freeze-dried and ready for liquid chromatography–mass spectrometry.
Liquid chromatography–mass spectrometry was performed on an Agilent 1200 LC/MSD instrument (Agilent Technologies Inc., Wilmington, DE, USA) equipped with an ion trap and a UV detector. The column used was Acquity ultraperformance liquid chromatography BEH C18 column (1.7 μm, 2.1 × 100 mm) (Waters Corporation). Eluent A was water/acetonitrile (85:15, v/v), and eluent B was water/acetonitrile (35:65, v/v). Both eluents contained 12 mM tributylamine and 38 mM ammonium acetate with pH adjusted to 6.5 with acetic acid. The column effluent entered the source of the electrospray ionization–mass spectrometry for continuous detection (Korir et al. 2008). The content of the disaccharides are calculated from the peak area of the extracted ion chromatogram calibrated to a standard curve for each disaccharide.
In vitro anticoagulation activity of bioengineered and pharmaceutical heparin
Stock solutions of both heparin samples were prepared in saline and added to platelet-poor pooled human plasma that had been collected over sodium citrate. Following re-calcification with calcium chloride and the addition of Platelin reagent (BioMerieux, Durham, NC, USA), clotting times were determined on an AMAX coagulation analyzer (Sigma).
Results
Control of the N-sulfo/N-acetyl ratio in the N-deacetylation and N-sulfonation step
The N-deacetylation reaction relied on NaOH treatment and was sampled every hour for 5 h. The N-deacetylated glucosamine residues were subsequently N-sulfonated with trimethylamine-sulfur trioxide. The resulting N-sulfo, N-acetyl heparosan were subjected to 1H NMR study for determining the N-sulfo/N-acetyl ratio of the products (N-sulfo/N-acetyl ratio = [1 − %N-acetylglucosamine]/%N-acetylglucosamine, where %N-acetylglucosamine = N-acetyl content). The H1 proton of the glucosamine was used for quantifying the N-sulfo/N-acetyl ratio, as it shows a peak at around 5.31 ppm as in N-acetylglucosamine and shifts to around 5.55 ppm when the N-acetyl was replaced with an N-sulfo group (Fig. 2a). The polysaccharide obtained after N-sulfonation was used for the 1H NMR study because it was more stable and had better solubility than the N-deacetylated heparosan, and the change in H1 proton chemical shift was more evident in the 1H NMR.
The N-acetyl content decreased with prolonged the N-deacetylation reaction time. In this study, we examined treatment with 2 M NaOH for periods of 1 to 5 h at 60 °C. A 3-h N-deacetylation reaction performed in triplicate yielded a form of product with N-acetyl content of 12.3–16.6% as determined from the NMR spectra. The mean value of N-acetyl content was 14.8%, with 95% confidence limits of 12.2% to 17.3% (Fig. 2b). These values closely match the N-acetyl content reported for pharmaceutical heparins of 11.9–17.6% (Guerrini et al. 2001).
Influence of the N-deacetylation step on molecular weight of the polysaccharide
K5 heparosan showed a number average molecular weight of 49 kDa, much larger than the molecular weight of commercial heparin (Fig. 3). The N-deacetylation reaction under alkaline conditions reportedly results in some chain depolymerization (Erbing et al. 1976). After a 3-h N-deacetylation reaction time, the number average molecular weight of the product N-deacetylated heparosan decreased to 9.7 kDa as determined by size exclusion chromatography (Fig. 3a). Since sulfo groups make up just under half of heparin’s molecular weight, the N-deacetylated heparosan should give rise to a bioengineered heparin with molecular weight properties similar to those reported for porcine intestinal heparins (Cesaretti et al. 2004) (Fig. 3b).
a Size exclusion chromatogram of K5 heparosan (top) and N-deacetylated K5 heparosan (bottom). MN, MW, and PDI calculated from the chromatogram for each sample are annotated on the figure. b Polyacrylamide gel electrophoresis gel of bovine lung heparin ladder (lane 1), bioengineered heparin (lane 2), and pharmaceutical porcine intestinal heparin (lane 3). MN, MW, and PDI calculated from the gel for each sample are annotated on the figure
The molecular weight of bioengineered heparin and pharmaceutical heparin are typically characterized by polyacrylamide gel electrophoresis, as size exclusion chromatography has been reported to be impacted by electrostatic repulsion between heparin and the column packing material in standard buffers (Guo et al. 2003). The availability of defined heparin oligosaccharide molecular weight standards provides a reliable way to determine heparin molecular weight using polyacrylamide gel electrophoresis. Molecular weights for pharmaceutical heparins determined by this polyacrylamide gel electrophoresis method range of MN and MW values from 9 to 12 kDa and 13–20 kDa, respectively. The values obtained for pharmaceutical heparin and bioengineered heparin in this study were MN 13 and 14 kDa, and MW 18 kDa (Fig. 3b). Because the chemical N-sulfonation and enzymatic O-sulfonation steps are under mild conditions, no further depolymerization is expected; thus, the molecular weight of the bioengineered heparin should be the molecular weight of N-deacetylated heparosan plus the added sulfo groups. The increase in molecular weight from 9.7 kDa for N-deacetylated heparosan to 13 kDa for bioengineered heparin after sulfonation closely matches the expected increase based on the extent of sulfonation. A molecular weight of the N-deacetylated heparosan of ∼10 kDa represents the optimal starting material for the preparation of a bioengineered heparin closely matching the molecular weight properties of porcine intestinal heparin. By carefully selecting the appropriate N-deacetylation reaction conditions, we obtained both the desired molecular weight properties (Fig. 3) and the optimal N-sulfo/N-acetyl ratio (Figs. 2b and 4). Heparin’s binding with both antithrombin and thrombin to form a ternary complex is chain-size dependent (Petitou et al. 1999). Thus, controlling the heparin molecular weight is critical in matching the anticoagulant activity of bioengineered heparin with pharmaceutical heparin.
Structural comparison of the bioengineered heparin with pharmaceutical heparin
1H NMR spectrum of the bioengineered heparin is similar to that of a pharmaceutical heparin prepared from porcine intestine (Fig. 4). The sharp peaks from 3.4 to 3.8 ppm, not present in the pharmaceutical heparin spectrum, correspond to a glycerol impurity in the bioengineered heparin, which is carried over from the O-sulfotransferase enzymes where it is used as a cryopreservative. Bioengineered heparin has comparable N-sulfoglucosamine and N-acetylglucosamine content to that of the pharmaceutical heparin (Table 1). The bioengineered heparin showed a lower iduronic acid content and higher glucuronic acid content compared to commercial heparin, and both numbers fell outside of the previously reported normal iduronic acid and glucuronic acid content variability range of different commercial heparins (Guerrini et al. 2001). This result suggests that the C5 epimerization step, conversion of glucuronic acid to iduronic acid, was insufficiently complete. The 3-O-sulfoglucosamine content of the bioengineered heparin was 8.3%, higher than the commercial heparin studied here, and slightly above the 4.9–7.2% range of 3-O-sulfoglucosamine content observed in different commercial heparin samples (Guerrini et al. 2001). The 3-O-sulfotransferase-1 generally acts on glucosamine units located between glucuronic acid (at the non-reducing side of glucosamine) and iduronic acid (at the reducing side of glucosamine). The higher glucuronic acid content and lower iduronic acid content in the bioengineered heparin may have resulted in the increased action of 3-O-sulfotransferase-1. The presence and location of the 3-O-sulfo group is important in that it is essential for interaction with antithrombin and corresponds to the critical central residue in the pentasaccharide antithrombin binding site (Kuberan et al. 2003b; Lindahl et al. 2005).
Disaccharide analysis with high performance liquid chromatography–mass spectrometry after heparinase II digestion afforded the disaccharide composition of the bioengineered heparin and commercial heparin (Fig. 5 and Table 2). All major disaccharide components present in pharmaceutical heparin can also be found in the bioengineered heparin except for ΔUA2S-GlcNAc6S (Table 3). The absence of ΔUA2S-GlcNAc6S in the digested bioengineered heparin sample may be due to (1) incomplete action of C5 epimerase; or (2) the action pattern of the recombinantly expressed hamster 2-O-sulfotransferase catalytic domain, which reportedly preferentially 2-O-sulfonates the iduronic acid with an N-sulfoglucosamine residue at its reducing end (Chen et al. 2005); or (3) the recombinant 6-O-sulfotransferases do not act well on IdoA2S-GlcNAc disaccharide; or (4) all of the IdoA2S-GlcNAc6S disaccharide sequences have been 3-O-sulfonated or are adjacent to 3-O-sulfonated dissacharide and are resistant to heparin lyase II, and thus not detected in high performance liquid chromatography–mass spectrometry dissacharide analysis.
Liquid chromatography–mass spectrometry of disaccharides the most commonly found in heparan sulfate/heparin. a Extracted ion chromatogram of the bioengineered heparin; b extracted ion chromatogram of the commercial heparin (0S = ΔUA-GlcNAc, NS = ΔUA-GlcNS, 6S = ΔUA-GlcNAc6S, 2S = ΔUA2S-GlcNAc, NS6S = ΔUA-GlcNS6S, NS2S = ΔUA2S-GlcNS, 2S6S = ΔUA2S-GlcNAc6S, TriS = ΔUA2S-GlcNS6S)
Anticoagulant activity of the bioengineered and pharmaceutical heparins
Activated partial thromboplastin time assays were used to assess the global in vitro anticoagulant activity of the heparin samples. The clotting times obtained at three concentrations of heparin showed statistically identical values (Fig. 6).
Discussion
The controlled N-deacetylation of heparosan, the first chemical step in the chemoenzymatic modification of heparosan chain to afford anticoagulant heparin, is especially important not only because it affects the N-acetyl content and molecular weight of the final heparin produced but it also has a profound impact on subsequent enzymatic steps. N-deacetylation with NaOH, when appropriately controlled, can provide an optimal N-acetyl/N-sulfo ratio in the resulting polysaccharide product as well as result in a partially depolymerized product of the desired molecular weight. The immediate impact of the heparosan N-deacetylation/N-sulfonation step in following enzymatic modifications is on the C5 epimerization step. Epimerization with C5 epimerase only occurs at glucuronic acid residues at the reducing side of N-sulfoglucosamine residues, and only with uronic acids that are neither O-sulfonated nor adjacent to O-sulfoglucosamine residues. Glucuronic acids located at the reducing side of N-acetylglucosamine are also resistant to C5 epimerase (Kusche et al. 1991; Li et al. 1997; Kuberan et al. 2003a, b). Concomitant 2-O-sulfonation, catalyzed by 2-O-sulfotransferase, predominantly occurs at the iduronic acid with an N-sulfoglucosamine residue at the reducing end (Chen et al. 2005). Subsequent 3-O-sulfonation is catalyzed by 3-O-sulfotransferase-1, which generally acts on glucosamine units located between glucuronic acid (at the non-reducing side of glucosamine) and iduronic acid (at the reducing side of glucosamine), generating an antithrombin binding site (Kuberan et al. 2003a). The 2-O-sulfonation of iduronic acid within the antithrombin-binding pentasaccharide limits 3-O-sulfotransferase-1 mediated 3-O-sulfonation of glucosamine residues at the reducing side of a 2-O-sulfo iduronic acid residue, while it has no effect at its non-reducing end (Zhang et al. 2001; Kuberan et al. 2003a). Thus, the action patterns of the enzymes are quite interdependent. The precursor polysaccharide sequences created by previous chemoenzymatic modification steps can impact the sequence of polysaccharides produced in the following enzymatic steps. In an effort to build bioengineered heparin that closely resembles porcine intestinal heparin, control of the chemical steps of heparosan N-deacetylation/N-sulfonation is critical.
In this study, the reaction time of aqueous NaOH with heparosan was carefully controlled to afford the appropriate level of N-deacetylation to obtain an N-acetyl/N-sulfo ratio that matched the pharmaceutical heparin. The molecular weight of the polysaccharide was similarly controlled to obtain the desired range. The structure, molecular weight, and anticoagulant activity of this bioengineered heparin have been improved to better match porcine intestinal heparin compared to previous versions of bioengineered heparin (Kuberan et al. 2003a; Chen et al. 2005, 2007; Zhang et al. 2008). The anticoagulant polysaccharides reported by Kuberan et al. in 2003 were completely N-deacetylated and had no N-acetylglucosamine residues, no molecular weight data were reported, and no anticoagulant activity levels were measured to compare with commercial heparin, although a gel mobility shift assay indicated binding with antithrombin (Kuberan et al. 2003a). The chemoenzymatically synthesized polysaccharides reported by Chen et al. in 2007 and Zhang et al. in 2008 also used per-N-sulfonated heparosan as starting material and thus do not resemble the commercial heparin due to the lack of N-acetylglucosamine residue in the polysaccharide chain (Chen et al. 2007; Zhang et al. 2008). The anticoagulant polysaccharides reported by Chen et al. in 2005 were synthesized by first chemically desulfonating commercial heparin, thus the synthetic route just represents a scientific laboratory research tool, but not viable for the commercial production of bioengineered heparin (Chen et al. 2005). In contrast, the bioengineered heparin presented here has comparable N-sulfoglucosamine and N-acetylglucosamine content to that of the pharmaceutical heparin and its molecular weight and anticoagulant activity closely match the pharmaceutical heparin. However, some properties of the bioengineered heparin that still require additional work are primarily associated with the fine chemical structure and require careful control of the enzymatic modification steps following chemical N-deacetylation and N-sulfonation. Future efforts will be directed to engineering improved recombinant enzymes and to the control of each enzymatic modification step required to produce a bioengineered heparin that is chemically and biologically equivalent to the porcine intestinal heparin.
References
Agnelli G, Piovella F, Buoncristiani P, Severi P, Pini M, D’Angelo A, Beltrametti C, Damiani M, Andrioli GC, Pugliese R, Iorio A, Brambilla G (1998) Enoxaparin plus compression stockings compared with compression stockings alone in the prevention of venous thromboembolism after elective neurosurgery. N Engl J Med 339:80–85
Casu B, Lindahl U (2001) Structure and biological interactions of heparin and heparan sulfate. Adv Carbohydr Chem Biochem 57:159–206
Casu B, Grazioli G, Razi N, Guerrini M, Naggi A, Torri G, Oreste P, Tursi F, Zoppetti G, Lindahl U (1994) Heparin-like compounds prepared by chemical modification of capsular polysaccharide from E. coli K5. Carbohydr Res 263:271–284
Cesaretti M, Luppi E, Maccari F, Volpi N (2004) Isolation and characterization of a heparin with high anticoagulant activity from the clam Tapes philippinarum: evidence for the presence of a high content of antithrombin III binding site. Glycobiology 14:1275–1284
Chen J, Avci FY, Munoz EM, McDowell LM, Chen M, Pedersen LC, Zhang L, Linhardt RJ, Liu J (2005) Enzymatic redesigning of biologically active heparan sulfate. J Biol Chem 280:42817–42825
Chen J, Jones CL, Liu J (2007) Using an enzymatic combinatorial approach to identify anticoagulant heparan sulfate structures. Chem Biol 14:986–993
Dahlback B (2000) Blood coagulation. Lancet 355:1627–1632
Erbing C, Granath K, Kenne L, Lindberg B (1976) A new method for the N-deacetylation of carbohydrates. Carbohydr Res 47:C5–C7
Gerard N, Pierre B (1961) Process of purifying heparin, and product produced therefrom. US patent #2989438
Guerrini M, Bisio A, Torri G (2001) Combined quantitative (1)H and (13)C nuclear magnetic resonance spectroscopy for characterization of heparin preparations. Semin Thromb Hemost 27:473–482
Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang ZQ, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R (2008) Oversulfated chondroitin sulfate is a contaminant in heparin associated with adverse clinical events. Nat Biotechnol 26:669–675
Guo X, Condra M, Kimura K, Berth G, Dautzenberg H, Dubin PL (2003) Determination of molecular weight of heparin by size exclusion chromatography with universal calibration. Anal Biochem 312:33–39
Kishimoto TK, Viswanathan K, Ganguly T, Elankumaran S, Smith S, Pelzer K, Lansing JC, Sriranganathan N, Zhao GL, Galcheva-Gargova Z, Al-Hakim A, Bailey GS, Fraser B, Roy S, Rogers-Cotrone T, Buhse L, Whary M, Fox J, Nasr M, Dal Pan GJ, Shriver Z, Langer RS, Venkataraman G, Austen KF, Woodcock J, Sasisekharan R (2008) Contaminated heparin associated with adverse clinical events and activation of the contact system. N Engl J Med 358:2457–2467
Korir AK, Limtiaco JF, Gutierrez SM, Larive CK (2008) Ultraperformance ion-pair liquid chromatography coupled to electrospray time-of-flight mass spectrometry for compositional profiling and quantification of heparin and heparan sulfate. Anal Chem 80:1297–1306
Kuberan B, Beeler DL, Lech M, Wu ZL, Rosenberg RD (2003a) Chemoenzymatic synthesis of classical and non-classical anticoagulant heparan sulfate polysaccharides. J Biol Chem 278:52613–52621
Kuberan B, Lech MZ, Beeler DL, Wu ZL, Rosenberg RD (2003b) Enzymatic synthesis of antithrombin III-binding heparan sulfate pentasaccharide. Nat Biotechnol 21:1343–1346
Kusche M, Hannesson HH, Lindahl U (1991) Biosynthesis of heparin. Use of Escherichia coli K5 capsular polysaccharide as a model substrate in enzymic polymer-modification reactions. Biochem J 275(Pt 1):151–158
Li J, Hagner-McWhirter A, Kjellen L, Palgi J, Jalkanen M, Lindahl U (1997) Biosynthesis of heparin/heparan sulfate. cDNA cloning and expression of D-glucuronyl C5-epimerase from bovine lung. J Biol Chem 272:28158–28163
Lindahl U (2000) ‘Heparin’—from anticoagulant drug into the new biology. Glycoconj J 17:597–605
Lindahl U, Li JP, Kusche-Gullberg M, Salmivirta M, Alaranta S, Veromaa T, Emeis J, Roberts I, Taylor C, Oreste P, Zoppetti G, Naggi A, Torri G, Casu B (2005) Generation of “Neoheparin” from E. coli K5 capsular polysaccharide. J Med Chem 48:349–352
Linhardt RJ (1991) Heparin: an important drug enters its seventh decade. Chem Ind 2:45–50
Linhardt RJ (2003) 2003 Claude S. Hudson award address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem 46:2551–2564
Linhardt RJ, Gunay NS (1999) Production and chemical processing of low molecular weight heparins. Semin Thromb Hemost 25(Suppl 3):5–16
Linhardt RJ, Toida T (1997) Heparin oligosaccharides: new analogues—development and applications. In: Nieforth ZJ, Wa KA (eds) Carbohydrates in drug design. Marcel Dekker, New York, pp 277–341
Linhardt RJ, Rice KG, Kim YS, Lohse DL, Wang HM, Loganathan D (1988) Mapping and quantification of the major oligosaccharide components of heparin. Biochem J 254:781–787
Liu H, Zhang Z, Linhardt RJ (2009) Lessons learned from the contamination of heparin. Nat Prod Rep 26:313–321
Loganathan D, Wang HM, Mallis LM, Linhardt RJ (1990) Structural variation in the antithrombin III binding site region and its occurrence in heparin from different sources. Biochemistry 29:4362–4368
Ly M, Wang Z, Laremore TN, Zhang F, Zhong W, Pu D, Zagorevski DV, Dordick JS, Linhardt RJ (2011) Analysis of E. coli K5 capsular polysaccharide heparosan. Anal Bioanal Chem 399:737–745
Munoz EM, Linhardt RJ (2004) Heparin-binding domains in vascular biology. Arterioscler Thromb Vasc Biol 24:1549–1557
Okuyama T, Yoshida K, Sakurai K, Ogura T, Horie K, Tawada A, Hara T (1975) Method of separating and recovering mucopolysaccharides from connective tissue of animals. US patent #3862003
Petitou M, Herault JP, Bernat A, Driguez PA, Duchaussoy P, Lormeau JC, Herbert JM (1999) Synthesis of thrombin-inhibiting heparin mimetics without side effects. Nature 398:417–422
Vidic H-J (1981) Process for the preparation of heparin. US patent #4283530
Wang Z, Ly M, Zhang F, Zhong W, Suen A, Hickey AM, Dordick JS, Linhardt RJ (2010) E. coli K5 fermentation and the preparation of heparosan, a bioengineered heparin precursor. Biotechnol Bioeng 107:964–973
Zhang L, Lawrence R, Schwartz JJ, Bai X, Wei G, Esko JD, Rosenberg RD (2001) The effect of precursor structures on the action of glucosaminyl 3-O-sulfotransferase-1 and the biosynthesis of anticoagulant heparan sulfate. J Biol Chem 276:28806–28813
Zhang ZQ, McCallum SA, Xie J, Nieto L, Corzana F, Jimenez-Barbero J, Chen M, Liu J, Linhardtt RJ (2008) Solution structures of chemoenzymatically synthesized heparin and its precursors. J Am Chem Soc 130:12998–13007
Acknowledgments
The authors are grateful for funding from the NIH in the form of grant # HL096972 and the support of the Bioengineered Heparin Consortium for supporting this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Z., Yang, B., Zhang, Z. et al. Control of the heparosan N-deacetylation leads to an improved bioengineered heparin. Appl Microbiol Biotechnol 91, 91–99 (2011). https://doi.org/10.1007/s00253-011-3231-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3231-5