Abstract
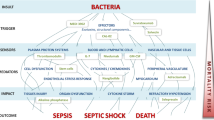
Sepsis (blood poisoning) is a severe infectious disease with high mortality, and no effective therapy is actually known. In the case of Gram-negative bacteria, endotoxins (lipopolysaccharides) are known to be responsible for the strong inflammation reaction leading to the systemic infection. Peptides based on endotoxin-binding domains of human or animal proteins represent a promising approach in sepsis research. Although so far no medicament is available, the progress in recent years might lead to a breakthrough in this field. In this review, recent investigations are summarised, which may lead to an understanding of the mechanisms of action of peptides to suppress the inflammation reaction in vitro and in vivo (animal models) and thus may allow the development of effective anti-septic drugs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis, blood poisoning, belongs to the most severe infectious diseases known. Thus, for example, the number of death cases alone in the USA is far beyond the number of 200,000 annually (Cross and Opal 1995). This corresponds to the number of 70,000 reported for Germany regarding the population sizes. However, new evaluation based on extrapolation on data from critical care units gives an estimate of 150,000 cases only for Germany (K. Reinhart, private communication), which must be seen in the light of the fact that frequently, the cause of death like organ failure or pneumonia is a result of a preceding sepsis.
The causative agents of sepsis are in more than 90% of the cases bacteria, and approximately, each one half results from Gram-negatives and Gram-positives (Opal and Cohen 1999). Moreover, it has been observed that viral infections such as the recently worldwide spreading influenza of serotype H1N1 (‘swine flu’) tend to be followed by a bacterial infection (‘super infection’), and the cases with lethal outcome were due to bacterial sepsis.
The ‘pathogenicity’ or ‘virulence factors’ of the bacteria are for Gram-negatives clearly endotoxins (lipopolysaccharides, LPS), whereas for Gram-positives, this is not completely known. It was observed that lipoteichoic acid (LTA) may give rise to a high production of cytokines such as tumour-necrosis-factor-α (TNF-α) (Yang et al. 2001). The study with highly purified or synthetic LTA, however, has shown that probably a lipoprotein (LP) contamination was the reason for the bioactivity of LTA. Therefore, regarding the data found in recent years (Hashimoto et al. 2007), it can be assumed that LP are responsible for the Gram-positive sepsis similar to the role of LPS for Gram-negatives, although the final proof as evidenced in the Gram-negative mouse model of sepsis is still lacking.
There are various therapeutic approaches to treat sepsis. However, except for some slight success found for a small cohort of patients with activated protein C (APC, drotrecogin), all clinical phase 3 studies failed to increase survival of the patients (Lefrant et al. 2010). Starting in the 1990s, antimicrobial peptides (AMPs) came into the focus of interest. These compounds were found in various species, ranging from bacteria, amoeba, insects to mammals. Furthermore, many AMPs were identified from the LPS-binding domain of proteins such as lipopolysaccharide-binding protein (LBP), lactoferrin, or Limulus anti-LPS factor (LALF) (Hoess et al. 1993), or mixtures of these. There is a variety of publications dealing with the antimicrobial action of the AMP, which, however, does not necessarily mean that the AMPs are equally potent to kill bacteria as to neutralise LPS. This represents the main problem of sepsis research: Conventional antibiotics, for example, may effectively kill bacteria, but in this way they liberate LPS and LP from the bacteria, which in many cases lead in critical care units to a worsening of the septic syndromes of the patients (Morrison 1998). Therefore, the challenge to develop a new effective anti-sepsis therapy is to find agents or combinations of agents which fulfil both tasks: killing of the bacteria and neutralisation of their liberated pathogenicity factors, essentially LPS and LP. In this review, the progresses found mainly in the last decade in an effective neutralisation of the pathogenicity factors by suitable peptides are summarised.
In a former review, the mode of action of a variety of antimicrobial and endotoxin-neutralising peptides was already presented (Andrä et al. 2006). Most of the findings described there will thus not be treated here.
Synthetic peptides derived from lytic polypeptides sharing the saposin fold
Activated cytotoxic T lymphocytes and natural killer (NK) cells of various species express saposin-like polypeptides, which exhibit antimicrobial as well as anti-cancer cell activities (Liepinsh et al. 1997). These polypeptides are cationic, 70–80 amino acid residues in length, and fold into a flexible α–helical structure, which is stabilised by three disulfide bonds connecting six cysteine residues in a conserved pattern. Two members of this family are in particular well characterised, i.e. porcine NK-lysin (Andersson et al. 1995) and human granulysin (Pena et al. 1997). Homologue polypeptides have been identified to date in other mammalian species, cattle (Endsley et al. 2004) and horse (Davis et al. 2005), and interestingly also in the nematode Caenorhabditis elegans and in protozoa (Leippe 1995), suggesting that these polypeptides are part of an ancient (immune) defence system. A direct interaction with or a response to LPS has been demonstrated for most of these compounds and porcine NK-lysin and granulysin have been used as templates for the generation of LPS-neutralising peptides (Andrä et al. 2004b), (Chen et al. 2007), (Brandenburg et al. 2010).
Porcine NK-lysin binds to LPS and protects mice from the lethal effects of LPS in galactosamine-sensitised mice, when mixed in a test tube and administered. However, protection got lost when LPS and NK-lysin where administered separately (Andersson et al. 1999). Moreover, an internal fragment of it (residues 39–65), termed NK-2, was a very potent inhibitor of the LPS-induced cytokine production of human mononuclear cells (Andrä et al. 2004b). Expression of channel catfish NK-lysin was gradually increased after challenge with Edwardsiella ictaluri, the causative agent of enteric septicemia of catfish (Wang et al. 2006). A synthetic peptide corresponding to the sequence of NK-2 exhibited antibacterial activity, although to a lesser extent than porcine NK-2, which may be due to a reduced net charge of +4 compared to +10. The inactivation of LPS by this peptide has not been investigated yet. A peptide of similar length and related sequence spanning residues 34–55 of bovine NK-lysin exhibited activity against Escherichia coli, Staphylococcus aureus and mycobacteria. Again, porcine NK-2 was superior over bovine NK-2 in particular against S. aureus (Endsley et al. 2004).
Besides killing of bacteria and directly interacting with endotoxins, Deng et al. (2005) demonstrated that granulysin, the human homologue of NK-lysin, also acts as a proinflammtory activator, which actively recruits immune cells to the site of inflammation and promotes the production of cytokines by these cells. Granulysin was chemoattractant for monocytes, certain T cells and mature dendritic cells at a concentration far below its direct antibacterial activity. Moreover, chemoattraction could be abrogated by pertussis toxin indicating the involvement of G-protein coupled receptors in this process. To understand the molecular basis behind the facets of polypeptide-mediated modulation of the biological activity of LPS and to exploit the natural compounds as lead structures for the development of anti-sepsis drugs, biophysical studies have been conducted.
Chen et al. (2007) have studied the interaction of granulysin-derived peptides in D-configuration (Gra-pep) with endotoxins. These peptides led to a significant inhibition of the LPS-induced TNFα production in human mononuclear cells, concomitant with a change of the aggregate structure of LPS into a multilamellar stack, as obtained by small-angle X-ray scattering (SAXS). Corresponding to this, cryo-electron micrographs showed an increase in the aggregation of LPS particles. The gel-to-liquid-crystalline phase behaviour, however, showed only slight changes due to the action of the peptides. Interestingly, there was a clear incorporation of the Gra-pep into the lipid matrix of phosphatidylserine and the peptides enhanced the incorporation of LPS into it. This is shown for the peptide G12.21 (rrvsrrpmrryrsrrprrlv), see the comparison of A and B in Fig. 1. These data imply that the cell activation, as well as its inhibition, is at least partially a membrane-driven process.
Intercalation of granulysin peptide G12.21 into phosphatidylserine (PS) liposomes, and the influence of LPS and lipopolysaccharide-binding-protein (LBP). Sequences of addition at 50, 100 and 150 s, respectively: Hepes buffer-LPS-LBP (a), G12.21-LPS-LBP (b), G12.21-Hepes-LBP (c) and Hepes-Hepes-LBP (d). Adapted from Chen et al. (2007)
Brandenburg et al. (2010) used microcalorimetry, Zeta potential measurements, FTIR spectroscopy, and small angle X-ray scattering to characterise the LPS interaction of nine porcine NK-2-derived peptides, which differ in length and net charge. They investigated the capability of the peptides to inhibit the LPS-induced production of TNFα by human mononuclear cells and correlated biological activity with peptide binding to LPS as well as the influence of the peptides on the phase transition of the LPS acyl chains and on the LPS aggregate structure. With decreasing length and net charge of the peptides the LPS-neutralising activity decreased considerably. This was not due to an inability of peptides to bind to LPS, which was indicated by a compensation of the negative LPS Zeta potential (surface charge) of LPS-aggregates also in the presence of inactive peptides. However, only binding of the most potent LPS-neutralising peptide (NK-2) to LPS led to an overcompensation of the Zeta potential. This observation indicates that besides an electrostatic attraction of the cationic peptides to the negatively charged LPS aggregate, an additional hydrophobic interaction takes place. In addition, all active peptides, also moderately active ones, led to a rearrangement of the LPS aggregate structure into a multilamellar stack. Most surprisingly, the substitution of a non-functional cysteine residue in NK-2 by a serine residue led to a significant decrease in activity (Table 1). This is noteworthy since the same substitution has been shown earlier to enhance the antibacterial activity against Gram-negative bacteria [Andrä et al. 2007b and data in Table 1]. Thus, antibacterial and LPS-neutralising activities of a given peptide are uncoupled to a certain degree. The authors conclude that effective peptide-mediated LPS neutralisation is driven by electrostatic and hydrophobic forces based on binding of lysine residues of the peptide to the LPS headgroup phosphates and a partially perturbation of the acyl chain region by hydrophobic amino acid side chains. The NMR structure of a peptide-LPS complex of a lactoferricin-derived peptide supports this thesis (Japelj et al. 2005). These interactions result in a high-affinity LPS-peptide complex unable to activate immune cells since interaction with natural LPS binding proteins such as LPB and CD14 is competitively inhibited (Andrä et al. 2004b).
Peptides based on lactoferrin
Lactoferrin (LF) is an iron-binding glycoprotein and is released from neutrophils during inflammation (Elass-Rochard et al. 1998). It was reported that LF binds to the lipid A part in a way that the LPS-induced cytokine induction in monocytes is reduced (Appelmelk et al. 1994). A detailed biophysical study showed a saturation of the [LPS]/[LF] binding at molar ratios of 1:5–1:10, as obtained by isothermal titration calorimetry (ITC) and Zeta potential measurement data (Brandenburg et al. 2001). SAXS measurements exhibited a conversion of the inverted cubic aggregate structure into a lamellar one, with slight expression of a multilamellar expression, i.e. only few lamellae. Interestingly, the LPS-induced TNFα secretion of MNC is clearly decreased in the presence of LF, whereas the data in the Limulus amebovcyte lysate (LAL) test at [LPS] = 10 ng/ml found an inhibition due to LF, but at lower LPS-concentrations no decrease, but partially even an increase of the LAL activity. From these data, it became clear that these two biological test systems do not necessary produce identical results (see later).
The antibacterial and endotoxin-neutralising activity of a lactoferricidin-derived peptides LF11 (FQWQRNIRKVR) and the acylated form lauryl-LF11 was investigated by Andrä et al. (2005). The addition of the acyl chain led to an increase in both activities, which could be understood, among others, by the better compensation of the negative charges of LPS by the binding of the lauryl-LF11, for LPS Re as well as LPS Ra. The investigation of the phase-transition behaviour gave no clear dependence on the kind of peptide, and the SAXS data showed a slight occurrence of reflections characteristic for multilamellae.
In a continuation of these investigations, peptides with sequences FWRIRIRR and FWRRFWRR and the corresponding lipopeptides with an octanoyl group at the N-terminal end were analysed and showed a significant ability to inhibit the LPS-induced TNFα production in human mononuclear cells, in particular at a [peptide]/[LPS] weight ratio of 100:1 (Brandenburg et al. 2010). The acylated compound showed also protection in an animal model of sepsis (50% survivors at [peptide]/[LPS] 500:1 weight ratio), whereas the non-acylated compounds were less effective. Again, from SAXS data a multilamellarisation of the LPS aggregates were found, and ITC experiments yielded saturation values at a ratio of [peptide]/[LPS] 2:1 M for the acylated compounds, whereas that of the non-acylated compound was larger than 3:1 M. These data indicate that the ability of a given peptide to inhibit the cytokine secretion directly correlates with a saturation of the peptide to LPS at low ratios. Interestingly, data from Förster resonance energy transfer spectroscopy showed that all peptides were able to incorporate into membranes made from the negatively charged phosphatidylserine, but also into LPS aggregates, but there was no correlation to their inhibition capacity. Disadvantageous, however, was the observation of a cytotoxic effect in HeLa cells in particular for the acylated compounds at 100 μg/ml.
Haversen et al. (2010) started with the peptide fragment from the amino acid residues 14–31 (sequence 14–31) from the N-terminal end, which corresponds to the antimicrobial α-helix of human lactoferrin and optimised this lead structure by downsizing, alanine scanning and the substitution of specific amino acids. By downsizing, they found that the original peptide sequence 14–31, could be reduced by five amino acids, and that the peptide sequence 19–31 showed to be the optimal length for antimicrobial activity. This peptide with the sequence KCFQWQRNMRKVR has the capacity to bind to lipid A/LPS. Alanine scanning of the peptide sequence 20–31 showed that the amino acids Cys20, Trp23, Arg28, Lys29 or Arg31 were crucial for the expression of full bacterial killing activity. Also a substitution of the neutral hydrophilic amino acids Gln24 and Asn26 for Lys and Ala enhanced significantly microbial activity. The impact of divalent cations like Ca2+ or Mg2+ was also considered and found that the antagonistic effect of these ions depends on the peptide structure. For specific optimised peptides a more than tenfold stronger fungicidal activity could be achieved.
The study presented by Haversen et al. shows that based on peptide lead structures derived from natural origins, it is possible to significantly enhance antimicrobial activity, by optimising the peptide length and the exchange of specific neutral amino acids.
Peptides based on Limulus anti-LPS factor and derivatives
The Limulus, the American horseshoe crab, has a very efficient system to detect bacterial endotoxin, the so-called LAL, a protein clotting cascade, which inhibits the uptake of bacteria into the animals (Levin 1987). This clotting system is commercially available as very sensitive test for LPS, but it must be noticed that the LAL test recognises also LPS structures, which are inactive in the human cytokine assay, for example antagonistic penta- and tetraacylated LPS and lipid A (Gutsmann et al. 2010a). The reason for this is the fact, that the recognition structure of the LAL test is the diglucosamine 4′-phosphate, independent of the number of acyl chains (tri- to heptaacyl chains). A further protein, the LALF, seems to be a regulation factor of the LAL system, but does not belong to it. The LALF was crystallised and the LPS-binding domain was identified (Hoess et al. 1993). Ried et al. (1996) synthesised peptides based on this domain and found that their inhibitory capacity was much larger in a cycled than in a linear form. They found that the complete binding sequence cLALF22 (amino acid sequence 31–52 of LALF: GCHYRIKPTFRRLKWKYKGKFWCG) had the highest ability to bind to lipid A, whereas the shortened analogues down to cLALF10 (LALF sequence 38–45, GCTFRRLKKCG) were much less active. In a following study, Dankesreiter et al. (2000) arrived at similar results. Beside the LALF part structures, they designed and synthesised also hybrid peptides, one part from LALF sequences, and the other one from AA sequences from the LPS-binding domains of LBP or BPI. For example, with hybrid LL10-H14 (sequence KPTFRRLKWKCRWKVRKSFFKLQC), they did not only get high inhibition of the LPS-induced cytokine secretion in Mono Mac cells and peripheral blood mononuclear cell, but also in a mouse model of sepsis at [LL10-H14]/[LPS] 100:1 wt.%. In a series of investigations, Andrä et al. performed a biophysical analysis of the interaction of LALF (in recombinant form called ENP = endotoxin-neutralising protein) and part structures with endotoxins (Andrä et al. 2004a); (Andrä et al. 2004c); (Andrä et al. 2007a). They found considerable inhibition of the biological activity of LPS at [ENP]/[LPS] 20 to 200:1 M. The gel to liquid-crystalline phase transition behaviour of lipid A and LPS was only slightly modified (increase in the phase-transition temperature), whereas the SAXS data were indicative of a change of the lipid A cubic structure into a more lamellar one. Importantly, determination of the Zeta potential showed its increase from −70 mV to positive values for lipid A and LPS Re, indicating an overcompensation of the negative head group charges of the endotoxins. LALF-derived cyclic peptides were analysed in the following studies (Andrä et al. 2004c), (Andrä et al. 2007a), starting with the compound with the complete LPS-binding domain (cLALF22) and various shortened analogues. The data consistently gave a change of the endotoxin aggregate structure (LPS Re and lipid A) from preferentially cubic into a multilamellar one (Fig. 2) for cLALF18, 20, and 22. Concomitantly, the morphologies of the LPS Re aggregates, as evidenced by freeze-fracture electron microscopy, were converted from ‘open egg shells’, i.e. spherical particles in the range 100–200 nm, into large stacks of some 100 nm size (Fig. 3), which corresponded to the multilamellae in the SAXS experiment with periodicities of 6.3–7.5 nm. ITC showed that the binding was, in all cases, an exothermic process driven by the Coulomb interaction of the positive charges of peptides with the negative charges of the endotoxins. Saturation occurred, in dependence on the applied peptide, at molar ratios of [cLALF-Pep]/[LPS] 1 to 2. Additionally, all peptides exhibited considerable membrane activity by incorporating into PS liposomes.
Small-angle X-ray scattering with synchrotron radiation of LPS Re/cLALF18 (a) and cLALF20 (b) mixtures at [LPS]/[cLALF] 2.5:1 M ratio. Plotted is the logarithm of the scattering intensity versus the scattering vectors (=1/d, d the spacings of the reflections). Adapted from Andrä et al. (2007a)
Freeze-fracture electron micrographs of LPS Re in the presence of cLALF18 at an [LPS]/[peptide] 3:1 M ratio, adapted from Andrä et al. (2007a). The figure shows two extended multilamellar stacks from left to right on the upper part and from the bottom to the middle at the left part of the picture
Pan et al. investigated the efficacy of the terminal part of the shrimp anti-LPS-factor, a synthetic peptides with 24 AA, cyclic and linear with a sequence Ac- ECKFTVKPYLKRFQVYYKGRMWCP, to act antimicrobially (Pan et al. 2007). Pre-treatment of mice with the cyclic compound led to a considerable enhancement of survival of mice, which had been infected with 1.28 106 cfu from Pseudomonas aeruginosa. The peptides were also able to decrease the bacterial-induced production of TNFα in the animals. Unfortunately, the peptides exhibited at a concentration of already 2 μg/ml significant cytotoxicity in HeLa, MCF-7, and HT1080 cell lines, as measured by the MTT test. A synthetic peptides derived from LALF, CLP-19 (CRKPTFRRLKWKIKFKFKC) was tested in vitro as well as in vivo and was able to provide considerable protection of endotoxic mice, concomitant with a reduction of TNFα concentrations in the animals (Ren et al. 2008).
A new series of 19-mer peptides, originally based on LALF peptides, but with essential changes in the sequence, were analysed biophysically (Kowalski et al. 2010). Peptide 19–2 (GCKKYRRFRWKFKGKFWFWCG) exhibited strong endotoxin binding, as deduced from ITC experiments, which resulted in a nearly complete blocking of the LPS-induced TNFα production of human MNC at [Pep]/[LPS] 100:1 wt.%. A slightly shortened variant exhibited much less anti-endotoxin, but considerably higher antibacterial activity. This illustrates again that the two parameters, antibacterial and anti-endotoxin activity, not necessarily correlate. A further improvement was obtained by slight modifications in the sequence, which led to a nearly complete inhibition of the LPS-induced cytokine secretion at a [Pep]/[LPS] 3:1 M ratio (Gutsmann et al. 2010b). The same peptide (Pep19–2.5: GCKKYRRFRWKFKGKFWFWG) and variants of it could protect animals in the mice model of sepsis already at a [Pep]/[LPS] ratio of 50:1 wt.% (25 ng LPS and 1,250 ng peptide), at a peptide concentration, which was far below any cytotoxicity. As mechanism of action, a change of lipid A/LPS cubic into a multilamellar structures was proposed, connected with an extremely low saturation value of binding at [peptide]/[LPS] = 0.3 M/M, i.e. the binding is already saturated with three peptide and ten LPS molecules. Thus, human binding proteins such as LBP and CD14 are no more able to bind to the LPS epitopes and thus cannot initiate the inflammation reaction. Interestingly, the lead structure Pep19–2.5 was able to inhibit also the cytokine secretion in human mononuclear cells induced by a lipoprotein SitC from Gram-positive source (from S. aureus) (unpublished data). Since lipoproteins are assumed to represent the ‘Gram-positive endotoxins’, our peptide 19–2.5 seems to be a general antiseptic agent.
Peptides based on defensins and cathelicidins
One important and very active antibacterial class of protein is the cathelicidins, which have been identified in various mammals including humans (Zanetti et al. 1995). They contain a highly conserved N-terminal domain called cathelin and a C-terminal domain that comprises an antimicrobial peptide. Human and rabbit cathelicidins are termed 18 kDa cationic antibacterial protein hCAP18 and rCAP18, respectively. CAP18 was originally isolated from rabbit granulocytes using the agglutination of LPS-coated erythrocytes as an assay (Larrick et al. 1991).
CAP18 is stored in the intracellular granules of neutrophilic granulocytes, which are essential in the primary defence against intruding microorganisms and is liberated into the phagocytic vacuoles during phagocytosis (Cowland et al. 1995). It has been shown that hCAP18 is present in seminal plasma in 70-fold higher concentration than in blood plasma (1.18 μg/ml) (Malm et al. 2000; Sorensen et al. 1997). The bactericidal C-terminal fragment of hCAP18 FALL-39 (hCAP18102–140) and its precursors have also been found in wound fluid (Frohm et al. 1996) and LL-37 (hCAP18104–140) in alveolar macrophages, bronchial epithelial cells and bronchial glands (Agerberth et al. 1999). The C-terminal domain of CAP18 exhibits LPS-binding, LPS-neutralising, antibacterial and anticoagulant activities (Hirata et al. 1995). These properties are shared with cationic antibiotics such as polymyxin B (PMB), defensins and the bactericidal/permeability-increasing protein (BPI). There are, however, no sequence homologies between these peptides. CAP18 shows a higher binding affinity (KD = 0.58 nM) to biotinylated LPS from Salmonella Minnesota than rBPI21 (KD = 3.75 nM) (de Haas et al. 1998). A major difference in the interaction of human and rabbit CAP18 with cell membranes is observed in their effect on human red blood cells: the hCAP18-fragment FALL-9 (hCAP18102–140) is haemolytic, whereas rCAP18106–142 is not (Travis et al. 2000). A number of groups studied variants of CAP18 including an optimization of the antimicrobial and the LPS-neutralising activity. Ciornei et al. compared the activities of LL-37 and fragments obtained by N-terminal truncation called 106 (aa 106–140) and 110 (aa 110–140). They could show that the two fragments 106 and 110 inhibited in the same manner the LPS-induced vascular nitric oxide production. However, the fragments were more active against bacteria in serum and had a decreased cytotoxicity caused by the removal of the hydrophopic amino acids of the N-terminus (Ciornei et al. 2005). Ogata et al. used another approach. They linked CAP18106–138 to immunoglobulin G via a bifunctional linker. Interestingly, they found out that EDTA enhanced the LPS capture capability of this complex leading to a decreased TNF production. In contrast, heparin decreased the LPS neutralising activity (Ogata et al. 1997). A 12 residue synthetic peptide derived by substantial modification of the bovine cathelicidin bactenecin showed enhanced innate immune regulatory activity and suppressed the LPS-induced TNF production (Wieczorek et al. 2010). Bowdish et al. demonstrated that a peptide based on LL-37 having no antimicrobial activity was able to protect in animal models of S. aureus and Salmonella infections (Bowdish and Hancock 2005). They propose that this is possible by exerting immunomodulatory properties.
Defensins are a class of cationic antimicrobial peptides that are intensively investigated as antimicrobial agents (Hancock and Sahl 2006). However, the LPS-neutralising properties have not been investigated in detail. There are only some studies describing the activity of beta defensins. Scott et al. 2000 investigated the activity of the human beta defensin-2 (hBD-2) to neutralise LPS (Scott et al. 2000). They compared the capability of various AMP to reduce the LPS-induced TNF-α release with the inhibition of LPS-LBP binding and found a linear correlation.
Based on the lead structure of β-defensin, Motzkus et al. (2006) have presented a functional analysis of a novel β-defensin denoted as DEFB123. This new peptide contains the β-defensin core region. Especially, the six conserved cysteine residues of human β-defensins are of relevance to host defence against bacterial, fungal and viral infections (Lehrer and Ganz 2002). DEFB123 is a 37 amino acid peptide (GTQRCWNLYGKCRYRCSKKERVYVYCINNKMCCVKPK). Motzkus et al. (2006) were able to show that DEFB123 exerted antimicrobial activity against a broad spectrum of Gram-positive as well as Gram-negative bacteria. DEFB123 showed also LPS-binding activity and its prevention of LPS-induced TNF-α secretion in a murine monocyte cell assay. The peptide was also tested in a murine model of acute sepsis and it was observed a prevention of LPS-induced mortality in mice (C57BL/6). Based on their results, the authors proposed that the physiological role of β-defensins may include interference with LPS-activity on macrophages (Motzkus et al. 2006).
Poymyxin B and other peptides
PMB is a cyclic lipo-decapeptide antibiotic with a seven amino acid peptide ring from Bacillus polymyxa. It contains a tripeptide side chain and a fatty acid tail of varying composition (6-methyloctanoic acid = PMB1, 6-methylheptanoic acid = PMB2, octanoic acid = PMB3, heptanoic acid = PMB4). Under physiological pH conditions, the five amino acid primary amino groups are ionised, leading to a positively charged molecule, up to maximal five positive charges (Garidel and Brandenburg 2009). The number of charges as well as the spatial position, in addition to the hydrophobic fatty acid tail, are prerequisites for the interaction of PMB with endotoxin molecules (Brandenburg et al. 2005), (Howe et al. 2007).
PMB is used for the treatment of various bacterial infections, in particular related to sepsis. One main concern using PMB was its toxicity and the severe side effects that were observed using this antibiotic (Michalopoulos and Falagas 2008). With the appearance, especially in the last years, of multi-drug resistant Gram-negative bacteria to most available antibiotics (Hornef et al. 2002), the use of PMB in the clinical treatment was re-evaluated, especially its toxic properties and clinical dosing (for more details see Falagas and Kasiakou 2006; Garidel and Brandenburg 2009). The main reason for this re-evaluation is due to the fact that PMB is seen by a number of clinicians as the “last option therapy”. To reduce or even avoid bacterial multi-resistance against PMB, it is recommended to use it with great care in clinical management applications.
PMB is also used “indirectly” as part of an extracorporeal hemoperfusion medical device (Cruz et al. 2007) with the peptide covalently immobilised to an insoluble carrier material surface fitted in polystyrene fibres. This medical device is used for selective blood purification from endotoxins. The advantage of this approach is due to the fact that PMB is not systemically present in the body of the patient, and thus avoiding direct PMB toxicity. Such medical device is used since ca. 15 years for the treatment of septic shock (for more details see the recent review by Garidel and Brandenburg 2009).
Recently, Pini et al. (2009) presented a study using a non-natural antimicrobial peptide (KKIRVRLSA) that has a strong capacity to neutralise LPS-induced cytokine release, and thus prevents septic shock in animals, which were infected with bacterial species of clinical interest. One important aspect using peptides is their resistance to proteolytic degradation in the body fluid of mammals. In order to achieve this, Pini et al. developed a tetrabranched peptide derivative with four peptide molecules linked by a lysine core, because the linearly branched versions were sensitive to proteolytic degradations, and thus were inactivated in vivo. The investigated tetrabranched antimicrobial peptide showed a strong selectivity for Gram-negative bacteria, with a MIC in the range of 0.3–3 10−6 M for multidrug resistant clinical isolates like Pseudomonas aeruginosa, Klebsiella pneumoniae or Acinetobacter baumannii.
As noted by Hancock and Sahl (2006) it is important to test microbial activity under relevant physiological conditions, because it is known that the antimicrobial activity, especially for cationic peptides, can be antagonised to a certain extent by divalent cations or even in the presence of monovalent cations at physiological relevant ionic strengths.
Therefore, Pini et al. have tested their 9-mer peptide in a sepsis animal model (mouse) and found a dose-dependent positive effect.
Conclusions
Various results described above are indicative, that the use of peptides may be a promising strategy for treating bacterial sepsis. It could be shown that amphiphilic and polycationic compounds of suitable chain length in the range 17–27 AA should be most adequate to neutralise endotoxins. Shorter analogues, as well as much longer compounds, may be efficient antimicrobial drugs, but they do not meet the requirements to bind effectively the lipid A part of LPS, a prerequisite for inactivation. Necessary conditions of endotoxin neutralisation are in all cases the conversion of their aggregate structures into multilamellar ones, a low saturation molar ratio of peptide/endotoxin binding and an overcompensation of the negative backbone charges of LPS by the peptides. In contrast, the change in the lipid A acyl chain-melting does not necessarily correlate with the biological effectivity of a given peptide. Of course, the selectivity, in pharmaceutical words, the therapeutical index must be high, i.e. the concentration at which effective endotoxin inactivation is observed must be considerable lower than any cytotoxic effect in human cells.
The results presented here must be discussed in the light of other antiseptic therapeutic trials. These range from the use of anti-LPS antibodies, in particular the IgM monoclonal antibody HA-1A (Baumgartner et al. 1990), the application of an antibody to tumour-necosis facftor α (Reinhart and Karzai 2001) over the administration of anticoagulants such as activated protein C (APC, drotrecogin alfa®, Dhainaut et al. 2004) to the use of LPS-antagonists such as E5564 (Tidswell et al. 2010). The former three therapeutic approaches were shown to be more or less ineffective, while the use of APC had in a subpopulation of septic patients a slightly beneficial effect, but with the risk of severe side effects such as serious increase in bleeding rates. The use of the LPS antagonist yielded a ‘trend towards a lower mortality rate’, but must be investigated further for the suitability in critical care units. This therapy, however, can of course only be effective in the case of Gram-negative induced sepsis, because Eritoran represents a TLR4-receptor antagonist (LPS signalling) and does not involve TLR2-antagonist necessary for blocking of Gram-positive lipoproteins. Also, the use of SALP as presented here has the advantage of a simultaneous anti-microbial activity as outlined above.
So far, no clinical phases with peptides are underway. We will start a clinical phase 1 in 2012 by utilising the above-described Pep19 series. One clinical phase is known which utilises recombinant human lactoferrin to treat severe sepsis (phase II study, drug talactoferrin alfa, published in ClinicalTrials, gov). However, up to now, no study results have been published.
References
Agerberth B, Grunewald J, Castanos-Velez E, Olsson B, Jornvall H, Wigzell H, Eklund A, Gudmundsson GH (1999) Antibacterial components in bronchoalveolar lavage fluid from healthy individuals and sarcoidosis patients. Am J Respir Crit Care Med 160:283–290
Andersson M, Gunne H, Agerberth B, Boman A, Bergman T, Sillard R, Jornvall H, Mutt V, Olsson B, Wigzell H (1995) NK-lysin, a novel effector peptide of cytotoxic T and NK cells. Structure and cDNA cloning of the porcine form, induction by interleukin 2, antibacterial and antitumour activity. EMBO J 14:1615–1625
Andersson M, Gunne H, Agerberth B, Boman A, Bergman T, Olsson B, Dagerlind A, Wigzell H, Boman HG, Gudmundsson GH (1996) NK-lysin, structure and function of a novel effector molecule of porcine T and NK cells. Vet Immunol Immunopathol 54:123–126
Andersson M, Girard R, Cazenave P (1999) Interaction of NK lysin, a peptide produced by cytolytic lymphocytes, with endotoxin. Infect Immun 67:201–205
Andrä J, Garidel P, Majerle A, Jerala R, Ridge R, Paus E, Novitsky T, Koch MHJ, Brandenburg K (2004a) Biophysical characterization of the interaction of Limulus polyphemus endotoxin neutralizing protein with lipopolysaccharide. Eur J Biochem 271:2037–2046
Andrä J, Koch MHJ, Bartels R, Brandenburg K (2004b) Biophysical characterization of endotoxin inactivation by NK-2, an antimicrobial peptide derived from mammalian NK-lysin. Antimicrob Agents Chemother 48:1593–1599
Andrä J, Lamata M, Martinez de Tejada G, Bartels R, Koch MHJ, Brandenburg K (2004c) Cyclic antimicrobial peptides based on Limulus anti-lipopolysaccharide factor for neutralization of lipopolysaccharide. Biochem Pharmacol 68:1297–1307
Andrä J, Lohner K, Blondelle SE, Jerala R, Moriyon I, Koch MHJ, Garidel P, Brandenburg K (2005) Enhancement of endotoxin neutralization by coupling of a C12-alkyl chain to a lactoferricin-derived peptide. Biochem J 385:135–143
Andrä J, Gutsmann T, Garidel P, Brandenburg K (2006) Mechanisms of endotoxin neutralization by synthetic cationic compounds. J Endotoxin Res 12:261–277
Andrä J, Howe J, Garidel P, Rössle M, Richter W, Leiva-Leon J, Moriyon I, Bartels R, Gutsmann T, Brandenburg K (2007a) Mechanism of interaction of optimized Limulus-derived cyclic peptides with endotoxins: thermodynamic, biophysical and microbiological analysis. Biochem J 406:297–307
Andrä J, Monreal D, Martinez de Tejada G, Olak C, Brezesinski G, Gomez SS, Goldmann T, Bartels R, Brandenburg K, Moriyon I (2007b) Rationale for the design of shortened derivatives of the NK-lysin-derived antimicrobial peptide NK-2 with improved activity against Gram-negative pathogens. J Biol Chem 282:14719–14728
Appelmelk BJ, An Y-Q, Geerts M, Thijs BG, De Boer HA, MacLaren DM, De Graaff J, Nuijens JH (1994) Lactoferrin is a lipid A-binding protein. Infect Immun 62:2628–2632
Baumgartner JD, Heumann D, Gerain J, Weinbreck P, Grau GE, Glauser MP (1990) Association between protective efficacy of anti-lipopolysaccharide (LPS) antibodies and suppression of LPS-induced tumor necrosis factor alfa and interleukin 6. J Exp Med 171:889–896
Bowdish DM, Hancock RE (2005) Anti-endotoxin properties of cationic host defence peptides and proteins. J Endotoxin Res 11:230–236
Brandenburg K, Jürgens G, Müller M, Fukuoka S, Koch MHJ (2001) Biophysical characterization of lipopolysaccharide and lipid A inactivation by lactoferrin. Biol Chem 382:1215–1225
Brandenburg K, David A, Howe J, Koch MHJ, Andrä J, Garidel P (2005) Temperature dependence of the binding of endotoxins to the polycationic peptides polymyxin B and its nonapeptide. Biophys J 88:1845–1858
Brandenburg K, Garidel P, Fukuoka S, Howe J, Koch MHJ, Gutsmann T, Andrä J (2010) Molecular basis for endotoxin neutralization by amphipathic peptides derived from the alpha-helical cationic core-region of NK-lysin. Biophys Chem 150:80–87
Chen X, Howe J, Andrä J, Rössle M, Richter W, da Silva AP, Krensky AM, Clayberger C, Brandenburg K (2007) Biophysical analysis of the interaction of granulysin-derived peptides with enterobacterial endotoxins. Biochim Biophys Acta 1768:2421–2431
Ciornei CD, Sigurdardottir T, Schmidtchen A, Bodelsson M (2005) Antimicrobial and chemoattractant activity, lipopolysaccharide neutralization, cytotoxicity, and inhibition by serum of analogs of human cathelicidin LL-37. Antimicrob Agents Chemother 49:2845–2850
Cowland JB, Johnsen AH, Borregaard N (1995) hCAP-18, a cathelin/pro-bactenecin-like protein of human neutrophil specific granules. FEBS Lett 368:173–176
Cross AS, Opal SM (1995) Endotoxin's role in Gram-negative bacterial infection. Curr Opin Infect Dis 8:156–163
Cruz DN, Bellomo R, Ronco C (2007) Clinical effects of polymyxin B-immobilized fiber column in septic patients. Contrib Nephrol 156:444–451
Dankesreiter S, Hoess A, Schneider-Mergener J, Wagner H, Mietke T (2000) Synthetic endotoxin-binding peptides block endotoxin-triggered TNF-α production by macrophages in vitro and in vivo and prevent endotoxin-mediated toxic shock. J Immunol 164:4804–4811
Davis EG, Sang Y, Rush B, Zhang G, Blecha F (2005) Molecular cloning and characterization of equine NK-lysin. Vet Immunol Immunopathol 105:163–169
de Haas CJ, Haas PJ, van Kessel KP, van Strijp JA (1998) Affinities of different proteins and peptides for lipopolysaccharide as determined by biosensor technology. Biochem Biophys Res Commun 252:492–496
Deng A, Chen S, Li Q, Lyu SC, Clayberger C, Krensky AM (2005) Granulysin, a cytolytic molecule, is also a chemoattractant and proinflammatory activator. J Immunol 174:5243–5248
Dhainaut JF, Yan SB, Joyce DE, Pettila V, Basson B, Brandt T, Sundin DP, Levis ML (2004) Treatment effects of drotrecogin alfa (activated) in patients with severe sepsis with or without overt disseminated intravascular coagulation. J Thromb Haemost 2:1924–1833
Elass-Rochard E, Legrand D, Salmon V, Roseanu A, Trif M, Tobias PS, Mazurier J, Spik G (1998) Lactoferrin inhibits the endotoxin interaction with CD14 by competition with the lipopolysaccharide-binding protein. Infect Immun 66:486–491
Endsley JJ, Furrer JL, Endsley MA, McIntosh MA, Maue AC, Waters WR, Lee DR, Estes DM (2004) Characterization of bovine homologues of granulysin and NK-lysin. J Immunol 173:2607–2614
Falagas ME, Kasiakou SK (2006) Toxicity of polymyxins: a systematic review of the evidence from old and recent studies. Crit Care 10:R27
Frohm M, Gunne H, Bergman AC, Agerberth B, Bergman T, Boman A, Liden S, Jornvall H, Boman HG (1996) Biochemical and antibacterial analysis of human wound and blister fluid. Eur J Biochem 237:86–92
Garidel P, Brandenburg K (2009) Current understanding of polymyxin B applications in bacteraemia/sepsis therapy prevention: clinical, pharmaceutical, structural and mechanistic aspects. Antiinfect Agents Medic Chem 8:367–385
Gutsmann T, Howe J, Zähringer U, Garidel P, Schromm AB, Koch MHJ, Fujimoto Y, Fukase K, Moriyon I, Martinez de Tejada G, Brandenburg K (2010a) Structural prerequisites for endotoxic activity in the Limulus test as compared to cytokine production in mononuclear cells. Innate Immun 16:39–47
Gutsmann T, Razquin-Olazaran I, Kowalski I, Kaconis Y, Howe J, Bartels R, Hornef M, Schürholz T, Rössle M, Sanchez-Gomez S, Moriyon I, Martinez de Tejada G, Brandenburg K (2010b) New antiseptic peptides to protect against endotoxin-mediated shock. Antimicrob Agents Chemother 54:3817–3824
Hancock RE, Sahl HG (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat Biotechnol 24:1551–1557
Hashimoto M, Furuyashiki M, Kaseya R, Fukada Y, Akimaru M, Aoyama K, Okuno T, Tamura T, Kirikae T, Kirikae F, Eiraku N, Morioka H, Fujimoto Y, Fukase K, Takashige K, Moriya Y, Kusumoto S, Suda Y (2007) Evidence of immunostimulating lipoprotein existing in the natural lipoteichoic acid fraction. Infect Immun 75:1926–1932
Haversen L, Kondori N, Baltzer L, Hanson LA, Dolphin GT, Duner K, Mattsby-Baltzer I (2010) Structure-microbicidal activity relationship of synthetic fragments derived from the antibacterial alpha-helix of human lactoferrin. Antimicrob Agents Chemother 54:418–425
Hirata M, Zhong J, Wright SC, Larrick JW (1995) Structure and functions of endotoxin-binding peptides derived from CAP18. Prog Clin Biol Res 392:317–326
Hoess A, Watson S, Siber GR, Liddington R (1993) Crystal structure of an endotoxin-neutralizing protein from the horseshoe crab, Limulus anti-LPS factor, at 1.5 A resolution. EMBO J 12:3351–3356
Hornef MW, Wick MJ, Rhen M, Normark S (2002) Bacterial strategies for overcoming host innate and adaptive immune responses. Nat Immunol 3:1033–1040
Howe J, Andrä J, Conde R, Iriarte M, Garidel P, Koch MHJ, Gutsmann T, Moriyon I, Brandenburg K (2007) Thermodynamic analysis of the lipopolysaccharide-dependent resistance of gram-negative bacteria against polymyxin B. Biophys J 92:2796–2805
Japelj B, Pristovsek P, Majerle A, Jerala R (2005) Structural origin of endotoxin neutralization and antimicrobial activity of a lactoferrin-based peptide. J Biol Chem 280:16955–16961
Kowalski I, Kaconis Y, Andrä J, Razquin-Olazaran I, Gutsmann T, Martinez de Tejada G, Brandenburg K (2010) Physicochemical and biological characterization of anti-endotoxin peptides and their Influence on lipid properties. Protein Pept Lett 17:1328–1333
Larrick JW, Morgan JG, Palings I, Hirata M, Yen MH (1991) Complementary DNA sequence of rabbit CAP18–a unique lipopolysaccharide binding protein. Biochem Biophys Res Commun 179:170–175
Lefrant JY, Muller L, Raillard A, Jung B, Beaudroit L, Favier L, Masson B, Dingemans G, Thevenot F, Selcer D, Jonquet O, Capdevila X, Fabbro-Peray P, Jaber S (2010) Reduction of the severe sepsis or septic shock associated mortality by reinforcement of the recommendations bundle: a multicenter study. Ann Fr Anesth Rèanim 29:621–628
Lehrer RI, Ganz T (2002) Defensins of vertebrate animals. Curr Opin Immunol 14:96–102
Leippe M (1995) Ancient weapons: NK-lysin is a mammalian homolog to pore-forming peptides of a protozoan parasite. Cell 83:17–18
Levin J (1987) The Limulus amebocyte lysate test: perspectives and problems. Prog Clin Biol Res 231:1–23
Liepinsh E, Andersson M, Ruysschaert JM, Otting G (1997) Saposin fold revealed by the NMR structure of NK-lysin. Nat Struct Biol 4:793–795
Malm J, Sorensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, Lilja H, Stahle-Backdahl M, Borregaard N, Egesten A (2000) The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun 68:4297–4302
Michalopoulos A, Falagas ME (2008) Colistin and polymyxin B in critical care. Crit Care Clin 24:377–391
Morrison DC (1998) Antibiotic-mediated release of endotoxin and the pathogenesis of Gram-negative sepsis. Prog Clin Biol Res 397:199–207
Motzkus D, Schulz-Maronde S, Heitland A, Schulz A, Forssmann WG, Jubner M, Maronde E (2006) The novel beta-defensin DEFB123 prevents lipopolysaccharide-mediated effects in vitro and in vivo. FASEB J 20:1701–1702
Ogata M, Fletcher MF, Kloczewiak M, Loiselle PM, Zanzot EM, Vermeulen MW, Warren HS (1997) Effect of anticoagulants on binding and neutralization of lipopolysaccharide by the peptide immunoglobulin conjugate CAP18106–138-immunoglobulin G in whole blood. Infect Immun 65:2160–2167
Opal SM, Cohen J (1999) Clinical Gram-positive sepsis: does it fundamentally differ from Gram-negative bacterial sepsis? Crit Care Med 27:1608–1616
Pan CY, Chao TT, Chen JC, Chen JY, Liu WC, Lin CH, Kuo CM (2007) Shrimp (Penaeus monodon) anti-lipopolysaccharide factor reduces the lethality of Pseudomonas aeruginosa sepsis in mice. Int Immunopharmacol 7:687–700
Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM (1997) Processing, subcellular localization, and function of 519 (Granulysin), a human late T cell sctivation molecule with homology to small, lytic, granule proteins. J Immunol 158: 2680–2688
Pini A, Falciani C, Mantengoli E, Bindi S, Brunetti J, Iozzi S, Maria RG, Bracci L (2009) A novel tetrabranched antimicrobial peptide that neutralizes bacterial lipopolysaccharide and prevents septic shock in vivo. FASEB J 24:1015–1022
Reinhart K, Karzai W (2001) Anti-tumor necrosis factor therapy in sepsis: update on clinical trials and lessons learned. Crit Care Med 7:S121–S125
Ren JD, Gu JS, Gao HF, Xia PY, Xiao GX (2008) A synthetic cyclic peptide derived from Limulus anti-lipopolysaccharide factor neutralizes endotoxin in vitro and in vivo. Int Immunopharmacol 8:775–781
Ried C, Wahl C, Miethke T, Wellnhofer G, Landgraf C, Schneider-Mergener J, Hoess A (1996) High affinity endotoxin-binding and neutralizing peptides based on the crystal structure of recombinant Limulus anti-lipopolysaccharide factor. J Biol Chem 271:28120–28127
Scott MG, Vreugdenhil ACE, Buurman WA, Hancock REW, Gold M (2000) Cationic antimicrobial peptides block the binding of lipopolysaccharide (LPS) to LPS binding protein. J Immunol 164:549–553
Sorensen O, Cowland JB, Askaa J, Borregaard N (1997) An ELISA for hCAP-18, the cathelicidin present in human neutrophils and plasma. J Immunol Meth 206:53–59
Tidswell M, Tillis W, LaRosa SP, Lynn M, Wittek AE, Kao R, Wheller J, Gogate J, Opal SM, Eritoran Sepsis Study Group (2010) Phase 2 trial od eritoran tetrasodium (E5564), a Toll-like receptor 4 antagonist, in patients with severe sepsis. Crit Care Med 38:72–83
Travis SM, Anderson NN, Forsyth WR, Espiritu C, Conway BD, Greenberg EP, McCray PB, Lehrer RI, Welsh MJ, Tack BF (2000) Bactericidal activity of mammalian cathelicidin-derived peptides. Infect Immun 68:2748–2755
Wang Q, Wang Y, Xu P, Liu Z (2006) NK-lysin of channel catfish: gene triplication, sequence variation, and expression analysis. Mol Immunol 43:1676–1686
Wieczorek M, Jenssen H, Kindrachuk J, Scott WR, Elliott M, Hilpert K, Cheng JT, Hancock RE, Straus SK (2010) Structural studies of a peptide with immune modulating and direct antimicrobial activity. Chem Biol 17:970–980
Yang SH, Tamai R, Akashi S, Takeuchi O, Akira S, Sugawara S, Takada H (2001) Synergistic effect of muramyldipeptide with lipopolysaccharide or lipoteichoic acid to induce inflammatory cytokines in human monocytic cells in culture. Infect Immun 69:2045–2053
Zanetti M, Gennaro R, Romeo D (1995) Cathelicidins: a novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett 374:1–5
Acknowledgements
The authors are indebted the German ministry BMBF for financial support (K.B. 01GU0824). J.A. acknowledges the financial support from the German Science Foundation (DFG) grant AN301/5-1.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brandenburg, K., Andrä, J., Garidel, P. et al. Peptide-based treatment of sepsis. Appl Microbiol Biotechnol 90, 799–808 (2011). https://doi.org/10.1007/s00253-011-3185-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3185-7