Abstract
Biological synthesis of therapeutic drugs beneficial for human health using microbes offers an alternative production strategy to the methods that are commonly employed such as direct extraction from source organisms or chemical synthesis. In this study, we evaluated the potential for yeast (Saccharomyces cerevisiae) to be used as a catalyst for the synthesis of tranilast and various tranilast analogs (cinnamoyl anthranilates). Several studies have demonstrated that these phenolic amides have antioxidant properties and potential therapeutic benefits including antiinflammatory, antiproliferative, and antigenotoxic effects. The few cinnamoyl anthranilates naturally produced in plants such as oats and carnations result from the coupling of various hydroxycinnamoyl-CoAs to anthranilic acid. In order to achieve the microbial production of tranilast and several of its analogs, we engineered a yeast strain to co-express a 4-coumarate/CoA ligase (4CL, EC 6.2.1.12) from Arabidopsis thaliana and a hydroxycinnamoyl/benzoyl-CoA/anthranilate N-hydroxycinnamoyl/benzoyltransferase (HCBT, EC 2.3.1.144) from Dianthus caryophyllus. This modified yeast strain allowed us to produce tranilast and 26 different cinnamoyl anthranilate molecules within a few hours after exogenous supply of various combinations of cinnamic acids and anthranilate derivatives. Our data demonstrate the feasibility of rapidly producing a wide range of defined cinnamoyl anthranilates in yeast and underline a potential for the biological designed synthesis of naturally and non-naturally occurring molecules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The worldwide drug market is large and is constantly expanding. Medical drugs used to treat human and animal diseases can be produced chemically or biologically. Even if the biological production is the preferred strategy, it is still rarely used due to the absence of known biosynthetic pathways, the toxicity of intermediate or final products, and poor yields or high recovery costs. Chemically produced drugs usually require large quantities of expensive and non-eco-friendly chemicals. For example, the drug tranilast (Fig. 1a), which belongs to the group of cinnamoyl anthranilate molecules, is manufactured only using organic synthesis methodologies. Tranilast and some of its analogs were recently shown to exhibit antioxidant, antigenotoxic, and antifibrotic activities (Fagerlund et al. 2009; Lee-Manion et al. 2009; Zammit et al. 2009). This synthetic drug (Rizaban, Kissei Pharmaceutical Co, Japan) is currently used in Japan and South Korea as an antihistamine to treat bronchial asthma, atopic dermatitis, allergic conjunctivitis, allergic rhinitis, and other allergic disorders (Azuma et al. 1976; Okuda et al. 1984; Komatsu et al. 1988). Tranilast is also used to treat hypertrophic scars, scleroderma, and other skin disease related to excessive fibrosis because it has the capacity to inhibit the release of chemical mediators from mast cells and macrophages and suppresses collagen deposition (reviewed in Isaji et al. 1998). More recently, tranilast was shown to both inhibit and increase the expression of proinflammatory and antiinflammatory cytokines, respectively, confirming its role in regulating mast cell and macrophage degranulation (Prud’homme 2007; Pae et al. 2002; Sun et al. 2010). Thus, health beneficial effects of tranilast have been assessed in vivo against the development of several disorders associated with proinflammatory leukocyte mediators, fibrogenesis, and tumorigenesis including atherosclerosis, restenosis after angioplasty, arthritis, lacrimal gland chronic GVHD, inflammatory bowel disease, multiple sclerosis, adhesions, fibrosis, and tumor angiogenesis, growth, and metastasis (Tamai et al. 2002; Platten et al. 2005; Oshitani et al. 2007; Chakrabarti et al. 2009; Cui et al. 2009; Guo et al. 2009; Ogawa et al. 2010; Shiota et al. 2010; Tan et al. 2010). Importantly, several years of clinical use have established that tranilast is well tolerated by most patients at doses of up to 600 mg/day for months (Konneh 1998).
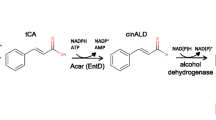
Structure of tranilast and related cinnamoyl anthranilates. a Six structural analogs to tranilast that exhibit antioxidant, antifibrotic, antigenotoxic effects are shown (Lee-Manion et al. 2009; Zammit et al. 2009). b Schematic representation of the enzymatic reactions catalyzed by Arabidopsis 4-coumarate/coenzyme A ligase (4CL5) and hydroxycinnamoyl/benzoyl-CoA/anthranilate N-hydroxycinnamoyl/benzoyltransferase (HCBT) for the biosynthesis of various cinnamoyl anthranilates. For the biological production of cinnamoyl anthranilates analogous to tranilast, recombinant yeast expressing 4CL5 and HCBT was grown in the presence of anthranilate and known substrates for 4CL5 (p-coumaric, caffeic, ferulic, or sinapic acid)
Identification of new genes, biochemical characterization of enzymes, and the combination of enzymes to generate biological pathways is a key element of synthetic biology for the engineering of foreign hosts that are able to biologically synthesize naturally and non-naturally occurring drugs. Additionally, high-yield production is usually achieved when biosynthetic pathways are heterologously expressed in microbes that are suitable for fermentor production such as yeast Saccharomyces cerevisiae or Escherichia coli. The expression of plant metabolic pathways in microbial organisms is an attractive strategy for the production of valuable natural products that accumulate at low concentrations, are difficult to extract, or originate from endemic plant species (Horwitz 1994; Trantas et al. 2009). Microbial expression systems have several advantages over chemical synthesis or direct extraction from plant tissue, e.g., reduced requirements for toxic chemicals and natural resources, consistent quality, scalability, simple extraction, and potential for higher synthesis efficiency (Chang and Keasling 2006). Advantages of S. cerevisiae over other microbial hosts include its food-grade status, the extensive knowledge for large scale production, the availability of genetic tools, and its suitability to express plant genes such as cytochrome P450 enzymes (Trantas et al. 2009; Limem et al. 2008). Remarkable examples of pharmaceutical metabolites produced in recombinant yeast strains expressing plant genes include the precursor of the antimalarial drug artemisinic acid and taxadiene (Ro et al. 2006; Engels et al. 2008), flavonoids, stilbenoids and phenylpropanoids (Vannelli et al. 2007; Limem et al. 2008), vitamin C (Branduardi et al. 2007), hydrocortisone (Szczebara et al. 2003), and serotonin derivates (Park et al. 2008).
Natural cinnamoyl anthranilates are produced by the amide condensation of anthranilate and (hydroxy)-cinnamoyl-CoA derivatives, and most of them were co-purified from oats and carnation plants (Ponchet et al. 1988; Collins 1989). We attempted to produce cinnamoyl anthranilates in S. cerevisiae by introducing two genes from two different plant species (Fig. 1b). The first gene encodes the hydroxycinnamoyl/benzoyl-CoA/anthranilate N-hydroxycinnamoyl/benzoyltransferase (HCBT), an enzyme from Dianthus caryophyllus, which has affinity for anthranilate and p-coumaroyl-CoA and is capable of producing N-(4′-hydroxycinnamoyl)-anthranilate in vitro (Yang et al. 1997). The second gene encodes 4-coumarate/CoA ligase five (4CL5) from Arabidopsis thaliana, which converts various hydroxycinnamic acids into the corresponding CoA thioesters (Hamberger and Hahlbrock 2004). This enzyme was required since the hydroxycinnamoyl-CoA thioesters are unstable, commercially unavailable, membrane impermeable, and not naturally produced in yeast. Additionally, in order to reduce the degradation of the 4CL5 substrates, we used a yeast strain lacking pad1, which encodes a phenylacrylic decarboxylase known to catalyze the decarboxylation of several hydroxycinnamic acids (Mukai et al. 2010). Our findings show that the engineered yeast strain was able to produce the pharmaceutical drug tranilast and a variety of known or uncharacterized analogs after incubation with anthranilate, 3-hydroxyanthranilate, and various natural or synthetic cinnamic acids.
Materials and methods
Chemicals
Ferulic acid, p-coumaric acid, 2,5-dimethoxycinnamic acid, 2,4-dimethoxycinnamic, and caffeic acid were purchased from TCI America (Portland, OR, USA). Cinnamic acid, sinapic acid, o-coumaric acid, m-coumaric acid, 3-hydroxy-4-methoxycinnamic acid, 3,4-dimethoxycinnamic acid, 3,4,5-trimethoxycinnamic acid, 3-methoxycinnamic acid, 4-methoxycinnamic acid, 2,3-dimethoxycinnamic acid, anthranilate, 3-hydroxyanthranilate, tranilast [N-(3′,4′-dimethoxycinnamoyl)-anthranilic acid], dithiothreitol, phenylmethanesulfonylfluoride, and protease inhibitor cocktail were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chromatographic solvents were HPLC grade and purchased from local suppliers.
Chemical synthesis of N-(4′-hydroxy-(E)-cinnamoyl)-anthranilate
N-4′-(Hydroxy-(E)-cinnamoyl)-anthranilate was prepared as described (Collins 1989). Briefly, acid 4-acetoxy-(E,Z)-cinnamoyl chloride was prepared from 4-hydroxy-(E,Z)-cinnamic acid by acetylation with acetic anhydride (Sigma-Aldrich, p-toluenesulfonic acid catalyst) and treatment of the recrystallized (hot MeOH) 4-acetoxy-(E,Z)-cinnamic acid with excess thionyl chloride (Sigma-Aldrich) according to the procedures of Fosdick and Starke (1940). Removal of excess thionyl chloride by repeated rotary evaporation and washing with acetone gave a crude acid chloride containing no detectable free 4-acetoxy-(E,Z)-cinnamic acid. The crude acid chloride was found suitable for subsequent reactions and was used without further purification. A solution of 135 mg (1 mmol) of anthranilic acid was condensed with the dried residue corresponding to (1 mmol) 4-acetoxy-(E,Z)-cinnamoyl chloride. After deacylation with mild alkali, the products were purified by repeated chromatography on a Sephadex LH-20 resin (GE Healthcare, Piscataway, NJ, USA) using glass columns and a gravity-flow isocratic elution in CHCl3–cyclohexane–MeOH–acetic acid (50:40:5:5 v/v/v/v by percentage) and CHCl3–cyclohexane–MeOH–acetic acid (50:35:105 v/v/v/v by percentage) to give N-4′-hydroxy-(E)-cinnamoyl-2-aminobenzoic acid (yield 235 mg (83%)) and a small amount of the Z isomer. Crystallization of the E isomer from hot acetone–water gave colorless rods: mp 219°C; C16H13NO4; M•+ 283; UV (MeOH) λmax (log ε) 218 (4.30), 294 s, (4.25), 302 s, (4.33), 329 (4.47) nm; UV (MeOH + NaOH) λmax (log ε) 213 (4.43), 233 s, (4.19), 306 s, (4.05), 314 (4.08), 371 (4.51) nm.
Generation of a shuttle vector for gene coexpression in yeast
We generated a yeast shuttle vector pDRf1-GW-P HXT7 , which contains a Gateway cloning cassette (Invitrogen, Carlsbad, CA, USA) inserted between the PMA1 promoter (P PMA1 ) and the ADH1 terminator (T ADH1 ), and carries a second yeast expression cassette inserted into the SphI restriction site at the 3′-end of T ADH1 . This cassette contains the HXT7 promoter (P HXT7 ) and the CYC1 terminator (T CYC1 ), both separated by a multicloning site containing a NotI restriction site (P HXT7 –T CYC1 ). The P HXT7 –T CYC1 and P PMA1 –T ADH1 expression cassettes are in the same orientation. To generate a pDRf1-GW-P HXT7 coexpression vector, the yeast shuttle vector p426 (Wieczorke et al. 1999) was first modified by site-directed mutagenesis (Kunkel 1985) to insert two SphI restriction sites at the 5′-end of P HXT7 and the 3′-end of T CYC1 using the following primers 5′-CGAAATTGTTCCTACGAGCTCGCATGCTTTTGTTCCCTTTAGTGAGG-3′ and 5′-GACTCACTATAGGGCGAATTGGCATGCGGCCGCAAATTAAAGCCTTC-3′, respectively. This vector was further modified to insert the unique NotI restriction site between P HXT7 and T CYC1 . The multicloning site and the sequence encoding a His-tag located between P HXT7 and T CYC1 was replaced by site-directed mutagenesis (Kunkel 1985) using the following primer 5′-CATAACTAATTACATGACTCGAGCGGCCGCCCGGGGGATCCACTAGA-3′. After mutagenesis, the P HXT7 –T CYC1 expression cassette was sequence-verified, digested with SphI (Fermentas Inc., Glen Burnie, MD, USA), and inserted into the unique SphI restriction site of pDRf1-GW located at the T ADH1 3′-end (Loqué et al. 2007).
Construction and expression of recombinant yeast harboring 4CL5 and HCBT
The 4CL5 gene (At3g21230) was cloned from A. thaliana (ecotype Columbia). Four microgram of total RNA was isolated from mixed organs of Arabidopsis plants using the RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) and used to perform an RT-PCR. First strand cDNAs were synthesized using the Transcriptor High Fidelity cDNA Synthesis kit (Roche, Indianapolis, IN, USA) and used to amplify the 4CL5 gene using the following oligonucleotides containing NotI restriction sites: forward, 5′-GCGGCCGCATGGTGCTCCAACAACAAACGC-3′; and reverse, 5′-GCGGCCGCCTATTTAGAGCACATGGTTTCC-3′ (NotI sites are underlined). The PCR product was subcloned into the pCR-Blunt vector (Invitrogen), digested with NotI restriction enzyme (Fermentas Inc.), gel-purified, and ligated into the pDRf1-GW-pHXT7 vector at the unique NotI restriction site located between pHXT7 and tCYC1 of the expression cassette. A clone showing correct orientation for the 4CL5 gene was selected, and the resulting vector was named pDRf1-4CL5-GW.
To clone the gene encoding HCBT, a gene sequence encoding the HCBT1 protein (O24645) without stop codon and flanked with the attB1 (5′-end) and attB2 (3′-end) Gateway recombination sites was synthesized and codon-optimized for yeast expression by GenScript (Piscataway, NJ, USA). The attB1-HCBT-attB2 fragment was remobilized into the Donor plasmid vector pDONR221-f1 (Lalonde et al. 2010) by in vitro BP recombination and transferred into the pDRf1-4CL5-GW and pDRf1-GW-pHXT7 vectors by in vitro LR recombination using the Gateway technology (Invitrogen). The resulting vectors were named pDRf1-4CL5-HCBT1 and pDRf1-HCBT1. A pDRf1-4CL5 control vector was also generated by in vitro LR recombination between the pDRf1-4CL5-GW vector and an ENTRY clone containing only a nucleotide sequence corresponding to a PvuII restriction site (CAGCTG) between the attL recombination sites. This six-nucleotide sequence consequently replaced both the ccdB and chloramphenicol resistance genes of the Gateway cassette in the pDRf1-4CL5-GW vector.
pDRf1-4CL5-HCBT1, pDRf1-HCBT1, and pDRf1-4CL5 were transformed into the S. cerevisiae pad1 knockout (MATa his3∆1 leu2∆0 met15∆0 ura3∆0 ∆pad1, ATCC 4005833; Winzeler et al. 1999) using the lithium acetate transformation method (Gietz and Woods 2002) and selected on solid medium containing Yeast Nitrogen Base (YNB) without amino acids (Difco 291940; Difco, Detroit, MI, USA) supplemented with 3% glucose and 1× dropout-uracil (CSM-ura; Sunrise Science Products, San Diego, CA, USA).
HCBT expression analysis
The codon optimized HCBT clone was synthesized without a stop codon, therefore generating an in-frame C-terminal tag corresponding to the PAFLYKVV peptide after translation of the attB2 site obtained after LR recombination. A polyclonal antibody was raised against an AttB2 peptide (DPAFLYKVVD) using rabbit as a host and purified using an affinity column (Biogenes, Berlin, Germany). The purified serum was named “universal antibody” since it can be used to quantify the expression level of any protein expressed with any Gateway destination vectors.Footnote 1
For soluble protein extraction, overnight cultures from single colonies were used to inoculate 50 mL of 2× yeast nitrogen base medium without amino acids (Difco) supplemented with 6% glucose and 2× CSM-Ura (Sunrise Science Products) at an OD600 = 0.15 and incubated at 30°C until it reached OD600 = 1. Cells were centrifuged at 4,500×g for 5 min at 4°C and washed with one volume of chilled water. The cell pellets were resuspended in 300 μL of CelLytic-Y yeast cell lysis/extraction reagent (Sigma-Aldrich) supplemented with 10 mM dithiothreitol, 2 mM phenylmethanesulfonylfluoride, and 2% protease inhibitor cocktail (v/v, P8215 Sigma, St. Louis, MO, USA). Approximately 200 μL of acid-washed glass beads (Sigma) was added to the mixture, which was then vortexed ten times for 30 s and centrifuged at 10,000×g for 5 min at 4°C to collect the supernatant. Samples were maintained on ice between vortexing steps. The supernatant containing soluble proteins was collected and used for immunoblotting.
Protein concentration was quantified using the Bradford (1976) method and bovine serum albumin as a standard. For electrophoresis, soluble protein (5 μg) was mixed with 0.2 M Tris–HCl, pH 6.5, 8% (w/v) SDS, 8% (v/v) β-mercaptoethanol, 40% (v/v) glycerol, and 0.04% (w/v) bromophenol blue and incubated at 40°C for 30 min. Proteins were separated by SDS-PAGE using 8–16% (w/v) polyacrylamide gradient gels (Invitrogen) and electrotransferred (100 V, 45 min) onto PVDF membranes (Thermo Fisher Scientific, Rockford, IL, USA). Blotted membranes were incubated 1 h in TBS-T (20 mM Tris–HCl, 150 mM NaCl, 0.1% (v/v) Tween 20, pH 7.6) containing 2% (w/v) non-fat milk powder and incubated overnight with the universal antibody (1:20,000) in TBS-T containing 2% (w/v) non-fat milk powder. Membranes were then washed in TBS-T for 30 min and incubated for 1 h with an anti-rabbit secondary antibody conjugated to horseradish peroxidase (1:20,000; Sigma-Aldrich) in TBS-T containing 2% (w/v) non-fat milk powder. Membranes were then washed in TBS-T for 30 min, and detection was performed by chemiluminescence using the SuperSignal West Dura Extended Duration Substrate (Thermo Fisher Scientific).
Production of cinnamoyl anthranilates
An overnight culture from a single colony of the pDRf1-4CL5-HCBT recombinant yeast grown on 2× YNB medium without amino acids supplemented with 6% glucose and 2× CSM-Ura was used to inoculated 15 mL of fresh minimal medium at an OD600 = 0.15 and shaken at 200 rpm in a 30°C room. When the 10-mL culture reached an OD600 = 1, all substrates were added at once to reach final concentrations of 500 μM for anthranilate and 3-hydroxyanthranilate, and 300 μM for the cinnamic acids except for 3-methoxycinnamic acid, 4-methoxycinnamic acid, and 2,5-dimethoxycinnamic acid, which were supplied at a final concentration of 50 μM due to their negative effect on cell growth at higher concentrations. The cultures were shaken at 200 rpm in a 30°C room for 15 h for the production of cinnamoyl anthranilates. As negative controls, yeast colonies harboring the pDRf1-HCBT1 or pDRf1-4CL5 vectors were grown using similar conditions.
Detection of cinnamoyl anthranilates
For the detection of cinnamoyl anthranilates, an aliquot of the culture medium was collected and cleared by centrifugation (21,000×g for 5 min at 4°C). The cleared medium was collected, mixed with an equal volume of cold methanol, and filtered using Amicon Ultra centrifugal filters (3,000 Da MW cutoff regenerated cellulose membrane; Millipore, Billerica, MA, USA) prior to LC-TOF MS analysis. For the analysis of the tranilast content in yeast cells, the cell pellet from the 10-mL culture was washed three times with water, resuspended in cold methanol–water (1:1, v/v), sonicated twice for 30 s, and centrifuged at 21,000×g for 5 min at 4°C. The supernatant was collected and filtered prior to LC-TOF MS analysis.
The separation of the cinnamoyl anthranilates was conducted on ZIC-HILIC columns (150 or 250 mm length, 2.1 mm internal diameter, and 3.5 μm particle size; from Merck SeQuant, and distributed via The Nest Group, Inc., Southborough, MA, USA) using an Agilent Technologies 1200 Series HPLC system (Agilent Technologies, Santa Clara, CA, USA). The temperature of the sample tray was maintained at 4°C by an Agilent FC/ALS Thermostat. The column compartment was set to 40°C. Analytes were eluted isocratically with a mobile phase composition of 50 mM ammonium acetate in water and acetonitrile (2:8, v/v). A flow rate of 0.1 mL/min was used throughout.
The HPLC system was coupled to an Agilent Technologies 6210 time-of-flight mass spectrometer (LC-TOF MS), via a 1/3 post-column split. A LAN card was used to establish the contact between both instrument setups in order to trigger the MS into operation upon the initiation of a run cycle from the MassHunter workstation (Agilent Technologies). Electrospray ionization (ESI) was conducted in the negative ion mode, and a capillary voltage of −3,500 V was utilized. MS experiments were carried out in full scan mode at 0.85 spectra/s and a cycle time of 1.176 s, for the detection of [M–H]− ions. The instrument was tuned for a range of 50–1,700 m/z. Prior to LC-TOF MS analysis, the TOF MS was calibrated via an ESI-L-low concentration tuning mix (Agilent Technologies). Internal reference mass calibration was utilized throughout the chromatographic run via an API TOF reference mass solution kit (Agilent Technologies). Data acquisition and processing were performed by the MassHunter software package.
Quantifications of tranilast and N-(4′-Hydroxycinnamoyl)-anthranilate released in the culture medium and accumulated in yeast cells were made by comparison with a standard curve prepared in methanol–water (1:1, v/v).
Results
Expression analysis of the HCBT enzyme in recombinant yeast
To verify HCBT expression, we conducted immunoblotting analysis on crude protein extracts obtained from recombinant yeast strains harboring pDRf1-HCBT and pDRf1-4CL5-HCBT, respectively. As shown in Fig. 2, a specific signal corresponding to an approximately 53-kDa protein was detected only in protein extracts derived from the yeast strain harboring the HCBT gene, which is in accordance with the predicted size of HCBT tagged with the AttB2 peptide.
Expression analysis of HCBT. Recombinant yeast cells grown to an OD600 = 1 were harvested by centrifugation for protein extraction, and 5 μg of soluble protein was analyzed using immunoblotting techniques. For protein extracts obtained from cells harboring the pDRf1-4CL5-HCBT or pDRf1-HCBT vectors, recombinant tagged HCBT was detected around 53 kDa using the universal antibody and according to the position of known markers. Protein extracts from yeast cells harboring the pDRf1-4CL5-GW or pDRf1 empty vectors were also analyzed as negative controls
Production of N-(4′-hydroxycinnamoyl)-anthranilate by the recombinant yeast
The HCBT enzyme was previously shown to catalyze the condensation of coumaroyl-CoA and anthranilate to produce N-(4′-hydroxycinnamoyl)-anthranilate in vitro (Yang et al. 1997). Yeast harboring the pDRf1-4CL5-HCBT vector was consequently grown for 15 h in the presence of coumaric acid (300 μM) and anthranilate (500 μM) as precursors, and the medium was analyzed by LC-TOF MS for the detection of the N-(4′-hydroxycinnamoyl)-anthranilate product. Negative control cultures of yeast harboring pDRf1-4CL5, pDRf1-HCBT, or pDRf1 empty vectors were also conducted using the same precursors. LC-TOF MS analysis of the pDRf1-4CL5-HCBT yeast culture medium revealed a peak which was not present in control cultures and which corresponds to N-(4′-hydroxycinnamoyl)-anthranilate by comparison with an authentic standard solution (Fig. 3). The absence of N-(4′-hydroxycinnamoyl)-anthranilate in the yeast expressing 4CL5 alone or HCBT without 4CL5 confirmed the requirement of the 4-coumarate/CoA ligase to produce 4-hydroxycinnamoyl-CoA and showed that the yeast strain was unable to produce cinnamoyl anthranilates without the HCBT gene. Using these non-optimized culture conditions, the N-(4′-hydroxycinnamoyl)-anthranilate content in the culture medium was estimated to be 14.5 mg/L, which corresponds to a concentration of 51 μM and a conversion yield of 17% based on the starting concentration of coumaric acid. Additionally, the N-(4′-hydroxycinnamoyl)-anthranilate content inside yeast cells accounted for approximately 1.5% of that of the medium (data not shown).
Detection of N-(4′-hydroxycinnamoyl)-anthranilate from the recombinant yeast culture medium. ESI-MS spectra were obtained after LC-TOF MS analysis of a the culture medium of recombinant yeast incubated with anthranilate and coumaric acid and b an authentic N-(4′-hydroxycinnamoyl)-anthranilate solution
Evaluation of the recombinant yeast strain for the production of tranilast analogs and N-(hydroxycinnamoyl)-hydroxyanthranilates using known 4CL5 natural substrates
The tranilast drug corresponds to N-(3′,4′-dimethoxycinnamoyl)-anthranilic acid for which one group of analogs feature various substitutions on the cinnamoyl moiety. For the production of such tranilast analogs, three known 4CL5 substrates (ferulic acid, sinapic acid, and caffeic acid) and anthranilate were supplied independently as precursors to the culture medium of recombinant yeast. This approach allowed the biological production of three different tranilast analogs, namely N-(3′-methoxy-4′-hydroxycinnamoyl)-anthranilic acid, N-(3′,5′-dimethoxy-4′-hydroxycinnamoyl)-anthranilic acid, and N-(3′,4′-dihydroxycinnamoyl)-anthranilic acid, respectively (Table 1).
Furthermore, in an independent experiment, we supplied 3-hydroxyanthranilate in combination with p-coumaric, ferulic, sinapic, or caffeic acid to the medium of different recombinant yeast cultures since HCBT was also shown to use this anthranilate derivate as a substrate (Yang et al. 1997). Four new N-(hydroxycinnamoyl)-3-hydroxyanthranilates were detected in the media after 15 h of incubation of the recombinant yeast in presence of these precursors (Table 1). LC-TOF MS analysis of the culture medium from each feeding experiment showed unique peaks that were not present in yeast control cultures harboring a pDRf1-4CL5 vector and fed with the same precursors (Fig. S1). The masses were determined for each extracted ion chromatographic peak and compared with the theoretical masses of the predicted compounds that were expected to be produced based on the nature of the precursors used. In all cases, the measured masses agree with the expected theoretical masses within less than 3 ppm mass error. The compounds exhibited exact mass measurements with high mass accuracies, and as a result, the identity of each hydroxycinnamoyl anthranilate was confirmed with a high degree of confidence. Additionally, the seven new molecules produced had similar retention times ranging from 6.22 to 7.20 min (Table 1).
Production of tranilast and additional analogs using various cinnamic acids as precursors
Tranilast corresponds to the 4′-methoxylated form of N-(3′-methoxy-4′-hydroxycinnamoyl)-anthranilic acid, which is produced by our recombinant yeast strain when grown in presence of ferulic acid (3-methoxy-4-hydroxycinnamic acid) and anthranilic acid (Figs. 1a and S1). Therefore, in order to synthesize tranilast biologically, we fed the yeast strain with 3,4-dimethoxycinnamic acid and tested the potential for the heterologously expressed genes encoding 4CL5 and HCBT to produce and utilize 3,4-dimethoxycinnamoyl-CoA, respectively. The extracted ion chromatograms obtained after LC-TOF MS analysis of both a synthetic tranilast solution and the culture medium collected after feeding the recombinant yeast with anthranilate and 3,4-dimethoxycinnamic acid clearly confirmed tranilast production by the recombinant yeast (Fig. 4). Using these non-optimized culture conditions, the tranilast content in the medium was estimated to be 670 μg/L, which corresponds to a concentration of 2.05 μM and a conversion yield of 0.67% based on the starting concentration of 3,4-dimethoxycinnamic acid. The tranilast content inside the yeast cells accounts for approximately 3.5% of the quantity found in the medium (data not shown). This result demonstrates that 4CL5 is able to convert the unnatural substrate 3,4-dimethoxycinnamic acid into 3,4-dimethoxycinnamoyl-CoA, the latter being subsequently conjugated to anthranilate by HCBT to form tranilast.
In order to further explore the diversity of tranilast analogs that could potentially be biologically produced with the recombinant yeast strain harboring 4CL5 and HCBT, a large variety of cinnamic acids derivatives were co-fed individually with anthranilate or 3-hydroxyanthranilate. These included cinnamic acid, isoferulic acid, o-coumaric acid, m-coumaric acid, and the 4-methoxy-, 3-methoxy-, 2,3-dimethoxy-, 2,5-dimethoxy-, 2,4-dimethoxy-, 3,4,5-trimethoxy-cinnamic acid derivates. We postulated that the corresponding cinnamoyl-CoA thioesters potentially produced by 4CL5 could be used as substrates by HCBT. This approach successfully led to the production of 18 additional cinnamoyl anthranilates that could be accurately identified from the culture medium using LC-TOF MS (Table 2, Fig. S2).
Discussion
We investigated the potential for yeast to produce various cinnamoyl anthranilates. Using an engineered yeast strain, we demonstrated the feasibility of synthesizing biologically as many as 27 molecules, including the pharmaceutical drug tranilast (Fig. 5). The diversity of cinnamoyl anthranilates produced in this study reflects the broad substrate affinity of 4CL5 from Arabidopsis. The conversion of cinnamic acid, isoferulic acid, 4-methoxy-, 3-methoxy-, 3,4-dimethoxy-, 2,3-dimethoxy-, 2,5-dimethoxy-, 2,4-dimethoxy-, and 3,4,5-trimethoxycinnamic acids by 4CL5 has never been reported before, although it is well known that some 4CL in plants can accept these other substrates (Knobloch and Hahlbrock 1975; Funk and Brodelius 1990). After verifying that HCBT is active on p-coumaroyl-CoA and anthranilate in yeast (Fig. 3), we further showed its capacity to couple anthranilate and 3-hydroxyanthranilate to a broader range of cinnamoyl-CoA thioesters (Table 2, Fig. S2). Our data confirm earlier reports showing that HCBT has affinity for p-coumaroyl-CoA and cinnamoyl-CoA in vitro, as well as for 3-hydroxyanthranilate albeit that the conversion rate was 20% of that of anthranilate (Yang et al. 1997; Reinhard and Matern 1989).
p-Cinnamoyl-anthranilate, caffeoyl-anthranilate, and feruloyl-anthranilate produced in this study are oat-specific natural products named avenanthramide D, E, and F, respectively (Collins and Mullin 1988). In this work, we also report on newly characterized cinnamoyl-3-hydroxyanthranilates that are closely related to the cinnamoyl-5-hydroxyanthranilate avenanthramides found in oats (Collins 1989). Avenanthramides are present at low concentrations in oat groats (2.5–42 mg/kg) and are difficult to purify individually (Bratt et al. 2003). Radical-scavenging activity has been recently shown for a wide range of avenanthramides in vitro, as well as antioxidant and antigenotoxic activities (Fagerlund et al. 2009; Lee-Manion et al. 2009). For example, caffeoyl-5-hydroxyanthranilate (avn C) is capable of attenuating reactive oxygen species production in tissues of exercised rats and enhances activities of antioxidative enzymes (Ji et al. 2003). Furthermore, similar to tranilast, avenanthramides are known to exert various antiinflammatory and antiproliferative processes, which have the potential to contribute to beneficial physiological effects (Liu et al. 2004; Nie et al. 2006; Sur et al. 2008). For example, avn C was shown to have antiproliferative effects on inflammation processes that contribute to atherosclerosis and restenosis after angioplasty (Guo et al. 2008). Interestingly, it was recently shown that p-coumaroyl-3-hydroxyanthranilate and caffeoyl-3-hydroxyanthranilate had antioxidant activities similar to those of their corresponding cinnamoyl-5-hydroxyanthranilate derivatives (Moglia et al. 2010). These results suggest that the cinnamoyl-3-hydroxyanthranilates biologically produced with 4CL-HCBT recombinant yeast could have similar health benefits as cinnamoyl-5-hydroxyanthranilate. Notably, we were unable to produce any hydroxycinnamoyl-5-hydroxyanthranilates when our 4CL5-HCBT yeast strain was grown in the presence of 5-hydroxyanthranilate and p-coumaric acid, caffeic acid, ferulic acid, or sinapic acids (data not shown). This result suggests that either HCBT does not use 5-hydroxyanthranilate as a substrate or that the substrate is not transported into the yeast cells. Replacement of HCBT with the oat-derived HHT1 in our engineered yeast strain could potentially lead to the synthesis of hydroxycinnamoyl-5-hydroxyanthranilates since the HHT1 enzyme was shown to use feruloyl-CoA and 5-hydroxyanthranilate as substrates for the production of feruloyl-5-hydroxyanthranilate (Yang et al. 2004). Finally, dihydroavenanthramide D (DHAvD), a synthetic hydrogenated analog of p-hydroxycinnamoyl-anthranilate, was found to reduce histamine-related skin disorders such as itching, redness, and wheal. DHAvD is used as an active ingredient in cosmetic products and was also demonstrated to block the development of type 1 diabetes in cytokine-treated mice (Heuschkel et al. 2008, 2009; Lv et al. 2009). Consequently, all three structurally related hydroxycinnamoyl-anthranilates (i.e. m-, o-, and p- substituted) produced in this study could share similar health benefits, or alternatively, be used as direct precursors for chemical hydrogenation (Schmaus et al. 2006).
Our system allows the selective production of tranilast analogs, including 24 molecules that have never been identified from natural sources so far. Structural variability of cinnamoyl anthranilates is of particular interest to screen for derivatives with improved biological activity (Zammit et al. 2009). In this respect, additional molecules can potentially be biologically produced using the same strategy because the HCBT enzyme is known to accept other substrates such as benzoyl-CoA and salicyloyl-CoA (Yang et al. 1997); a plant 4CL enzyme active on benzoic acid was recently isolated and characterized in Arabidopsis (Kliebenstein et al. 2007).
The tranilast production presented in this study using yeast as a catalyst could be further optimized in various ways, in particular for the endogenous synthesis of anthranilate and 3,4-dimethoxycinnamic acid. Endogenous overproduction of anthranilate could be achieved directly from the conversion of glucose as recently demonstrated for an engineered strain of E. coli (Balderas-Hernández et al. 2009). Endogenous production of p-coumaric acid from phenylalanine can be accomplished in yeast by expressing plant phenylalanine-ammonia lyase, cinnamic acid 4-hydroxylase, and cytochrome P450 reductase genes (Vannelli et al. 2007; Trantas et al. 2009). Furthermore, expression of the Arabidopsis p-coumaric acid 3-hydroxylase (CYP98A3) gene in yeast allows the conversion of p-coumaric acid into caffeic acid (Nair et al. 2002). Caffeic acid represents a possible precursor for the production of 3,4-dimethoxycinnamic acid via two methoxylation reactions, which could be catalyzed by the bacterial O-methyltransferases SafC (Nelson et al. 2007). Alternatively, coexpression of one of the well-characterized plant caffeic acid 3-O-methyltransferases with ferulic acid 4-O-methyltransferase could lead to the production of 3,4-dimethoxycinnamic acid from caffeic acid. Highly active ferulic acid 4-O-methyltransferase has not been discovered yet; however, recent work based on site-directed mutagenesis allowed successful design of 4-O-methyltransferases with defined substrate specificities (Bhuiya and Liu 2010). Finally, optimizing 4CL activity for the conversion of 3,4-dimethyoxycinnamic acid should be considered to improve the biological synthesis of tranilast. The identification of residues involved in such activity already offers a potential for the engineering of 4CL enzymes (Lindermayr et al. 2003). All molecules produced by the recombinant yeast could be identified from the culture medium. This accumulation suggests the presence of an export mechanism potentially involving non-specific transporters. Identifying and over-expressing such transporters could further increase export of cinnamoyl anthranilates from yeast cells and would prevent any potential intracellular toxicity. A specific transporter could be also isolated from oats since cinnamoyl anthranilates typically accumulate in the oat cell wall (Okazaki et al. 2004).
Notes
Vector name derived from the Gateway manual, Invitrogen.
References
Azuma H, Banno K, Yoshimura T (1976) Pharmacological properties of N-(3′,4′-dimethoxycinnamoyl) anthranilic acid (N-5′), a new anti-atopic agent. Br J Pharmacol 58:483–488
Balderas-Hernández VE, Sabido-Ramos A, Silva P, Cabrera-Valladares N, Hernández-Chávez G, Báez-Viveros JL, Martínez A, Bolívar F, Gosset G (2009) Metabolic engineering for improving anthranilate synthesis from glucose in Escherichia coli. Microb Cell Fact 2:8–19
Bhuiya MW, Liu CJ (2010) Engineering monolignol 4-O-methyltransferases to modulate lignin biosynthesis. J Biol Chem 285:277–285
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Branduardi P, Fossati T, Sauer M, Pagani R, Mattanovich D, Porro D (2007) Biosynthesis of vitamin C by yeast leads to increased stress resistance. PLoS ONE 2:e1092
Bratt K, Sunnerheim K, Bryngelsson S, Fagerlund A, Engman L, Andersson RE, Dimberg LH (2003) Avenanthramides in oats (Avena sativa L.) and structure-antioxidant activity relationships. J Agric Food Chem 51:594–600
Chakrabarti R, Subramaniam V, Abdalla S, Jothy S, Prud’homme GJ (2009) Tranilast inhibits the growth and metastasis of mammary carcinoma. Anticancer Drugs 20:334–345
Chang MC, Keasling JD (2006) Production of isoprenoid pharmaceuticals by engineered microbes. Nat Chem Biol 2:674–681
Collins FW (1989) Oat phenolics: avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J Agric Food Chem 37:60–66
Collins FW, Mullin WJ (1988) High performance liquid chromatographic determination of avenanthramides, N-aroylanthranilic acid alkaloids from oats. J Chromatogr 445:363–370
Cui H, Gensini M, Kataria R, Twaddle T, Zhang J, Wadsworth S, Petrilli J, Rodgers K, diZerega G, Cooper K (2009) Reducing post-surgical adhesions utilizing a drug-enhanced device: sodium carboxymethylcellulose aqueous gel/poly(p-dioxanone) and tranilast. Biomed Mater 4:015001
Engels B, Dahm P, Jennewein S (2008) Metabolic engineering of taxadiene biosynthesis in yeast as a first step towards Taxol (paclitaxel) production. Metab Eng 10:201–206
Fagerlund A, Sunnerheim K, Dimberg LH (2009) Radical-scavenging and antioxidant activity of avenanthramides. Food Chem 113:550–556
Fosdick LS, Starke AC Jr (1940) Some alkamine esters of 4-acetylferulic and 3, 4-dimethoxycinnamic acids. J Am Chem Soc 62:3352–3355
Funk C, Brodelius PE (1990) Phenylpropanoid metabolism in suspension cultures of Vanilla planifolia Andr.: III. Conversion of 4-methoxycinnamic acids into 4-hydroxybenzoic acids. Plant Physiol 94:102–108
Gietz RD, Woods RA (2002) Tranformation of yeast by the LiAc/SS carrier DNA/PEG method. Methods Enzymol 350:87–96
Guo W, Wise ML, Collins FW, Meydani M (2008) Avenanthramides, polyphenols from oats, inhibit IL-1β-induced NF-kB activation in endothelial cells. Free Radical Biol Med 44:415–429
Guo T, Chen WQ, Zhang C, Zhao YX, Zhang Y (2009) Chymase activity is closely related with plaque vulnerability in a hamster model of atherosclerosis. Atherosclerosis 207:59–67
Hamberger B, Hahlbrock K (2004) The 4-coumarate:CoA ligase gene family in Arabidopsis thaliana comprises one rare, sinapate-activating and three commonly occurring isoenzymes. Proc Natl Acad Sci USA 101:2209–2214
Heuschkel S, Wohlrab J, Schmaus G, Neubert RH (2008) Modulation of dihydroavenanthramide D release and skin penetration by 1,2-alkanediols. Eur J Pharm Biopharm 70:239–247
Heuschkel S, Wohlrab J, Neubert RH (2009) Dermal and transdermal targeting of dihydroavenanthramide D using enhancer molecules and novel microemulsions. Eur J Pharm Biopharm 72:552–560
Horwitz SB (1994) How to make taxol from scratch. Nature 367:593–594
Isaji M, Miyata H, Ajisawa Y (1998) Tranilast: a new application in the cardiovascular field as an antiproliferative drug. Cardiovasc Drug Rev 16:288–299
Ji LL, Lay D, Chung E, Fu Y, Peterson DM (2003) Effects of avenanthramides on oxidant generation and antioxidant enzyme activity in exercised rats. Nutr Res 23:1579–1590
Kliebenstein DJ, D’Auria JC, Behere AS, Kim JH, Gunderson KL, Breen JN, Lee G, Gershenzon J, Last RL, Jander G (2007) Characterization of seed-specific benzoyloxyglucosinolate mutations in Arabidopsis thaliana. Plant J 51:1062–1076
Knobloch KH, Hahlbrock K (1975) Isoenzymes of p-coumarate: CoA ligase from cell suspension cultures of Glycine max. Eur J Biochem 52:311–320
Komatsu H, Kojima M, Tsutsumi N, Hamano S, Kusama H, Ujiie A, Ikeda S, Nakazawa M (1988) Study of the mechanism of inhibitory action of tranilast on chemical mediator release. Jpn J Pharmacol 46:43–51
Konneh M (1998) Tranilast, kissei pharmaceuticals. IDrugs 1:141–146
Kunkel TA (1985) Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci USA 82:488–492
Lalonde S, Sero A, Pratelli R, Pilot G, Chen J, Sardi MI, Parsa SA, Kim D-Y, Acharya BR, Stein EV, Hu H-C, Villiers F, Takeda K, Yang Y, Han YS, Schwacke R, Chiang W, Kato N, Loqué D, Assmann SM, Kwak JM, Schroeder JI, Rhee SY, Frommer WB (2010) A membrane protein/signaling protein interaction network for Arabidopsis version AMPv2. Front Physio 1:24. doi:10.3389/fphys.2010.00024
Lee-Manion AM, Price RK, Strain JJ, Dimberg LH, Sunnerheim K, Welch RW (2009) In vitro antioxidant activity and antigenotoxic affects of avenanthramides and related compounds. J Agric Food Chem 57:10619–10624
Limem I, Guedon E, Hehn A, Bourgaud F, Ghedira L, Engasser J-M, Ghoul M (2008) Production of phenylpropanoid compounds by recombinant microorganisms expressing plant-specific biosynthesis genes. Process Biochem 43:463–479
Lindermayr C, Fliegmann J, Ebel J (2003) Deletion of a single amino acid residue from different 4-coumarate:CoA ligases from soybean results in the generation of new substrate specificities. J Biol Chem 278:2781–2786
Liu L, Zubik L, Collins FW, Marko M, Meydani M (2004) The antiatherogenic potential of oat phenolic compounds. Artherosclerosis 175:39–49
Loqué D, Lalonde S, Looger LL, von Wirén N, Frommer WB (2007) A cytosolic trans-activation domain essential for ammonium uptake. Nature 446:195–198
Lv N, Song MY, Lee YR, Choi HN, Kwon KB, Park JW, Park BH (2009) Dihydroavenanthramide D protects pancreatic beta-cells from cytokine and streptozotocin toxicity. Biochem Biophys Res Commun 387:97–102
Moglia A, Comino C, Lanteri S, de Vos R, de Waard P, van Beek TA, Goitre L, Retta SF, Beekwilder J (2010) Production of novel antioxidative phenolic amides through heterologous expression of the plant’s chlorogenic acid biosynthesis genes in yeast. Metab Eng 12:223–232
Mukai N, Masaki K, Fujii T, Kawamukai M, Lefuji H (2010) PAD1 and FDC1 are essential for the decarboxylation of phenylacrylic acids in Saccharomyces cerevisiae. J Biosci Bioeng 109:564–569
Nair RB, Xia Q, Kartha CJ, Kurylo E, Hirji RN, Datla R, Selvaraj G (2002) Arabidopsis CYP98A3 mediating aromatic 3-hydroxylation. Developmental regulation of the gene, and expression in yeast. Plant Physiol 130:210–220
Nelson JT, Lee J, Sims JW, Schmidt EW (2007) Characterization of SafC, a catechol 4-O-methyltransferase involved in saframycin biosynthesis. Appl Environ Microbiol 73:3575–3580
Nie L, Wise ML, Peterson DM, Meydani M (2006) Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis 186:260–266
Ogawa Y, Dogru M, Uchino M, Tatematsu Y, Kamoi M, Yamamoto Y, Ogawa J, Ishida R, Kaido M, Hara S, Matsumoto Y, Kawakita T, Okamoto S, Tsubota K (2010) Topical tranilast for treatment of the early stage of mild dry eye associated with chronic GVHD. Bone Marrow Transplant 45:565–569
Okazaki Y, Isobe T, Iwata Y, Matsukawa T, Matsuda F, Miyagawa H, Ishihara A, Nishioka T, Iwamura H (2004) Metabolism of avenanthramide phytoalexins in oat. Plant J 39:560–572
Okuda M, Ishikawa T, Saito Y, Shimizu T, Baba S (1984) A clinical evaluation of N-5′ with perennial-type allergic rhinitis—a test by the multi-clinic, intergroup, double-blind comparative method. Ann Allergy 53:178–185
Oshitani N, Yamagami H, Watanabe K, Higuchi K, Arakawa T (2007) Long-term prospective pilot study with tranilast for the prevention of stricture progression in patients with Crohn’s disease. Gut 56:599–600
Pae HO, Jeong SO, Koo BS, Ha HY, Lee KM, Chung HT (2002) Tranilast, an orally active anti-allergic drug, up-regulates the anti-inflammatory heme oxygenase-1 expression but down-regulates the pro-inflammatory cyclooxygenase-2 and inducible nitric oxide synthase expression in RAW264.7 macrophages. Biochem Biophys Res Commun 371:361–365
Park M, Kang K, Park S, Kim YS, Ha S-H, Lee SW, Ahn M-J, Bae J-M, Back K (2008) Expression of serotonin derivative synthetic genes on a single self-processing polypeptide and the production of serotonin derivatives in microbes. Appl Microbiol Biotechnol 81:43–49
Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, Gupta R, Lee LY, Kidd BA, Robinson WH, Sobel RA, Selley ML, Steinman L (2005) Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science 310:850–855
Ponchet M, Favre-Bonvin J, Hauteville M, Ricci P (1988) Dianthramides (N-benzoyl and N-paracoumarylanthranilic acid derivatives) from elicited tissues of Dianthus caryophyllus. Phytochemistry 27:725–730
Prud’homme GJ (2007) Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab Invest 87:1077–1091
Reinhard K, Matern U (1989) The biosynthesis of phytoalexins in Dianthus caryophyllus L. cell cultures: induction of benzoyl-CoA:anthranilate N-benzoyltransferase activity. Arch Biochem Biophys 275:295–301
Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, Keasling JD (2006) Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature 440:940–943
Schmaus G, Joppe H, Herrmann M, Sabater-Luntzel C, Vossing T (2006) Anthranilic acid amides and derivatives thereof as cosmetic and pharmaceutical agents. U.S. Patent 20060089413
Shiota N, Kovanen PT, Eklund KK, Shibata N, Shimoura K, Niibayashi T, Shimbori C, Okunishi H (2010) The anti-allergic compound tranilast attenuates inflammation and inhibits bone destruction in collagen-induced arthritis in mice. Br J Pharmacol 159:626–635
Sun X, Suzuki K, Nagata M, Kawauchi Y, Yano M, Ohkoshi S, Matsuda Y, Kawachi H, Watanabe K, Asakura H, Aoyagi Y (2010) Rectal administration of tranilast ameliorated acute colitis in mice through increased expression of heme oxygenase-1. Pathol Int 60:93–101
Sur R, Nigam A, Grote D, Liebel F, Southall MD (2008) Avenanthramides, polyphenols from oats, exhibit anti-inflammatory and anti-itch activity. Arch Dermatol Res 25:1–6
Szczebara FM, Chandelier C, Villeret C, Masurel A, Bourot S, Duport C, Blanchard S, Groisillier A, Testet E, Costaglioli P, Cauet G, Degryse E, Balbuena D, Winter J, Achstetter T, Spagnoli R, Pompon D, Dumas B (2003) Total biosynthesis of hydrocortisone from a simple carbon source in yeast. Nat Biotechnol 21:143–149
Tamai H, Katoh K, Yamaguchi T, Hayakawa H, Kanmatsuse K, Haze K, Aizawa T, Nakanishi S, Suzuki S, Suzuki T, Takase S, Nishikawa H, Katoh O (2002) The impact of tranilast on restenosis after coronary angioplasty: the Second Tranilast Restenosis Following Angioplasty Trial (TREAT-2). Am Heart J 143:506–513
Tan SM, Zhang Y, Cox AJ, Kelly DJ, Qi W (2010) Tranilast attenuates the up-regulation of thioredoxin-interacting protein and oxidative stress in an experimental model of diabetic nephropathy. Nephrol Dial Transplant. doi:10.1093/ndt/gfq355
Trantas E, Panopoulos N, Ververidis F (2009) Metabolic engineering of the complete pathway leading to heterologous biosynthesis of various flavonoids and stilbenoids in Saccharomyces cerevisiae. Metab Eng 11:355–366
Vannelli T, Wei Qi W, Sweigard J, Gatenby AA, Sariaslani FS (2007) Production of p-hydroxycinnamic acid from glucose in Saccharomyces cerevisiae and Escherichia coli by expression of heterologous genes from plants and fungi. Metab Eng 9:142–151
Wieczorke R, Krampe S, Weierstall T, Freidel K, Hollenberg CP, Boles E (1999) Concurrent knock-out of at least 20 transporter genes is required to block uptake of hexoses in Saccharomyces cerevisiae. FEBS Lett 464:123–128
Winzeler EA, Shoemaker DD, Astromoff A et al (1999) Functionnal characterization of the S. cerevisiae genome by deletion and parallel analysis. Science 285:901–906
Yang Q, Reinhard K, Schiltz E, Matern U (1997) Characterization and heterologous expression of hydroxycinnamoyl/benzoyl-CoA:anthranilate N-hydroxycinnamoyl/benzoyltransferase from elicited cell cultures of carnation, Dianthus caryophyllus L. Plant Mol Biol 35:777–789
Yang Q, Trinh HX, Imai S, Ishihara A, Zhang L, Nakayashiki H, Tosa Y, Mayama S (2004) Analysis of the involvement of hydroxyanthranilate hydroxycinnamoyltransferase and caffeoyl-CoA 3-O-methyltransferase in phytoalexin biosynthesis in oat. Mol Plant-Microb Interact 17:81–89
Zammit SC, Cox AJ, Gow RM, Zhang Y, Gilbert RE, Krum H, Kelly DJ, Williams SJ (2009) Evaluation and optimization of antifibrotic activity of cinnamoyl anthranilates. Bioorg Med Chem Lett 19:7003–7006
Acknowledgments
This work was part of the DOE Joint BioEnergy Institute (http://www.jbei.org/) supported by the U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, through contract DE-AC02-05CH11231 between Lawrence Berkeley National Laboratory and the U.S. Department of Energy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1426 kb)
Rights and permissions
About this article
Cite this article
Eudes, A., Baidoo, E.E.K., Yang, F. et al. Production of tranilast [N-(3′,4′-dimethoxycinnamoyl)-anthranilic acid] and its analogs in yeast Saccharomyces cerevisiae . Appl Microbiol Biotechnol 89, 989–1000 (2011). https://doi.org/10.1007/s00253-010-2939-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2939-y