Abstract
The development of biotechnological processes using novel two-phase systems based on molten salts known as ionic liquids (ILs) got into the focus of interest. Many new approaches for the beneficial application of the interesting solvent have been published over the last years. ILs bring beneficial properties compared to organic solvents like nonflammability and nonvolatility. There are two possible ways to use the ILs: first, the hydrophobic ones as a substitute for organic solvents in pure two-phase systems with water and second, the hydrophilic ones in aqueous two-phase systems (ATPS). To effectively utilise IL-based two-phase systems or IL-based ATPS in biotechnology, extensive experimental work is required to gain the optimal system parameters to ensure selective extraction of the product of interest. This review will focus on the most actual findings dealing with the basic driving forces for the target extraction in IL-based ATPS as well as presenting some selected examples for the beneficial application of ILs as a substitute for organic solvents. Besides the research focusing on IL-based two-phase systems, the “green aspect” of ILs, due to their negligible vapour pressure, is widely discussed. We will present the newest results concerning ecotoxicity of ILs to get an overview of the state of the art concerning ILs and their utilisation in novel two-phase systems in biotechnology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In biotechnology, two-phase systems consisting of organic solvent and water are often used as substrate reservoir or for product recovery. Major challenges using this technique are the inactivation of the biocatalyst and the environmental pollution by the organic solvent. A promising alternative for the application of organic solvents in biotechnology are ionic liquids (ILs).
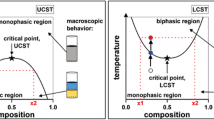
ILs are salts with unique physical properties, such as a negligible vapour pressure, low viscosity and high thermal stability, depending on their structure. Below 100 °C, ILs exist in the liquid state (Seddon 1997). Walden (1914) described the compound ethylammonium nitrate as the first known IL in 1914. For the next 70 years, there was no interest in the research on this topic. It was not until the 1980s when new ILs like metal halide anions or aluminates were studied (Wilkes et al. 1982). These ILs are very unstable against water and therefore not in the focus of interest for application in biotechnology (Fig. 1). With the beginning of the new century, a new generation of water-stable ILs with anions like [BF4], [PF6], [Tf2N] or halides were synthesised (Wasserscheid and Welton 2008). There are many examples for the use of these kind of ILs in biocatalysis (e.g., Kragl et al. 2002, Sheldon et al. 2002, Park and Kazlauskas 2003, Pinto et al. 2008). But not only catalysis, also product recovery is an important part of any biotechnological production. The manufacturing costs of a product depend largely on the costs emerging during extraction and purification processes. Therefore, there always is a demand for new, fast, simple and nonpolluting downstream processing techniques in the industrial biotechnology. Aqueous two-phase systems (ATPS) offer an attractive alternative to conventional extraction methods for the separation of biomolecules. ATPS are usually formed by mixing two polymers or a polymer and a salt with water. A phase separation occurs when the two polymers or the polymer and the salt are incompatible. The driving force of the phase separation in this solvent system is the enthalpy of the interactions between the polymer molecules. If the interaction enthalpy is bigger than the loss in entropy according to the phase separation, the ATPS is formed (Flory 1953). These ATPS are used for product recovery and downstream processing in biotechnology, e.g. the recovery of enzymes from fermentation media (Naganagouda and Mulimani 2008). It was shown that not only polymers like poly-ethylene-glycole (PEG) or Dextran but also ILs in combination with a cosmothrophic inorganic salt are able to form ATPS (Shehong et al. 2005). Against the background of the recently shown enhanced stability, activity and enantioselectivity of enzymes in aqueous IL solutions, IL-based ATPS are particularly suitable for the extraction of biocatalysts or their products (Dreyer and Kragl 2008, Dreyer and Kragl 2009). There are also recent examples for the recovery of biomolecules and catalytic products from aqueous media by using a pure IL phase (Jiang et al. 2007). One-phase systems with various amounts of water and ILs will be not treated here; the reader is referred to the original papers or reviews (van Rantwijk and Sheldon 2007, Kragl et al. 2002).
Hydrophilic IL-based ATPS
Proteins are one of the most important biotechnological products on the world market. They are widely used as pharmaceuticals like insulin or somatropin and as detergent supplement like proteases and lipases. More and more industry applies enzymes as biocatalysts for effective and ecofriendly production of chiral and very pure compounds like alcohols, sugars or lipids. These biotechnologically very interesting proteins are mostly produced by microorganisms like yeast or Echerichia coli in bioreactors. To recover the target proteins from the fermentation broth, several different recovery protocols exist, depending on the target protein and the desired grade of purity. The extraction with classic(PEG)-based ATPS is already used in the industry (Rito-Palomares 2004). To combine the beneficial properties of ILs and PEG with respect to the well-known stabilisation of active enzymes in solution by ILs as well as by PEG, the design of IL-based ATPS is crucial. For the efficient development of protein recovery protocols, a prediction of the possible partitioning behaviour of different model proteins is desirable. Therefore, it is necessary to understand the driving forces of protein partitioning in IL-based ATPS.
Susanne Dreyer and coworkers experimented with the IL Ammoeng™ 110 which consists of cations with oligo-ethylene groups (Dreyer and Kragl 2008). It was shown that it is (Fig. 2) possible to efficiently form ATPS with Ammoeng™ 110 and K2HPO4/KH2PO4 as an organic buffer salt. The partitioning behaviour of four model proteins [bovine serum albumin (BSA), lysozyme, trypsin and myoglobin] at different temperatures and pH values was investigated. The published data show that the surface charge of the proteins and the molecular weight are the most important factors influencing the partitioning behaviour of the proteins. Bigger proteins like BSA are extracted into the IL-containing phase, while smaller proteins like myoglobin stay in the buffer phase. It was also shown that the negative charges on the protein surface have a strong influence on the partitioning behaviour of the protein itself (Dreyer and Kragl 2009). The suggested model to describe partitioning behaviour of proteins in IL-based ATPS states that electrostatic interactions between negatively charged amino acid residues on the protein’s surface and the cation of the IL are the driving forces for the extraction (Fig. 2; Dreyer and Kragl 2008). Therefore, it is possible to adapt the system for the extraction of different target proteins by changing the pH value of the system. The optimal parameters for a specific target protein can be calculated. It was not only possible to extract an alcohol dehydrogenase from cell extract, but even to use the IL- and enzyme-containing phase to perform catalytic reactions with higher product yields and higher enzyme stability compared to pure buffer systems (Dreyer and Kragl 2008).
Zhuo et al. (2007) extracted BSA using an aqueous two-phase system based on 1-butyl-3-methylimidazolium chloride ([Bmim][Cl]) and K2HPO4. They showed that there are no chemical reactions between [Bmim][Cl] and BSA during the extraction process. Their protein-partitioning model also identifies the electrostatic interactions between the positively charged [Bmim]+ and the negatively charged BSA surface as one of the main driving forces for the extraction. With increasing K2HPO4 concentration in the lower phase of the ATPS, they also observed a salting-out effect of the BSA into the IL-rich upper phase. According to their model, the salting-out effect and the electrostatic interactions are the most important driving forces for protein partitioning in IL-based ATPS.
Contrary to these findings, a study by Pei et al. (2009) suggests hydrophobic interactions as the main driving force for protein extraction into the IL-containing phase of the ATPS, while electrostatic interactions and salting-out only assist during the extraction process. The group experimented with imidazolium-based ILs which differed in the number of C-atoms in the cation ([C4mim]Br, [C6mim][Br], [C8mim][Br]). They studied the effect of temperature on the extraction efficiency of BSA in an IL-based ATPS (Pei et al. 2009). A higher temperature favours protein extraction into the IL-containing phase. The researchers concluded that the extraction process is endothermic. They calculated that during the extraction process, the ∆G t value is negative while the ∆H t value and the T∆S t value are positive (Pei et al. 2009). They also calculated that the T∆S t value is always greater than the ∆H t value, so the reaction is controlled by entropy changes, which is characteristic for hydrophobic interactions (Pei et al. 2009). They also studied possible conformational changes of Trypsin and BSA during the extraction. There were no major conformational changes or chemical reactions between the IL and the protein.
ATPS can be a powerful tool for protein extraction in combination with biocatalytical reactions. Depending on the properties of the target protein’s surface, it seems to be possible to judge on how to compose a suitable ATPS.
Besides the important research on protein extraction with IL-based ATPS, other groups investigate the recovery of antibiotics with these new systems. Antibiotics are drugs which help to contain bacterial infections and are prescribed very often. Penicillin is one of the most widely used antibiotics. It is produced in fed-batch fermentation by the fungus Penicillium crysogenum (Patnaik 2001). Because penicillin is a pharmaceutical applied to humans, a very high purity after the downstream processing is crucial. The product is mostly purified by liquid extraction with butyl acetate as extractant.
Lui et al. (2005) extracted penicillin G from fermentation broth with an ATPS based on [Bmim][BF4] and NaH2PO4 as inorganic salt. They investigated the effects of pH value and the concentration of the phase-forming components on the extraction efficiency of the ATPS. With increasing phosphate concentration in the lower phase, a salting-out effect occurs and over 90% of the penicillin G is extracted into the IL-containing upper phase (Lui et al. 2005). By increasing the [Bmim][BF4] concentration in the upper phase up to 40% (v/v) they reached the maximum yield of extraction. Contrary to the standard penicillin G extraction process with butyl acetate, which is carried out at pH values around 2, the IL-based ATPS extraction is performed at pH values between 4 and 5 (Lui et al. 2005). Because penicillin G is not very stable in hydrophilic environment under acidic conditions, this new extraction method is a gentler possibility to recover the product. One year later, the same group optimised this extraction system for penicillin G with [Bmim][Cl] as IL (Lui et al. 2006). Again, they successfully showed that it is possible to extract penicillin G from filtrated fermentation broth. They reached the maximum extraction yield of 93% with 40% (w/w) NaH2PO4∙2H2O, 50,000 u/ml penicillin G and 20% (w/w) [Bmim][Cl] (Lui et al. 2006).
Another approach to use ILs in ATPS to support antibiotic extraction was realised by Jiang and coworkers. They first applied a conventional ATPS formed by NaH2PO4 buffer and the polymer imidazolium-PEG (I-PEG) to extract penicillin G from fermentation media (Yangyang et al. 2009). One of the main challenges using ATPS for product recovery is to recover the phase-building compounds; in this case, the I-PEG. In a classic approach, one would precipitate the phase-building compound with an organic solvent and centifugate or use a pH-sensitive or temperature-sensitive polymer (Annunziata et al. 2000, Harris et al. 1991). With those methods, the phase-forming compounds are effectively removed, but the application is very expensive because the compounds cannot be recycled and reused. The group developed a three-step process in which the I-PEG is removed by addition of the IL [Bmim][PF6] (Yangyang et al. 2009). In the first step, they used a classic ATPS to extract the antibiotic as described above. In the second step, they gave the IL to the polymer-containing phase of the ATPS. At a pH value of 8–9 the IL extracts the I-PEG via hydrophobic interaction into a new phase (Yangyang et al. 2009). After the removal of the antibiotic-containing upper phase they introduced in a third step fresh water at pH value of 5.5–6 (Yangyang et al. 2009). I-PEG is protonised and extracted from the IL phase into the fresh water phase (Yangyang et al. 2009). The big advantage of the system is the ability to recycle all of the polymer and the added IL.
The antibiotic recovery with ATPS can be an effective and cost-reducing alternative to the classic extraction methods with organic solvents. The purity and extraction yield of the product recovery is equal to classic methods and the added phase-forming compounds can easily be recycled. Nevertheless a lot of research has still to be done on the topic, especially for upscaling and getting more data for various types of antibiotics.
Hydrophobic IL-based two-phase systems
An alternative, very interesting field of research is the application of ILs in two-phase systems with a pure IL phase. Here hydrophobic ILs often replace organic solvents in extraction or biocatalysis.
Jiang et al. (2007) developed a process for the separation, recovery and hydrolysis of penicillin G using two different ILs and phosphate buffer. The enzymatic hydrolysis of penicillin G by penicillin acylase is an important reaction to access 6-aminopenicillanic acid (6-APA), a resource for the production of semisynthetic antibiotics like ampicillin (Jiang et al. 2007). The commonly used integrated process is a two-phase system based on aqueous buffer and butyl acetate. The reaction occurs in the buffer phase while the by-product of the hydrolysis, phenylacetic acid (PAA), is extracted into the organic phase (Jiang et al. 2007). The main problem with this kind of process is the low pH value (4) which decreases the stability of the enzyme and even inhibits its activity. To overcome these disadvantages, the group replaced the organic solvent butyl acetate with ILs (Jiang et al. 2007). In a first step a IL-based ATPS is formed with phosphate buffer and the hydrophilic IL [C4mim][BF4] to extract penicillin into the IL-containing upper phase (Jiang et al. 2007). In a second step, the more hydrophobic IL [C4mim][PF6] is added to the upper phase of the ATPS. Phase separation occurs and a new two-phase system with an IL phase, containing both different ILs, and an aqueous phase, containing the enzyme and penicillin, is formed (Jiang et al. 2007). The reaction takes place in the buffer phase and the by-product PAA is extracted into the IL phase. After the hydrolysis is completed, the hydrophilic [C4mim][BF4] is recovered by raising the temperature of the system and can be reused to form a new ATPS, the residual [C4mim][PF6] can be reused to form a new two-phase system (Jiang et al. 2007). The great advantage of this new process is that the reaction system now has a pH value of 5, which is beneficial for the activity and stability of the penicillin acylase (Jiang et al. 2007). Again, the replacement of organic solvent by IL has proven to be a powerful tool to optimise a process.
Soto et al. (2005) presented a new liquid–liquid extraction method based on NaH2PO4 buffer and the IL 1-methyl-3-octylimidazolium tetrafluoroborate ([Omim][BF4]) for ampicillin and amoxicillin. They tested the partition coefficient at different pH values and showed that extraction of the antibiotics into the IL phase is more effective at higher pH values. They concluded that the reason for this finding is the fact that both antibiotics exist in their anionic forms at pH 8, carrying two negative charges each (Soto et al. 2005). At lower pH values, the antibiotics are in their zwitterionic forms carrying a positive and a negative charge. These results support the theory that electrostatic interactions between the anionic form of the antibiotic and the [Omim]+ cation of the IL is the main driving force for the extraction process (Soto et al. 2005).
Four years earlier, Cull et al. (2000) successfully proved that the commonly used organic solvent butyl acetate, used for the erythromycine-A liquid–liquid extraction, can be replaced by the IL [Bmim][BF4]. The extraction efficiency with butyl acetate is best at pH values higher than the pK value of erytromycine A, because the molecule is better soluble in organic solvents when it is deprotonated (Cull et al. 2000). This is not the case for the extraction with the IL. At pH values higher than 9, the erythromycine A retransfers into the buffer phase (Cull et al. 2000). Therefore, it is possible to use this behaviour for antibiotic recovery from the [Bmim][BF4] phase by removing the old buffer phase and adding a new buffer with a pH value over 9 in a third step (Cull et al. 2000). It was shown that the IL [Bmim][BF4] can easily replace butyl acetate in liquid–liquid extraction of erythromycine A and even supports an easy way for product recovery from the IL phase by just switching the pH value (Cull et al. 2000).
Another target of interest in liquid–liquid extractions are amino acids. Wang et al. (2005) experimented with imidazolium based ILs ([C4mim][PF6], [C6mim][PF6], [C6mim][BF4]) for the extraction of five model amino acids (tryptophan, phenylalanine, tyrosine, leucine and valine) from aqueous buffer. They tested the partitioning coefficient at different pH values. The presented data shows that the best extraction into the IL phase occurs when the pH value of the buffer is higher than the pK2 value of the tested amino acid (Wang et al. 2005). Because the amino acids switch to their anionic form at high pH values, the data indicate that electrostatic interaction between the [C4mim]+ cation and the anionic form of the amino acid is a main driving force for amino acid partitioning (Wang et al. 2005). The researchers also found that the partitioning coefficients of the amino acids into [C6mim]PF6 are much higher than into [C6mim]BF4 under the same conditions, especially at low pH values (Wang et al. 2005). According to the scientists, this indicates that the anion of the IL has an important role during the extraction process. The higher charge density of BF -4 compared to PF -6 results in a higher extraction of the cationic amino acid form into the IL phase (Wang et al. 2005). They did not find any correlation between the polarity of the tested ILs and the partition coefficients (Wang et al. 2005). The pH value of the aqueous phase has the greatest influence on the partitioning behaviour of the amino acids (Wang et al. 2005). It was concluded that the electrostatic interactions between the cationic form of the amino acids and the anion of the IL are responsible for the extraction at low pH value (Wang et al. 2005).
To conclude, the wide field of extraction methods using ILs, it is important to know that still research has to be done concerning the molecular driving forces that are responsible for the extraction process. Most likely it seems to be a mix of electrostatic and hydrophobic interactions between the target molecule and the IL, depending on which IL is used to form the two-phase system and on the chemical properties of the target molecule (Fig. 3; Dreyer and Kragl 2008, Pei et al. 2009).
The IL Ammoeng™ 110 is hydrophilic and in aqueous solution the cation interacts with negatively charged chemical groups of the target molecule (Fig. 3; Dreyer and Kragl 2009). The more hydrophobic IL [C8mim] [Br] shows a different behaviour. For it has a long alkyl chain here, the hydrophobic interactions between the IL cation and the target molecule are the main driving force for the extraction process (Fig. 3; Pei et al. 2009).
Non-water-miscible ILs are also of interest as a secondphase solvent for biocatalytic reactions. We firstly described the imidazolium-based IL [Bmim] [(CF3SO2)2N] and compared it to methyl tert-butyl ether (MTBE) in a biphasic reaction system with phosphate buffer in the alcohol dehydrogenase (ADH) catalysed reduction of prochiral 2-octanone to (R)-2-octanol (Eckstein et al. 2004). It was shown that the reaction is much faster in the system containing the IL compared to that containing the organic solvent (Eckstein et al. 2004). After the identification of the partitioning coefficients, it was shown that the coefficients for the substrates 2-propanol and acetone did not differ in the MTBE/buffer system (Eckstein et al. 2004). Contrary to that, in the IL/buffer system, acetone is extracted into the IL phase. It was concluded that the faster reaction is the result of the more effective cofactor recycling due to the extraction of acetone into the IL phase. Because of that, the equilibrium of the NADP+ regeneration in the aqueous phase switches towards the oxidation of 2-propanol and new NADPH is regenerated faster compared to the MTBE/buffer system (Eckstein et al. 2004). ILs can be a beneficial substitute for organic solvents in biphasic reaction systems.
Hydrophobic ILs can also be applied in whole-cell biocatalysis as shown by Weuster-Botz and coworkers in 2007 and 2004. They tested the biocatalytic conversion of ketones to alcohol with ADH from Lactobacillus brevis with different recombinant cells of yeast, E. coli and Lactobacillus kefir (Pfruender et al. 2004, Weuster-Botz 2007). Like in the Eckstein paper, the IL phase is used as a substrate reservoir and for the in situ extraction of the hydropohobic products. The membrane integrity of the microorganisms as well as the reaction yield were tested with the ILs [Bmim][PF6] [Bmim][NTF] and [Oma][NTF] and were compared to different organic solvents like MTBE, n-octanol, diisopropylether and n-decanol (Weuster-Botz 2007). In all cases the membrane integrity in the IL-based systems was significantly higher compared to the organic solvent-based systems and therefore less toxic towards the bacteria. The yield of the reaction was about ten times higher in the IL-based systems compared to a pure aqueous buffer system and even 100 times higher than in the MTBE/buffer system (Weuster-Botz 2007). It was shown, that the tested ILs are less toxic towards the cells than the organic solvents.
Lou et al. (2009) tested different water immiscible ILs in a two-phase whole-cell bioreduction of 4-(trimethylsilyl)-3-butyn-2-one (TMSB) to (S)-4-(trimethylsilyl)-3-butyn-2-ol (S-TMSBOL) by Candida parapsilosis cells. In this system the IL phase again serves as substrate reservoir and for the in situ extraction of the product. They analysed ILs with methylimidazolium-based cations with different alkyl-chain length (C2 to C7) and [PF6] or [Tf2N] as anion. It was shown that the yield of product S-TMSBOL is about 85% in [C4Mim][PF6]/buffer two-phase system while the yield in pure buffer is only around 33% (Lou et al. 2009). With increasing length of the alkyl chain in the cation of the IL, the reaction yield slightly decreases. The authors conclude that this is due to the higher viscosity of the IL phase and therefore the decrease of the substrate and product mass transfer rate between the phases (Lou et al. 2009). They also showed the beneficial effect that the toxicity of the substrate and the product towards the cells is reduced in the IL-based two-phase system compared to the aqueous buffer system. This happens because the substrate concentration in the aqueous phase is severely lower in the two-phase system (Lou et al. 2009). The better performance of the whole-cell biocatalysis in the IL-based two-phase system is due to excellent solvent probabilities of the IL towards the Substrate and the product as well as the good biocompatibility of the IL itself (Lou et al. 2009).
ILs - a green solvent?
In the beginning, the green aspect of the ILs has often been picked out as a central benefit of ILs compared to organic solvents. Due to the negligible vapour pressure of the ILs, they are less air-polluting than organic solvents. On the other hand, there are various kinds of organic solvents used during the IL production or recycling process, which reduces the ecofriendliness of the application of ILs in biotechnological processes (Zhu et al. 2009). For a final evaluation, a complete life cycle analysis has to be done when an IL turned out to yield the superior process. Of special importance will be the ILs behaviour in the environment, especially its degradation behaviour. So far, only a few reliable results are available on this matter (Zhang et al. 2010). More information is available on the acute toxicity of ILs especially for water living organisms (Ranke et al. 2007, Wells and Coombe 2006). The research on the toxicity of imidazolium and pyridinium based ILs revealed that the length of the alkyl chain play an important role (Table 1). It was shown, that with increasing hydrophobicity of the cation, the IL gets more toxic (Docherty and Kulpa 2005). However, some of the early studies were hampered by impurities in the ILs. Therefore, the search for novel types of more ecofriendly ILs is still ongoing. One approach to reduce the toxicity of ILs is the application of natural educts like amino acids or sugars for IL synthesis. Several novel types of ILs were realised by Ohno and Fukumoto (2007) and by Tao et al. (2005) using amino acids, while Handy and Okello (2003) reports the application of fructose-based ILs in organic synthesis. Another approach is to reduce the use of organic solvents during the IL synthesis. Synthesis of [Amim]-based ILs was achieved without organic solvent using a microwave (Keskin et al. 2007). Despite these successes, still much research on the field of biodegradability of ILs as well as on their behaviour in the environment has to be done to further foster the application of these promising solvents (Gathergood et al. 2006).
The presented examples for the beneficial use of ILs in biocatalysis and product recovery show that, in many cases, the substitution of flammable and toxic organic solvents is not only good for the environment but also result in higher yields or an easier product recovery compared to the classic systems. Even if ILs can perform better than organic solvents in biotechnological applications, much research needs to be done, especially concerning the recovery of the IL itself due to its high price. In addition, upscaling and adaptation of the newly developed processes to larger scale has to be done. For volatile compounds, separation from the IL is easy. Otherwise, extraction or membrane filtration might be used. Nevertheless, the research in the field of ILs for extraction and biocatalysis is very interesting and the first results presented here are very promising and let us look forward for the findings to come.
References
Annunziata R, Benaglia M, Cinquini M, Cozzi F, Tocco G (2000) A poly(ethylene glycol)-supported quaternary ammonium salt: an efficient, recoverable, and recyclable phase-transfer catalyst. Org Lett 2(12):1737–1739
Cull SG, Holbrey JD, Vargas-Mora V, Seddon KR, Lye G (2000) Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol Bioeng 69(2):227–233
Docherty KM, Kulpa CF (2005) Toxicity and antimicrobial activity of imidazolium and pyridinium ionic liquids. Green Chem 7:185–189
Dreyer S, Kragl U (2008) Ionic liquids for aqueous two-phase extraction and stabilization of enzymes. Biotechnol Bioeng 99(6):1416–1424
Dreyer S, Kragl U (2009) Driving forces of protein partitioning in an ionic liquid-based aquaeous two-phase system. Biochem Eng 46:176–185
Eckstein M, Liese A, Kragl U (2004) Use of an ionic liquid in a two-phase system to improve an alcohol dehydrogenase catalysed reduction. Chem Commun 9:1084–1085
Flory PJ (1953) Principles of polymer chemistry. Cornell University Press, Ithaca, NY
Gathergood N, Scammells PJ, Garcia MT (2006) Biodegradable ionic liquids—Part III. The first readily biodegradable ionic liquids. Green Chem 8(2):156–160
Handy ST, Okello M (2003) Fructose-derived ionic liquids: recyclable homogeneous supports. Tetrahedron Lett 44(46):8399–8402
Harris PA, Karlstrom G, Tjerneld F (1991) Enzyme purification using temperature-induced phase formation. Bioseparation 2(4):237–246
Jiang YY, Xia HS, Guo C, Mahmood I, Liu HZ (2007) Enzymatic hydrolysis of penicillin in mixed ionic liquids/water two-phase system. Biotechnol Prog 23(4):829–835
Keskin S, Kayrak-Talay D, Akman U, Hortacsu O (2007) A review of ionic liquids towards supercritical fluid applications. J Supercrit Fluids 43(1):150–180
Kragl U, Eckstein M, Kaftzik N (2002) Enzyme catalysis in ionic liquids. Curr Opin Biotechnol 13:565–571
Lou WY, Chen L, Zhang BB, Smith TJ, Zong MH (2009) Using a water-immiscible ionic liquid to improve asymmetric reduction of 4-(trimethylsilyl)-3-butyn-2-one catalysed by immobilized Candida parapsilosis CCTCC M203011 cells. BMC Biotechnol 9(90)
Lui Q, Xuesheng H, Wang Y, Yang P, Xia H, Yu J (2005) Extraction of penicillin G by aqueous two-phase system of [Bmim]BF6/NaH2PO4. Chin Sci Bull 50(15):1582–1585
Lui Q, Yu J, Li WL, Hu XS, Xia HS, Liu HZ, Yang P (2006) Partitioning behavior of penicillin G in aqueous two phase system formed by ionic liquids and phosphate. Sep Sci Technol 41(12):2849–2858
Naganagouda K, Mulimani VH (2008) Aqueous two-phase extraction (ATPE): an attractive and economically viable technology for downstream processing of Aspergillus oryzae α-galactosidase. Biochemistry 43(11):1293–1299
Ohno H, Fukumoto K (2007) Amino acid ionic liquids. Acc Chem Res 40:1122–1129
Park S, Kazlauskas RJ (2003) Biocatalysis in ionic liquids—advantages beyond green technology. Curr Opin Biotechnol 14:432–437
Patnaik PR (2001) Penicillin fermentation: mechanisms and models for industrial-scale bioreactors. Crit Rev Microbiol 27(1):25–39
Pei YC, Wang JJ, Wu K, Xuan XP, Lu XJ (2009) Ionic liquid-based aqueous two-phase extraction of selected proteins. Sep Purif Technol 64(3):288–295
Pfruender H, Amidjojo M, Kragl U, Weuster-Botz D (2004) Efficient whole-cell biotransformation in a biphasic ionic liquid/water system. Angew Chem Int Ed 43:4529–4531
Pinto PCAG, Saraiva MLMF, Lima JLFC (2008) Oxidoreductase behavior in ionic liquids: a review. Anal Sci 24:1231–1238
Ranke J, Stolte S, Stormann R, Arning J, Jastorff B (2007) Design of sustainable chemical products—the example of ionic liquids. Chem Rev 107:2183–2206
Rito-Palomares M (2004) Practical application of aqueous two-phase partition to process development for the recovery of biological products. J Chromatogr B 807:3–11
Seddon KR (1997) Ionic liquids for clean technology. J Chem Technol Biotechnol 68(4):351–356
Shehong L, Chiyang H, Huwei L, Kean L, Feng L (2005) Ionic liquid-based aqueous two-phase system, a sample pretreatment procedure prior to high-performance liquid chromatography of opium alkaloids. J Chromatogr 826(1–2):58–62
Sheldon RA, Madeira R, Sorgedrager MJ, van Rantwijk F, Seddon KR (2002) Biocatalysis in ionic liquids. Green Chem 4:147–151
Soto A, Arce A, Khoshkbarchi MK (2005) Partitioning of antibiotics in a two-liquid phase system formed by water and a room temperature ionic liquid. Sep Purif Technol 44(3):242–246
Tao GH, He L, Sun N, Kou V (2005) New generation ionic liquids: cations derived from amino acids. Chem Commun28:3562–3564
van Rantwijk F, Sheldon RA (2007) Biocatalysis in ionic liquids. Chem Rev 107:2757–2785
Walden P (1914) Molecular weights and electrical conductivity of several fusedsalts. Bull Acad Imperial Sci (St. Petersburg) 1800:405–422
Wang YP, Yang Z, Zhiguo H (2005) Recovery of amino acids by imidazolium based ionic liquids from aqueous media. Green Chem 7:196–202
Wasserscheid P, Welton T (2008) Ionic liquids in synthesis, 2nd edn. Wiley-VCH, Weinheim, Germany
Wells AS, Coombe VT (2006) On the freshwater ecotoxicity and biodegradation properties of some common ionic liquids. Org Process Res Dev 10:794–798
Weuster-Botz D (2007) Process intensification of whole-cell biocatalysis with ionic liquids. Chem Rec 7:334–340
Wilkes JS, Levisky JA, Wilson RA, Hussey CL (1982) Dialkylimidazolium chloroaluminate melts: a new class of room-temperature ionic liquids for electrochemistry, spectroscopy and synthesis. Inorg Chem 21:1263–1264
Yangyang J, Hansong X, Jiang Y, Chen G, Huizhou L (2009) Hydrophobic ionic liquids-assisted polymer recovery during penicillin extraction in aqueous two-phase system. Chem Eng J 147:22–26
Zhang C, Wang H, Malhotra SV, Dodge CJ, Francis AJ (2010) Biodegradation of pyridinium-based ionic liquids by an axenic culture of soil Corynebacteria. Green Chem 12:851
Zhu S, Chen R, Wu Y, Chen Q, Zhang X, Yu Z (2009) A mini-review on greenness of ionic liquids. Chem Biochem 23:207–211
Zhuo D, Yong-Liang Y, Jian-Hua W (2007) Extraction of proteins from biological fluids by use of an ionic liquid/aqueous two-phase system. Chem Eur J 13(7):2130–2137
Acknowledgements
Financial support for this work is provided by The Deutsche Forschungsgemeinschaft within the priority program ‘SPP 1191 Ionic Liquids’ and the BMBF within the ‘Programm Spitzenforschung – REMEDIS’.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oppermann, S., Stein, F. & Kragl, U. Ionic liquids for two-phase systems and their application for purification, extraction and biocatalysis. Appl Microbiol Biotechnol 89, 493–499 (2011). https://doi.org/10.1007/s00253-010-2933-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2933-4