Abstract
In Streptomyces coelicolor, the sco2127 gene is located upstream of the gene encoding for glucose kinase. This region restores sensitivity to carbon catabolite repression (CCR) of Streptomyces peucetius var. caesius mutants, resistant to 2-deoxyglucose (DogR). In order to search for the possible mechanisms behind this effect, sco2127 was overexpressed and purified for protein–protein interaction studies. SCO2127 was detected during the late growth phase of S. coelicolor grown in a complex media supplemented with 100 mM glucose. Pull-down assays using crude extracts from S. coelicolor grown in the same media, followed by far-western blotting, allowed detection of two proteins bound to SCO2127. The proteins were identified by MALDI-TOF mass spectrometry as SCO5113 and SCO2582. SCO5113 (BldKB) is a lipoprotein ABC-type permease (∼66 kDa) involved in mycelium differentiation by allowing the transport of the morphogenic oligopeptide Bld261. SCO2582, is a putative membrane metalloendopeptidase (∼44 kDa) of unknown function. In agreement with the possible role of SCO2127 in mycelium differentiation, delayed aerial mycelium septation and sporulation was observed when S. coelicolor A3(2) was grown in the presence of elevated glucose concentrations (100 mM), an effect not seen in a Δ-sco2127 mutant derived from it. We speculate that SCO2127 might represent a key factor in CCR of mycelium differentiation by interacting with BldKB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Streptomyces coelicolor mutants, isolated by their ability to utilize glycerol or arabinose for growth in the presence of the glucose analog, 2-deoxyglucose (DOG), are insensitive to carbon catabolite repression (CCR) (Hodgson 1982). CCR insensitivity in these mutants (DogR) seems to be due to both, a failure to utilize glucose and low glucose kinase (Glk) activity (Angell et al. 1994), which prevents production of glucose catabolites (Ramos et al. 2004). The DogR phenotype can be restored to the wild type by transforming the mutants with a 2.9 kb BclI fragment of S. coelicolor DNA containing two complete open reading frames encoding for Glk and SCO2127, a protein of unknown function (Ikeda et al. 1984; Angell et al. 1992). In addition to the S. coelicolor DogR mutants, the effect of glkA and sco2127 has been individually analyzed in Streptomyces peucetius var. caesius DogR mutants (deficient in glucose transport, Glk activity, and CCR). A complete reversion of the DogR mutant phenotype can be observed when this strain is transformed with sco2127 alone (Guzmán et al. 2005a). This result was unexpected since sco2127 encodes neither for a Glk (Angell et al. 1992) nor a glucose permease (GlcP) (Bertram et al. 2004). Dot blot analysis of the S. peucetius var. caesius DogR mutant, suggested that sco2127 encodes for a protein which stimulates transcription of glk and probably that of the glucose permease gene as well (Guzmán et al. 2005a). However, analysis of the predicted SCO2127 amino acid sequence does not suggest the presence of DNA binding motifs. Therefore, the possibility of a direct interaction of SCO2127 with other proteins to stimulate either glk transcription or enzyme activity was envisaged as its possible mechanism of action. Thus, the aim of this work was to evaluate the ability of SCO2127 to bind proteins from crude extracts of S. coelicolor grown in the presence of glucose.
Materials and methods
Microorganisms and culture conditions
S. coelicolor A3(2) M145 (SCP-1, SCP-2, prototroph) was obtained from the John Innes Centre (Norwich, UK). The S. coelicolor sco2127 null mutant (∆sco2127) was constructed from the wild-type S. coelicolor M145 strain by the PCR-targeting procedure reported by Gust et al. (2002). The apramycin resistance cassette from pIJ773 was used for SCO2127 interruption into the SC6E10 cosmid by using Escherichia coli BW25113/pIJ790 containing the λ-RED system. The oligonucleotides primer pairs used for amplification corresponded to forward: 36 nucleotides upstream from the initiation codon ATG, including 20 nucleotides for the FRT sequence after ATG. For reverse: 36 nucleotides downstream from the stop codon TCA (including 19 nucleotides of the FRT sequence) of the 575 pb sco2127 gene. They were: 5′ AACGTCGTAAGGACGAACCGTAGTCAGGA GTCCGTCATGATTCCGGGGATCCGTCGACC 3′ (forward) and reverse 5′ GAGCCC CGCTAAGGGCAACCGTACCCGAGGCAGGACTCATGTAGGCTGGAGCTGCTTC 3′. The isolated kanamycin-sensitive and apramycin-resistant mutants were PCR analyzed to confirm sco2127 substitution by the aac (3)IV-oriT cassette. The oligonucleotides used for this purpose were: forward 5′ AGAGCTCGAGCCTGGTCC 3′ and 5′ TCC ACGATGGCCTCGGGTGT 3′ for reverse. To avoid polar effects in the isolated mutants containing the cassette, this was further excised from the interrupted SC6E10 cosmid by passing through E. coli BT340, and the region was transferred to Streptomyces by protoplast transformation (Gust et al. 2002). As a control, the S. coelicolor sco2127 null mutant (∆sco2127) was complemented to the wild-type phenotype by transformation with the integrative plasmid pSET152 (Bierman et al. 1992), containing the S. coelicolor sco2127 gene (pSET2127). This plasmid resulted from sco2127 ligation to the pSET152 polylinker region. Previously, sco2127 was amplified using cosmid SC6E10 as template sequence. The oligonucleotides primer pairs used for this purpose were: forward 5′ GGGGGCGATGGAACCCGCGGATCCCCGGAGCGCGGGCCCGTT 3′ and 5′ TCAG TCCAGGTCGATGCGCTGCCCCGGGCC 3′ for reverse. This region included the sco2127 promoter.
For seed cultures, approximately 106 spores (maintained in 20% glycerol) were used to inoculate 250-ml Erlenmeyer flasks containing 50 ml YM medium [0.4% yeast extract, 1.0% malt extract (Guzmán et al. 2005a)] supplemented with 0.4% glucose. The seed cultures were incubated for 48 h at 29°C under agitation (180 rpm). The cells were collected by centrifugation (10,400×g for 10 min), washed two times, and resuspended in 5 ml sterile saline solution (NaCl 0.75%). For SCO2127 synthesis and purification studies, 250-ml Erlenmeyer flasks containing 50 ml YM medium with 100 mM glucose (YMG100) were inoculated with 1 ml of the washed seed cultures and incubated under similar conditions. At desired times, cells were collected by centrifugation (10,400×g) and disrupted by sonication (eight 30-s 60-W pulses, leaving 1 min between each pulse), centrifuged at 13,400×g for 10 min and the sample supernatants (20 μg total protein) used for western blot analyses. E. coli M-15 (containing the pREP4 plasmid which encodes for the lac repressor in trans, ensuring a tightly regulated expression) was obtained from QIAexpress (Qiagen).
For protein determination, samples were processed as previously reported (Segura et al. 1996), and assayed by the Lowry method, using bovine serum albumin as standard (Lowry et al. 1951). Remaining glucose was enzymatically determined in the culture medium, as previously described (Escalante et al. 1999).
Expression and purification of His6-tagged SCO2127
The sco2127 gene was subcloned into pQE30 and its transcription product (His6-SCO2127) was overexpressed. This procedure allowed purification of SCO2127 (with a Ni-NTA-Sepharose resin) and production of polyclonal antibodies. In western blot assays, the antibodies gave a positive reaction against protein extracts from both S. coelicolor and S. peucetius var. caesius, appearing as a single band of 34 kDa (Chávez et al. 2009). On the other hand, no reaction was obtained in extracts from the S. coelicolor ∆sco2127 (null) mutant.
Anti-SCO2127 antibody production
For anti-SCO2127 antibody production, 2 ml of an emulsion containing 50 mg pure SCO2127 in Freund’s complete adjuvant (1:1) were injected intradermally to a healthy female New Zealand white rabbit aged 3–4 months (body weight ∼2.5 kg) following the protocol described by Hu et al. (2002).
Immunoblotting
The supernatant samples containing 15 μg protein were subject to SDS-PAGE using standard procedures (Laemmli 1970). The proteins were transferred using an Electrophoretic Transfer Cell (Mini Trans-Blot®, Biorad-170-3930) to a PVDF membrane (Immobilon P, Millipore). The membrane (with transferred proteins) was incubated at room temperature for 1 h in a plastic bag containing ∼10 ml anti-SCO2127 antibodies (diluted 1:5,000) in blocking buffer [3% (w/v) Difco skim milk, 0.05% (v/v) Tween 20 (Sigma) in PBS]. After incubation, the membrane was washed three times (1 min each) with buffered Tween (BT) [0.05% (v/v) Tween 20 (Sigma) in PBS] and incubated with 3% skim milk containing goat anti-rabbit IgG (H + L) HRP conjugate (Zymed) (diluted 1:10,000 with BT). The membrane was washed as before and finally developed with 0.05% (w/v) 3,3′-diaminobenzidine tetrahydrochloride (Sigma), 0.02% nickel chloride hexahydrate, and 0.03% hydrogen peroxide. The procedure was stopped with distilled water, and the bound proteins were visualized.
Pull-down assay
SCO2127-His6 was used as bait to identify interactions with proteins from intracellular S. coelicolor crude extracts. For bait protein immobilization, 100 μl (20 mg protein/ml) of the purified SCO2127-His6 was transferred to a 1.5-ml Eppendorf tube containing 250 μl of Ni-NTA-Sepharose and diluted to 1 ml with pull-down buffer (0.5 M NaCl, 0.05 M imidazol, and 0.02 M KH2PO4). The mixture was incubated at room temperature for 10 min with gentle rocking motion on a rotating platform.
Concomitantly, lysates from S. coelicolor were prepared from cells previously grown (48 h) in 2.8-l Fernbach flask containing 500 ml YMG100 medium (Segura et al. 1996). Cell lysates (prey lysates) were transferred to sterile tubes and stored on ice.
To determine the possible interactions between SCO2127-His6 and S. coelicolor extracts, the prey lysates (800 μl containing 500 μg protein/ml) were placed into 1.5-ml Eppendorf tubes containing 250 μl of the Ni-NTA-Sepharose with the immobilized bait protein (SCO2127-His6). To allow protein–protein interaction, the samples were incubated for 2 h at 4°C with gentle rocking and centrifuged at 500×g for 3 min, discarding the supernatant. The Ni-NTA-Sepharose pellet was washed three times with 1 ml of pull-down buffer, mixing the suspension thoroughly for 3 min with gentle rocking. The samples were centrifuged discarding the washed volume. The bait–prey complex was eluted from the Ni-NTA-Sepharose with 100 μl of Tris–SDS buffer 4× (6.2 μM Tris–HCl pH 6.8, 339 μM glycerol, 6.9 μM SDS, 71.1 μM β-mercaptoethanol, and 1.86 nM bromophenol blue) with MilliQ water (1:1), and the sample was boiled for 5 min before being applied to a 10% SDS-PAGE for further analysis.
Far-western assay
Prey lysates from S. coelicolor cells, previously grown for 48 h in YMG100, were electrophoresed in 10% SDS-PAGE and blotted to PVDF membranes as described above. Using a plastic bag, the blot was immersed for 2 h in ∼10 ml blocking buffer and washed three times (1 min each) with BT. The membrane was then exposed to SCO2127-His6 (20 μg/ml in BT), incubated for 2 h at room temperature (on an orbital shaker with gentle rotation), and washed two times with the same buffer. To detect possible interactions between SCO2127-His6 and the proteins present in the S. coelicolor lysates, the blot was immersed again in BT (∼10 ml) containing 0.5 μg/ml of anti-histidine monoclonal antibody (Roche Applied Science, Indianapolis, IN) and incubated for 1 h at room temperature with gentle agitation. After washing (two times with the same buffer), the blot was exposed to a secondary antibody [rabbit anti-mouse IgG (H + L) conjugated to alkaline phosphatase (Zymed, San Francisco, CA)] (∼10 ml diluted 1:2,000 in BT). Under these conditions, the blot was incubated for 1 h, washed two times with the same buffer, and the bound proteins were visualized by incubation with BCIP/NBT (Perkin Elmer Life Sciences, Inc.) for about 7 min (Edmonson and Dent 2001).
Identification of SCO2127 binding proteins
From the pull-down assay, those proteins bound to SCO2127 were carefully excised from the SDS-PAGE gel and dissolved in 20 μl of a methanol–water mixture (70:30). The proteins were identified by matrix-assisted laser desorption/ionization (MALDI-TOF) mass spectrometry (Henzel and Stults 1996) on a Bruker-Daltonics-Autoflex MALDI-TOF mass spectrophotometer (Bruker-Flanzen Analytical, Gmbh, Germany) in the positive-ion mode, as reported by Jensen et al. (1998). Protein sequence comparisons were carried out by employing the Basic Local Alignment Search Tool (BLAST) program (Altschul et al. 1997), with the algorithm set to “blastp” (protein–protein BLAST).
Microscopy observations
Electron microscopy observations were carried out using scanning electron microscopy. For this purpose, agar blocks of S. coelicolor M-145 and its ∆sco2127 mutant derivative, grown for 48 h on solid YMG100 medium, were cut out and fixed with 2.5% glutaraldehyde in 0.05 M sodium cacodylate buffer pH 7.2 and left overnight at room temperature. The glutaraldehyde-fixed mycelium was post-fixed for 1 h in 1% osmium tetroxide in 0.05 M cacodylate buffer at room temperature. After washing, the samples were dehydrated by successive transfers in 30%, 50%, 70%, 90%, and 100% ethanol, 15–60 min each, and critical point dried. Finally, the mycelium samples were gold coated, and images were recorded using a JEOL scanning electron microscope JSM-6310LV (JEOL, Tokyo, Japan).
Results
Time course of SCO2127 synthesis
S. coelicolor grew well in YMG100 medium (Fig. 1a). Under this condition, glucose is consumed in a linear fashion during both the logarithmic and the stationary growth phases. In order to establish the relationship between growth phase and SCO2127 protein expression, cellular extracts of this microorganism grown under these conditions were analyzed by western blot assays. As can be seen in Fig. 1b, SCO2127 was initially detected during the pre-stationary growth phase of the cultures. Detection of SCO2127 coincided with the late stage of active glucose consumption. As shown in the same figure, the expressed protein (34 kDa) exhibited a stronger signal at 72 h fermentation.
Binding of SCO2127 to different S. coelicolor proteins
In order to understand the presence of SCO2127 at the pre-stationary and late growth phases, extracts of S. coelicolor grown for 48 h in YMG100 medium were exposed to immobilized SCO2127 and after elution, analyzed by SDS-PAGE. As can be seen in Fig. 2a, lane 3, at least three bands were detected after elution of the extract from the Ni-NTA-Sepharose bound to SCO2127.
a Pull-down assay. SDS-PAGE (10%) of S. coelicolor prey proteins eluted from Ni-NTA-Sepharose with immobilized SCO2127 bait protein and stained with Coomassie blue. Lane 1 positive control of SCO2127-Ni-NTA-Sepharose. Lane 2 negative control of S. coelicolor crude extracts previously exposed to Ni-NTA-Sepharose. Lane 3 S. coelicolor crude extracts exposed to SCO2127-Ni-NTA-Sepharose. M molecular weight marker. b Far-western assay, showing SCO2127-6×His bound to S. coelicolor crude extracts fixed onto a PVDF membrane and visualized using rabbit anti-mouse antibodies conjugated to alkaline phosphatase against anti-his antibodies
To identify the protein bands from the pull-down experiment, they were excised from the SDS-PAGE gel and analyzed by MALDI-TOF mass spectrometry. After digestion, the generated peptides matched two S. coelicolor proteins (Table 1). The first band (a, ∼78 kDa) contained two proteins which were identified as SCO2582 and SCO2127. The second band (b, ∼66 kDa) was identified as SCO5113 and the third one (c, ∼47 kDa) as SCO2582.
In order to confirm the possible binding of SCO2127 to the above-mentioned proteins (SCO5113 and SCO2582), S. coelicolor extracts were evaluated by far-western assays. As can be seen in Fig. 2b, the anti-SCO2127 antibody recognized not only SCO2127, but also two other protein bands from the intracellular extracts. The size of these bands corresponded to the SCO5113 and SCO2582 proteins evidenced in the pull-down experiment (Fig. 2a). SCO5113 is a lipoprotein ABC-type permease, also known as BldKB, involved in mycelium differentiation (Nodwell and Losick 1998). SCO2582 is a hypothetical protein of unknown function.
Effect of glucose on mycelia differentiation
Considering that one of the proteins bound to SCO2127 was the lipoprotein BldKB, which seems to be responsible for the transport of a morphogenetic oligopeptide (Bld261) involved in the early morphological differentiation of S. coelicolor (Nodwell and Losick 1998), we investigated a possible role for SCO2127 in this process.
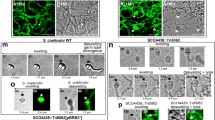
As can be seen in Fig. 3a, after 48 h incubation, a delayed septation and sporulation were observed when S. coelicolor M145 strain was grown in the presence of 100 mM glucose. Conversely, septum formation and sporulation were present in the null ∆sco2127 mutant when grown under the same conditions (Fig. 3b).
To verify that mutant phenotype was solely due to deletion of sco2127, the mutant was transformed with an integrative plasmid containing the S. coelicolor sco2127 gene (pSET2127). This fully restored sensitivity of aerial mycelium formation to 100 mM glucose, producing a bald-type phenotype, underlining that morphological differences of Δsco2127 were indeed due to deletion of sco2127.
Discussion
For several years, our research group has been interested in the properties of SCO2127 and its role in CCR (Guzmán et al. 2005a, b). In S. peucetius var. caesius, this region (576 bp) is located upstream of the glkA gene and pleiotropically stimulates Glk activity, glucose transport, and sensitivity to CCR (Guzmán et al. 2005a).
In the present work, we found that S. coelicolor expresses SCO2127 during the pre- and stationary growth phase when grown in a complex medium supplemented with 100 mM glucose. It was notorious that even when the supplemented glucose started to be consumed during the exponential growth phase, SCO2127 was not detected until the pre-stationary phase of growth. This result contrasted with a previous report for cells of this microorganism grown in a minimal chemically defined medium, where SCO2127 was mainly present during the exponential growth phase and showed a glucose-dependent production (Chávez et al. 2009). These results may suggest a different role for SCO2127 depending on the nutritional conditions present in the culture media.
Although binding of SCO2127 to the glucose permease (GlcP) and Glk was expected to occur, the use of pull-down and far-western assays against crude extracts of S. coelicolor grown in YMG100 medium showed interaction of SCO2127 to other proteins identified by mass spectrometry as BldKB and SCO2582. BldKB is part of a periplasmic ABC-type permease, necessary for the transport of the oligopeptide Bld261, which promotes early morphological differentiation of S. coelicolor on rich but not minimal medium (Claessen et al. 2006). It has been established that ABC-type permeases are also able to transport a wide variety of compounds, including the herbicide bialaphos, peptides, amino acids, and carbohydrates (Holland and Blight, 1999). Recently, Bentley et al. (2002) and Bertram et al. (2004), identified more than 50 genes encoding for carbohydrate transport systems in S. coelicolor A3(2), most of which are ABC-type permeases. Moreover, these permeases are capable of incorporating more than one carbohydrate (Hillerich and Westpheling, 2006) and also display some additional functions, such as morphogenesis and antibiotic production. Consequently, a deletion in BldKB in S. coelicolor produces a bald mutant phenotype (Claessen et al. 2006).
A similar example has been reported for agl3EFG, which encodes for an ABC transporter involved in carbohydrate transport. Inappropriate expression of this ABC transporter leads to defects in morphogenesis and antibiotic production (Hillerich and Westpheling 2006). Like bldKB, the agl3EFG transporter may serve two functions, carbohydrate transport and morphogenesis. One additional case is related to S-adenosylmethionine (SAM). It is well known that in S. coelicolor, SAM exerts control of morphological and physiological differentiation by inducing BldKB, SCO5477, and SCO5260 (Okamoto et al. 2003). The lack of SAM in deletion mutants of these genes (Δsco5260 and Δsco5477) prevented production of actinorhodin, undecylprodigiosin, and sporulation. SCO5260 and SCO5477 seem to be ABC transporter components likely coupling the accumulation of SAM to differentiation (Shin et al. 2007).
SCO2127 also binds to another protein identified as SCO2582, a 44-kDa polypeptide with a predicted amino acid sequence containing a conserved domain corresponding to the peptidase M48 superfamily, also known as metalloproteases (EC:33.4.24). The same sequence is homologous to a membrane putative zinc-dependent protease (84% identity) from Streptomyces griseus (e−161) (http://www.uniprot.org/uniprot/Q9RDK4). As with other metalloproteases, analysis of the amino acid sequence of this peptide evidenced the zinc-binding sequence HEXXH characteristic of the metzincin subfamily (Kurisu et al. 1997). Intracellular proteases in prokaryotic cells perform many functions, including cleavage of signal peptides during protein export, inactivation of regulatory proteins, and removal of aberrant proteins (Gottesman 1999). For these reasons, it can be speculated that SCO2582 might be part of the Bld261 secretory system, possibly engaged in the processing and maturation of this oligopeptide.
Additionally, BldKB and SCO2582 are reported as membrane proteins (Claessen et al. 2006; http://www.uniprot.org/uniprot/Q9RDK4) which likely have a soluble transition phase during their maturation. It is probably during this phase, when they are susceptible to bind SCO2127 and therefore amenable to regulation.
Regardless of the mechanism, it is predictable that the absence of SCO2127 might affect binding with BldKB and deregulate aerial mycelium formation. In agreement with this possibility, aerial mycelium formation and septation were not observed in the S. coelicolor M145 strain grown with 100 mM glucose (repressive conditions), but they were present in a Δ-sco2127 null mutant derived from it. Repression of mycelium differentiation due to the presence of glucose has been reported for S. griseus and S. coelicolor (Ueda et al. 2000). Therefore, we propose that SCO2127 represents a key factor in CCR of mycelium differentiation by modulating BldKB function in cultures of S. coelicolor cells grown in the presence of glucose.
References
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Angell S, Schwartz E, Bibb JM (1992) The glucose kinase gene of Streptomyces coelicolor A3(2): its nucleotide sequence, transcriptional analysis and role in glucose repression. Mol Microbiol 6:2833–2844
Angell S, Lewis CG, Buttner MJ, Bibb JM (1994) Glucose repression in Streptomyces coelicolor A3(2): a likely regulatory role for glucose kinase. Mol Gen Genet 244:135–143
Bentley SD, Chater KF, Cerdeno-Tarraga AM, Challis GL, Thomson NR, James KD, Harris DE, Quail MA, Kieser H, Harper D, Bateman A, Brown S, Chandra G, Chen CW, Collins M, Cronin A, Fraser A, Goble A, Hidalgo J, Hornsby T, Howarth S, Huang CH, Kieser T, Larke L, Murphy L, Oliver K, O’Neil S, Rabbinowitsch E, Rajandream MA, Rutherford K, Rutter S, Seeger K, Saunders D, Sharp S, Squares R, Squares S, Taylor K, Warren T, Wietzorrek A, Woodward J, Barrell BG, Parkhill J, Hopwood DA (2002) Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141–147
Bertram R, Schlicht M, Mahr K, Nothaft H, Saier MH Jr, Titgemeyer F (2004) In silico and transcriptional analysis of carbohydrate uptake systems of Streptomyces coelicolor A3(2). J Bacteriol 186:1362–1373
Bierman M, Logan R, O’Brien K, Seno ET, Nagaraja Rao R, Schoner BE (1992) Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene 116:43–49
Chávez A, García-Huante Y, Ruiz B, Langley E, Rodríguez-Sanoja R, Sanchez S (2009) Cloning and expression of the sco2127 gene from Streptomyces coelicolor M145. J Ind Microbiol Biotechnol 36:649–654
Claessen D, de Jong W, Dijkhuizen L, Wösten HA (2006) Regulation of Streptomyces development: reach for the sky! Trends Microbiol 14:313–319
Edmonson DG, Dent SYR (2001) Identification of protein interactions by far western analysis. In: Coligan JE, Dunn BM, Ploegh HL, Speicher DW, Wingfield PT (eds) Current protocols in protein science. Wiley, New York, pp 19.7.1–19.7.10
Escalante L, Ramos I, Imriskova I, Langley E, Sanchez S (1999) Glucose repression of anthracycline formation in Streptomyces peucetius var. caesius. Appl Microbiol Biotechnol 52:572–578
Gottesman S (1999) Regulation by proteolysis: developmental switches. Curr Opin Microbiol 2:142–147
Gust B, Kieser T, Chater KF (2002) REDIRECT© technology: PCR-targeting system in Streptomyces coelicolor. John Innes Centre, Norwich
Guzmán S, Carmona A, López R, Escalante L, Ruiz B, Rodríguez-Sanoja R, Sanchez S, Langley E (2005a) Pleiotropic effect of the SCO2127 gene on the glucose uptake, glucose kinase activity and carbon catabolite repression in Streptomyces peucetius var. caesius. Microbiology-SGM 151:1717–1723
Guzmán S, Ramos I, Moreno E, Ruiz B, Rodríguez-Sanoja R, Escalante L, Langley E, Sanchez S (2005b) Sugar uptake and sensitivity to carbon catabolite repression in Streptomyces peucetius var. caesius. Appl Microbiol Biotechnol 69:200–206
Henzel WJ, Stults JT (1996) Unit 16.2. Matrix assisted laser desorption/ionization time-of-flight mass analysis of peptides. In: Coligan JE, Dunn BM, Ploegh HL, Speicher DW, Wingfield PT (eds) Current protocols in protein science. Wiley, New York, pp 16.1.1–16.2.11
Hillerich B, Westpheling J (2006) A new GntR family transcriptional regulator in Streptomyces coelicolor is required for morphogenesis and antibiotic production and controls transcription of an ABC transporter in response to carbon source. J Bacteriol 188:7477–7487
Hodgson D (1982) Glucose repression of carbon source uptake and metabolism in Streptomyces coelicolor and its perturbation in mutants resistant to 2-deoxyglucose. J Gen Microbiol 128:2417–2430
Holland IB, Blight MA (1999) ABC-ATPases, adaptable energy generators fuelling transmembrane movement of a variety of molecules in organisms from bacteria to humans. J Mol Biol 293:381–399
Hu YX, Guo JY, Shen L, Chen Y, Zhang ZC, Zhang YL (2002) Get effective polyclonal antisera in one month. Cell Res 12:157–160
Ikeda H, Seno ET, Bruton CJ, Chater KF (1984) Genetic mapping, cloning and physiological aspects of the glucose kinase gene of Streptomyces coelicolor. Mol Gen Genet 196:501–507
Jensen ON, Larsen MR, Roepstorff P (1998) Mass spectrometric identification and microcharacterization of proteins from electrophoretic gels: strategies and applications. Proteins 2:74–89
Kurisu G, Kinoshita T, Sugimoto A, Nagara A, Kai Y, Kasai N, Harada S (1997) Structure of the zinc endoprotease from Streptomyces caespitosus. J Biochem (Tokyo) 121:304–308
Laemmli U (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:6801–6805
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Nodwell J, Losick R (1998) Purification of an extracellular signaling molecule involved in production of aerial mycelium by Streptomyces coelicolor. J Bacteriol 180:1334–1337
Okamoto S, Lezhava A, Hosaka T, Okamoto-Hosoya Y, Ochi K (2003) Enhanced expression of S-adenosylmethionine synthetase causes overproduction of actinorhodin in Streptomyces coelicolor A3(2). J Bacteriol 185:601–609
Ramos I, Guzmán S, Escalante L, Imriskova I, Rodríguez-Sanoja R, Sanchez S, Langley E (2004) The glucose kinase alone cannot be responsible for carbon source regulation in Streptomyces peucetius var. caesius. Res Microbiol 155:267–274
Segura D, Gonzalez R, Rodriguez-Sanoja R, Sandoval T, Escalante L, Sanchez S (1996) Streptomyces mutants insensitive to glucose repression showed deregulation of primary and secondary metabolism. Asia Pac J Mol Biol Biotechnol 4:30–36
Shin S-K, Park H-S, Kwon H-J, Yoon H-J, Suh J-W (2007) Genetic characterization of two S-adenosylmethionine-induced ABC transporter reveals their roles in modulations of secondary metabolism and sporulation in Streptomyces coelicolor M145. J Microbiol Biotechnol 17:1818–1825
Ueda K, Endo K, Takano H, Nishimoto M, Kido Y, Tomaru Y, Matsuda K, Beppu T (2000) Carbon-source-dependent transcriptional control involved in the initiation of cellular differentiation in Streptomyces griseus. Anton Leeuw Int J G 78:263–268
Acknowledgements
We are indebted to M. A. Ortíz and L. Escalante for their assistance during the elaboration of this work. This work was supported in part by the grants IN 209210 from PAPIIT, Dirección General de Asuntos del Personal Académico, UNAM and CB2008-100564-IIBO from Consejo Nacional de Ciencia y Tecnología, Mexico.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chávez, A., Forero, A., Sánchez, M. et al. Interaction of SCO2127 with BldKB and its possible connection to carbon catabolite regulation of morphological differentiation in Streptomyces coelicolor . Appl Microbiol Biotechnol 89, 799–806 (2011). https://doi.org/10.1007/s00253-010-2905-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2905-8