Abstract
Pseudomonas chlororaphis GP72 is a root-colonizing biocontrol strain isolated from the green pepper rhizosphere that synthesizes two phenazine derivatives: phenazine-1-carboxylic acid (PCA) and 2-hydroxyphenazine (2-OH-PHZ). The 2-OH-PHZ derivative shows somewhat stronger broad-spectrum antifungal activity than PCA, but its conversion mechanism has not yet been clearly revealed. The aim of this study was to clone and analyze the phenazine biosynthesis gene cluster in this newly found strain and to improve the production of 2-OH-PHZ by gene disruption and precursor addition. The conserved phenazine biosynthesis core operon in GP72 was cloned by PCR, and the unknown sequences located upstream and downstream of the core operon were detected by random PCR gene walking. This led to a complete isolation of the phenazine biosynthesis gene cluster phzIRABCDEFG and phzO in GP72. Gene rpeA and phzO were insertionally mutated to construct GP72AN and GP72ON, respectively, and GP72ANON collectively. The inactivation of rpeA resulted in a fivefold increase in the production of PCA, as well as 2-OH-PHZ. The addition of exogenous precursor PCA to the broth culture, to determine the conversion efficiency of PCA to 2-OH-PHZ under current culture conditions, revealed that PCA had a positive feedback effect on its own accumulation, leading to enhanced synthesis of both PCA and 2-OH-PHZ. The production of 2-OH-PHZ by GP72AN increased to about 170 μg ml−1, compared with just 5 μg ml−1 for the wild type. The hypothesis of biosynthetic pathway for 2-OH-PHZ from PCA was confirmed by identification of 2-hydroxyphenazine-1-carboxylic acid as an intermediate in the culture medium of the high-phenazine producing GP72AN mutant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pseudomonas chlororaphis GP72 is a root-colonizing biocontrol strain isolated from the green pepper rhizosphere that shows broad-spectrum antifungal activity against several phytopathogens of agricultural significance (Liu et al. 2006a, b). This capability is primarily dependent on production of two phenazine compounds, phenazine-1-carboxylic acid (PCA) and 2-hydroxyphenazine (2-OH-PHZ). PCA has been widely studied and is found in a number of different Pseudomonas strains (Chin-A-Woeng et al. 2003), including Pseudomonas fluorescens, Pseudomonas aeruginosa, and Pseudomonas chlororaphis. In contrast, 2-OH-PHZ is found primarily in P. chlororaphis strains and requires the presence of the modifying gene phzO, which is responsible for the production of 2-OH-PHZ from PCA. One exception is the strain P. aurantiaca PB-St2, which can also produce 2-OH-PHZ, but the gene involved has not yet been characterized (Samina et al. 2009). A number of studies have shown that the bacteriostatic and fungistatic activity of 2-OH-PHZ is stronger than that of PCA (Smirnov and Kiprianova 1990; Delaney et al. 2001). For this reason, it would be quite useful to determine the conversion mechanism leading from PCA in order to enhance the production of 2-OH-PHZ.
The hypothesis of biosynthetic pathway leading to 2-OH-PHZ was first described in P. chlororaphis 30–84 (Delaney et al. 2001). In this pathway, 2-OH-PHZ was produced from PCA by the action of a single enzyme, PhzO, which catalyzed the conversion of PCA to 2-hydroxyphenazine-1-carboxylic acid (2-OH-PCA). This latter compound then spontaneously decarboxylated to form 2-OH-PHZ. However, the author failed to isolate the compound 2-OH-PCA. Later studies of P. chlororaphis strains confirmed the existence of 2-OH-PCA by high-performance liquid chromatography (HPLC) and the absence of 2-OH-PCA in a phzO-inactivated mutant, further verifying this to be an intermediate in the biosynthetic pathway of 2-OH-PHZ (Veselova et al. 2008; Kumaresan et al. 2005). However, the details of the conversion mechanism remain controversial as other reports indicated that 2-OH-PHZ synthesis also apparently involved the activity of a second enzyme, PhzE (Maddula et al. 2008). Another study suggested that PhzO could only effectively catalyze the conversion of PCA to 2-OH-PHZ in the presence of two other enzymes, PhzC and PhzD (Dwivedi and Johri 2003).
At present, the regulation of phenazine production in P. chlororaphis strains is based on limited information about the GacA/GacS system (Chancey et al. 2002) and the quorum sensing regulators PhzI/PhzR and CsaI/CsaR (Veselova et al. 2008). The only negative regulator reported to inhibit the synthesis of phenazine compounds is rpeA (Whistler and Pierson 2003). RpeA represses phenazine biosynthesis even when the accumulation of quorum sensing signals is sufficient and a functional GacA/GacS regulation system is required to activate phenazine production. However, the interaction between RpeA and its targets is unclear, and its role in phenazine production needs to be further examined.
The phenazine biosynthesis gene cluster in P. chlororaphis GP72 was first cloned and analyzed in the present study. A rpeA − strain GP72AN was constructed, leading to a higher production of both PCA and 2-OH-PHZ. When precursor PCA was added exogenously to the broth culture, a positive feedback of PCA was observed. The higher accumulation of phenazines allowed quantification of the intermediate, 2-OH-PCA, whose role was confirmed by construction of rpeA −, phzO − mutant strain GP72ANON. The biosynthetic pathway of 2-OH-PHZ in GP72 closely resembled that of P. chlororaphis 30–84.
Materials and methods
Bacterial strains, plasmids, and growth conditions
Escherichia coli strains DH5α and SM10 and P. chlororaphis GP72 (deposited in China General Microbiological Culture Collection Center with a collection number of 1748) were from our laboratory collections. E. coli was routinely grown at 37°C in Luria–Bertani (LB) medium (Sambrook et al. 1989). P. chlororaphis GP72 and its mutants were incubated at 28°C in LB or King’s medium B (King et al. 1954), respectively. Antibiotics in the LB medium were used at the following concentrations: ampicillin (Ap, 100 μg ml−1), tetracycline (Tc, 125 μg ml−1), gentamicin (Gm, 40 μg ml−1), chloromycetin (Cm, 250 μg ml−1) for Pseudomonas, and spectinomycin (Sp, 100 μg ml−1), tetracycline (Tc, 15 μg ml−1), gentamicin (Tm, 10 μg ml−1) for E. coli. The plasmids pMD19-T simple (TAKARA, Dalian, People’s Republic of China), pUCGm (Hoang et al. 1998), pEX18Tc (Keen et al. 1998), and pMMB207 (Morales et al. 1991) were used in the cloning and gene disruption study. LB broth was supplemented with 5% sucrose to counter-select the suicide plasmid pEX18Tc. The primers used in this study are listed in Table 1.
DNA manipulations
Plasmid DNA was isolated from E. coli and Pseudomonas using a spin plasmid preparation kit (BioDev-Tech, Beijing, People’s Republic of China) according to the manufacturer’s instructions. Genomic DNA was isolated from Pseudomonas using a Genomic DNA isolation kit (BioDev-Tech, Beijing, People’s Republic of China). The LA Taq polymerase with GC buffer and all restriction enzymes were purchased from TAKARA (TAKARA, Dalian, People’s Republic of China). Agarose gel electrophoresis was performed as described by Sambrook et al. 1989.
Cloning of the phenazine biosynthesis gene cluster in P. chlororaphis GP72
The gene sequence of the phenazine synthesis cluster (phzIRABCDEFG) and the downstream sequence phzO were determined by standard PCR (Ausubel et al. 1995) from P. chlororaphis GP72 genomic DNA (oligonucleotide primers are shown in Table 1) using the Mastercycler (Eppendorf, Germany). The annealing temperature was 54°C for phzIR1 and phzIR2, 58°C for phzAE1 and phzAE2, and 56°C for phzFG1, phzFG2, and PHZO10 (Delaney et al. 2001). The gene sequence upstream of phzIRABCDEFG was determined by random PCR gene walking (Pilhofer et al. 2007). The cycling program started with only one specific primer IRR3 and included 5-min initial denaturation at 94°C followed by 40 cycles of 94°C for 40 s, 64°C for 40 s, 72°C for 3 min, and the random primer was then added to the mixture, and 1 cycle was run of 94°C for 40 s, 30°C for 40 s, 72°C for 3 min, and then 72°C for an additional 10 min. The gene sequence downstream of phzO was determined using the same methods and materials using the specific primer OF1 and an annealing temperature of 54°C.
The PCR product was resolved by electrophoresis on 1.2% (w/v) agarose gel, eluted with AxyPrep DNA Gel Extraction Kit (Axygen, Hangzhou, People’s Republic of China) and cloned into the Pmd19-T simple vector. All cloned fragments were sequenced at Invitrogen (Shanghai, People’s Republic of China). Sequence similarities were searched at GenBank (National Centre for Biotechnology Information, NIH, USA) using BLAST programs (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Chromosomal inactivation of rpeA in GP72
Primers rpeAE1 and rpeAE2 were designed to clone the gene rpeA from strain GP72 at the annealing temperature 50°C, while primer Gm2 was used to clone the gentamycin resistance gene aacC1 in plasmid pUCGm at 56°C.
The PCR products of rpeA were cloned into a pMD19-T simple vector and were inserted by the fragment containing aacC1 at a filled ClaI site. The insertion was confirmed by sequencing at Invitrogen.
The 2.6-kb EcoRI–XbaI fragment was then subcloned into the suicide vector pEX18Tc. The resulting plasmid pEX18TcAG was mobilized from E. coli SM10 into strain GP72 by biparental mating. Transconjugants were selected on sucrose LB plates containing Sp and Gm to counter-select E. coli SM10. After a second crossing-over, Gm-resistant, Tc-sensitive, and sucrose-resistant recombinants were obtained. The resultant chromosomally inactivated rpeA-inactivated mutant, designated GP72AN, was confirmed by PCR and sequencing at Invitrogen. Figure 1 shows the physical map of the gene disruption of rpeA.
Chromosomal double inactivation of rpeA and phzO in GP72
The method of gene disruption was similar to that mentioned above. Primers OP1 and OP2 were designed to clone gene phzO at 48°C. Primers Cm1 and Cm2 were designed to clone the chloromycetin-resistant cassette from plasmid pMMB207 at 48°C.
The resultant phzO − mutant GP72ON and rpeA −, phzO − double mutant GP72ANON were confirmed by PCR and sequencing at Invitrogen.
Fermentation processing
The cells of strain GP72 stored in a −70°C freezer were activated twice at 28°C for 12 h with LB agar media. One colony was then inoculated into a 25-ml flask containing 5 ml King’s B broth and incubated overnight. A 2.5 ml sample (5% inoculum) of this culture was inoculated into 50 ml King’s B media in a 250-ml flask as a seed culture and incubated for 10 h. A 2.5-ml sample of the seed culture was inoculated into 50 ml King’s B broth in a 250-ml flask, and the fermentation process was initiated. The fermentation temperature was 28°C, and shaking speed was 180 rpm throughout the fermentation time. There are triplicate experiments for each fermentation test. The result value is expressed as mean ± standard derivation.
In vivo transformation of PCA
The PCA solution was prepared at a final concentration of 0.3–0.5 mg ml−1 from a 25 mM stock solution in 55 (w/v) NaHCO3 (Delaney et al. 2001). Baffled shake flasks were used instead of the standard ones in the PCA transformation test. PCA solution was then added to the fermentation broth at the time of 0, 4, 8, 12, and 16 h after the inoculation, at different initial concentrations, in order to determine the optimal conditions for 2-OH-PHZ accumulation. Samples were extracted and analyzed for phenazine content by HPLC.
Quantification of phenazine compounds
The fermentation broth was adjusted to pH 2.0 with 6 N HCl, before centrifugation at 9700×g force for 5 min using an Eppendorf Minispin centrifuge. Cellular debris was removed, and clear supernatant was collected and extracted with equal volume of ethyl acetate with vigorous shaking (Liu et al. 2006b). The collected organic layer was mixed with 10% volume distilled water and shaken vigorously. Finally, the organic phase containing 2-OH-PHZ and PCA was removed and evaporated under vacuum. The residue was dissolved in methanol for further analysis.
The extracted substances were analyzed by HPLC (KNAUER LC8A, Germany) on a C-18 reverse phase column (Agilent, USA) at 30°C with an ultraviolet (UV) light detector using 50% methanol plus 50% 5 mM NH4AC (pH 5.0) solution as the mobile phase at a flow rate of 1 ml min−1. The retention times for PCA and 2-OH-PHZ were approximately 3.2 and 16.5 min, respectively.
Nucleotide sequence accession numbers
Nucleotide sequences of the phenazine biosynthesis gene cluster in P. chlororaphis GP72 and the gene rpeA were submitted to the GENBANK database under accession numbers HM594285 and HM623278.
Results
Cloning and analysis of the phenazine biosynthesis gene cluster in P. chlororaphis GP72
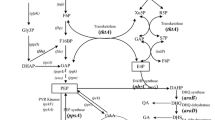
In total, 12418 bp of the gene cluster were cloned and sequenced using degenerate primers PCR and random PCR gene walking method. The homology of the genes between Pseudomonas strains was analyzed using the GenBank of NCBI (see Table 2). Other than the seven classic PCA synthesis genes, the only gene involved in the phenazine biosynthesis pathway in GP72 is phzO, which may make unique modifications to PCA to form 2-OH-PHZ.
The nucleotides located upstream of the operon may encode a fosmidomysin resistance protein (GENBANK CP_000076.1, 86% identical), a major facilitator superfamily MFS_1 (CP_000094.1, 82% identical) or it might be an nif operon and flanking genes (AJ_297527.2, 83% identical), while the downstream nucleotides may encode a gamma-glutamyltranspeptidase precursor (AF_230879.1, 97% identical; and CP_00076.1, 84% identical). These nucleotides would not appear to be relevant to the biosynthesis of phenazines in GP72.
The phenazine biosynthesis gene cluster ultimately obtained from P. chlororaphis GP72 is shown in Fig. 1.
Identification and analysis of rpeA in P. chlororaphis GP72
The gene rpeA in P. chlororaphis GP72 shared 95% identity with that of P. chlororaphis 30–84, having an open reading frame of 1,302 bp and encoded a putative peptide of 433 amino acids. The BLASTX search (http://blast.ncbi.nlm.nih.gov/Blast.cgi) showed that the rpeA from strain GP72 shares 86% sequence similarity to a periplasmic sensor signal transduction histidine kinase in P. fluorescens and 60% similarity to an integral membrane sensor signal transduction histidine kinase in P. putida. Thus, RpeA might belong to a histidine kinase family, but its function or mechanism is not clear, even in the previously reported strains.
Effect of rpeA on phenazines production
As determined by HPLC analysis, the production of both PCA and 2-OH-PHZ was substantially increased in the rpeA − strain GP72AN (Fig. 2). In the wild-type GP72WT, the production of PCA and 2-OH-PHZ was relatively low, at about 22.0and 4.5 μg ml−1, respectively. When the gene rpeA was inactivated, the production of these phenazines increased to 105 and 24.6 μg ml−1, or nearly fivefold the levels of the wild type, indicating a strong inhibitory effect of rpeA on phenazine production (Fig. 4).
HPLC analysis of Pseudomonas chlororaphis GP72WT and GP72AN culture after 108 h fermentation. a HPLC analysis of the GP72WT (GP72 wide type) and b GP72AN (GP72 rpeA-inactivation mutant), retention times for phenazine-1-carboxylic acid (PCA) and 2-hydroxyphenazine (2-OH-PHZ) were 3.2 and 16.5 min, respectively
The ability of GP72AN to produce red pigments also exceeded that of GP72WT, as indicated by the bright red-orange color of the cultures (Fig. 3). No differences were noted in either the swimming or the swarming ability of the GP72AN strain compared to the wild type (data not shown).
In vivo transformation of PCA to 2-OH-PHZ
As shown in Fig. 5, when 150 μg ml−1 PCA was added at different times to broth cultures of GP72AN, phenazine production was essentially unchanged among the different test groups. However, more phenazines were produced in the cultures containing added PCA than in the control groups. The control groups produced about 426 μg ml−1 PCA, while the groups with 150 μg ml−1 PCA added reached a final concentration of 656 μg ml−1 PCA. Net PCA production was about 500 μg ml−1 in the groups containing added PCA, which exceeded that of the control groups. The groups with added PCA also produced more 2-OH-PHZ (126 μg ml−1) than did the control groups (58 μg ml−1).
However, PCA added in excess to the culture inhibited the net production of phenazines by the microorganisms, as shown in Table 3. Net phenazine production (include PCA and 2-OH-PHZ) showed a steady increase for additions of PCA of 400–600 μg ml−1. About a 50% greater amount of phenazines (compared with the quantity of PCA added into the culture) was produced when 400 μg ml−1 PCA was added. Below that amount, for example, when 300 μg ml−1 exogenous PCA was added into the culture, the net production of phenazines was equivalent to that of the control groups. Above that amount, at 800 μg ml−1 PCA, the net production of phenazines was less than that of the control groups. On the other hand, the quantity of 2-OH-PHZ remained steady up to a final concentration of PCA of about 800 μg ml−1 and then slightly dropped when PCA exceeded 960 μg ml−1.
Identification of the intermediate 2-OH-PCA
In GP72WT culture, only two main chemical compounds were purified and identified by HPLC, PCA, and 2-OH-PHZ (Fig. 2). Their retention times were 3.2 and 16.5 min, respectively. However, another chemical compound with a retention time of about 7.8 min was found in the GP72AN culture, which accumulated much higher levels of phenazines. Using high performance liquid chromatography–mass spectrometry and the computer simulation of dipole moment of phenazine derivatives, this unknown compound was identified as 2-OH-PCA, the putative intermediate of the conversion of PCA to 2-OH-PHZ. In fact, GP72WT also produced this compound, but its quantity was so small that it could barely be detected by HPLC.
As shown in Fig. 6, the production of 2-OH-PCA rose with increases in PCA, and both reached their peak at about 40 h during the fermentation process. The production of 2-OH-PCA subsequently decreased as the level of 2-OH-PHZ increased. By 72 h of fermentation, 2-OH-PCA could no longer be detected, and the quantity of 2-OH-PHZ stopped increasing. Actually, the emergence of 2-OH-PHZ occurred about 20 h after the appearance of PCA and 2-OH-PCA. This was in agreement with the situation seen in P. chlororaphis 30–84, in which artificially synthesized 2-OH-PCA spontaneously turned into 2-OH-PHZ in a sodium phosphate buffer solution at pH 6–8 after 18 h (Delaney et al. 2001).
Role of PhzO in the conversion of 2-OH-PHZ
When the phzO gene was insertionally inactivated, no compounds other than PCA could be detected. Since 2-OH-PHZ is derived from PCA, it is predictable that the quantity of PCA will rise when the conversion is broken off. As shown in Fig. 4, Simultaneous blockage of PCA conversion led to an accumulation of PCA in the phzO − strain GP72ON when compared to the wild type. In the double mutant rpeA − , phzO − strain GP72ANON, the yield of PCA was the highest among the four strains.
Inactivation of phzO also led to reduced levels of red pigment in the GP72ON and GP72ANON mutants (Fig. 3). Since most phenazines are colored, the change in color in strain GP72 and its derivatives might be directly associated with their ability to produce phenazines.
Discussion
P. chlororaphis GP72 is a recently isolated biocontrol strain that can produce PCA and 2-OH-PHZ. Thus, the PCA biosynthesis operon phzABCDEFG and phzO, catalyzing the reaction of PCA to 2-OH-PHZ (Delaney et al. 2001), were apparently active in GP72. However, other phenazine modifying genes can also be found near the core operon in Pseudomonas strains. Gene phzH was located downstream of the core operon in P. chlororaphis PCL1391 catalyzed the conversion of PCA to phenazine-1-carboxamide (PCN) (Chin-A-Woeng et al. 2001); PhzM catalyzed the conversion of PCA to pyocyanin (PYO) with the help of PhzS in Pseudomonas aeruginosa PAO1 (Dmitri et al. 2001). Therefore, we conducted a random PCR gene walking experiment to uncover any modifying genes near the operon of strain GP72. Results showed that the only phenazine-modifying gene was phzO. The gene cluster resembled that of P. chlororaphis 30–84, but these strains differed in other aspects that made it valuable to further investigate phenazine production in strain GP72 (Liu et al. 2006b).
At present, few regulatory networks have been revealed in P. chlororaphis strains, and the only reported repressor is RpeA, which inhibits the expression of PhzB through as yet unknown channels that are not part of the quorum sensing regulations (Whistler and Pierson 2003). In the present study, phenazine production was quantified by HPLC, and the rpeA − strain GP72AN was shown to produce about fivefold greater amounts of PCA and 2-OH-PHZ than the wild type, confirming the role of RpeA as a repressor of phenazine synthesis. Previous studies in our lab have revealed another open reading frame (designated rpeB) located upstream of rpeA. There is a 4-bp gap between the two ORFs. Gene rpeBand rpeA might constitute a two-component regulatory system. Based on the predicted amino acid sequence, at about 34% identity, RpeA/RpeB might be the homologous to RstA/RstB of E. coli, which down-regulates the expression of three RpoS-controlled genes by reduction in RpoS at the cellular level (Cabeza et al. 2007). In P. chlororaphis PCL1391, RpoS has been reported to be a positive regulator of the production of PCN, another member of the phenazine family (Girard et al. 2006). Therefore, RpeA in strain GP72 quite possibly represses synthesis of phenazines by reduction in cellular RpoS levels. Another sigma factor, RpoN, has also been reported to stimulate the production of phenazines in strain GP72 (Liu et al. 2008). However, the rpoN − strain showed differences in swimming and swarming motilities compared to the wild type, which was not observed in this study with GP72AN. The interaction between RpeA and RpoS/RpoN needs to be tested in future work.
More phenazines were produced in the cultures containing added PCA than in the control groups, indicating an enhanced synthesis in response to added PCA and suggesting a positive feedback of PCA on its own accumulation at a proper concentration of PCA. Two hypotheses can be proposed to explain this autoinduction. First, PCA has been reported to serve as an electron acceptor in the electron transport chain of P. strains, receiving electrons from NADH and transferring them to oxygen (Price-Whelan et al. 2006). Thus, the addition of exogenous PCA may have accelerated electron transport and provided more energy for the growth of the microorganisms, with the eventual result of stimulating phenazine production. The second hypothesis suggests an autoinductive role of PCA in the activation of gene transcription, analogous to effects of 2,4-diacetylphloroglucinol (DAPG) and pyoluteorin (PLT) in the autoinduction regulation system. In the P. fluorescens strain CHA0, DAPG dissociates the repressor PhlF from its binding to the phlA promoter region and thus stimulates its own production (Schnider-Keel et al. 2000), while PLT enhances its own production and the transcriptional activity of three pyoluteorin biosynthetic genes (Brodhagen et al. 2004). Whether the autoinduction role of PCA on its own biosynthesis was due to a higher rate of metabolism or the activation of gene transcription is not yet clear and needs to be determined in future work.
Because 2-OH-PHZ was derived from PCA, its quantity would be expected to increase when PCA accumulated. However, the maximum amount of 2-OH-PHZ indicated that saturation of enzyme PhzO might be occurring under the current culture conditions and fermentation process. In present study, the production of the potential biocontrol agent, 2-OH-PHZ, was ultimately elevated to about 170 μg ml−1, compared with 5 μg ml−1 in the wild type. Work on optimization is in now in progress for further enhancement of 2-OH-PHZ production.
Three main hypotheses have been put forward to explain the biosynthetic mechanism underlying synthesis of 2-OH-PHZ. First, based on the studies of P. chlororaphis 30–84, 2-OH-PHZ was proposed to derive directly from PCA, through the action of only one enzyme, PhzO, that catalyzed the conversion of PCA to 2-OH-PCA. The latter compound then spontaneously decarboxylated to 2-OH-PHZ (Delaney et al. 2001). Another hypothesis proposed that conversion of 2-OH-PCA to 2-OH-PHZ is an enzymatic process requiring the action of PhzE (Dwivedi and Johri 2003). A third study indicated that, since a fragment containing only phzO failed to convert PCA into 2-OH-PCA, this conversion required additional regions containing part of phzC and phzD, in addition to phzO (Maddula et al. 2008).
In the present study, the intermediate 2-OH-PCA was isolated and identified in the strain GP72WT and GP72AN but was absent in the phzO − strain GP72ON or in the rpeA −, phzO − strain GP72ANON. These findings confirmed a role for phzO in the conversion of PCA to 2-OH-PHZ. The production of 2-OH-PCA increased with the accumulation of PCA and decreased with the growing amounts of 2-OH-PHZ, also indicating a clear precursor-product relationship between these compounds in the process of formation of 2-OH-PHZ.
In addition, a previous study of PhzO expression in E. coli DH5α by our lab showed that PhzO alone, without the help of PhzC or PhzD, was sufficient to catalyze the conversion of PCA to 2-OH-PHZ (Shen et al. 2009). These results support a direct conversion mechanism for PCA to 2-OH-PHZ (Delaney et al. 2001).
References
Ausubel FM, Kingstons RE, Moore DD, Seidman JG, Smith JA, Struhl K (1995) Short protocols in molecular biology. Wiley, New York
Brodhagen M, Henkels MD, Loper JE (2004) Positive autoregulation and signaling properties of pyoluteorin, an antibiotic produced by the biological control organism Pseudomonas fluorescens Pf-5. Appl Environ Microbiol 70:1758–1766
Cabeza ML, Aguirre A, Soncini FC, Véscovi EG (2007) Induction of RpoS degradation by the two-component system regulator RstA in Salmonella enteric. J Bacteriol 189:7335–7342
Chancey ST, Wood DW, Pierson EA, Pierson LS III (2002) Survival of GacS/GacA mutants of the biological control bacterium Pseudomonas aureofaciens 30–84 in the wheat rhizosphere. Appl Environ Microbiol 68:3308–3314
Chin-A-Woeng TFC, Thomas-Oates JE, Lugtenberg BJJ, Bloemberg GV (2001) Introduction of the phzH gene of Pseudomonas chlororaphis PCL1391 extends the range of biocontrol ability of phenazine-1-carboxylic acid-producing Pseudomonas spp. strains. Mol Plant-Microb Interact 14:1006–1015
Chin-A-Woeng TFC, Bloemberg GV, Lugtenberg BJJ (2003) Phenazines and their role in biocontrol by Pseudomonas bacteria. New Phytol 157:503–523
Delaney SM, Mavrodi DV, Bonsall RF, Thomashow LS (2001) PhzO, a gene for biosynthesis of 2-hydroxylated phenazine compounds in Pseudomonas aureofaciens 30–84. J Bacteriol 183:318–327
Dmitri VM, Robert FB, Shannon MD, Marilyn JS, Greg P, Thomashow LS (2001) Functional analysis of genes for biosynthesis of pyocyanin and phenazine-1-carboxamide from Pseudomonas aeruginosa PAO1. J Bacteriol 183(21):6454–6465
Dwivedi D, Johri BN (2003) Antifungals from fluorescent Pseudomonads: biosynthesis and regulation. Curr Sci 85:1693–1703
Girard G, Rij ETV, Lugtenberg BJJ, Bloemberg GV (2006) Regulatory roles of psrA and rpoS in phenazine-1-carboxamide synthesis by Pseudomonas chlororaphis PCL1391. Microbiology 152:43–58
Hoang TT, Karkhoff-Schweizer RAR, Kutchma AJ, Schweizer HP (1998) A broad-host-range Flp-FRT recombination system for site-specific excision of chromosomally-located DNA sequences: application for isolation of unmarked Pseudomonas aeruginosa mutants. Gene 212:77–86
Keen NT, Tamaki S, Kobayashi D, Keen DT (1998) Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191–197
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fluorescein. J Laboratoral Clin Med 44:301–307
Kumaresan K, Subramanian M, Vaithiyanathan S, Sevagaperumal N, GOPAL C, Dilantha FWG (2005) Broad spectrum action of phenazine against active and dormant structures of fungal pathogens and root knot nematode. Arch Phytopathol Plant Prot 38:69–76
Liu HM, Dong D, Peng HS, Zhang XH, Xu XQ (2006a) Genetic diversity of phenazine- and pyoluteorin-producing pseudomonads isolated from green pepper rhizosphere. Arch Microbiol 185:91–98
Liu HM, He YJ, Jiang HX (2006b) Characterization of a phenazine-producing strain Pseudomonas chlororaphis GP72 with broad-spectrum antifungal activity from green pepper rhizosphere. Curr Microbiol 54:302–306
Liu HM, Yan A, Zhang XH, Xu YQ (2008) Phenazine-1-carboxylic acid biosynthesis in Pseudomonas chlororaphis GP72 is positively regulated by the sigma factor RpoN. World J Microbiol Biotechnol 24:1961–1966
Maddula VSRK, Pierson EA, Pierson LS III (2008) Atering the ratio of phenazines in Pseudomonas chlororaphis strain 30–84: effects on biofilm formation and pathogen inhibition. J Bacteriol 190:2759–2766
Morales VM, Backman A, Bagdasarian M (1991) A series of wide-host-range low-copy-number vectors that allow direct screening for recombinants. Gene 97:39–47
Pilhofer M, Bauer AP, Schrallhammer M, Richter L, Ludwig W, Schleifer KH, Petroni G (2007) Characterization of bacterial operons consisting of two tubulins and a kinesin-like gene by the novel two-step gene walking method. Nucleic Acids Res 35:e135
Price-Whelan A, Dietrich LEP, Newman DK (2006) Rethinking ‘secondary’ metabolism: physiological roles for phenazine antibiotics. Nat Chem Biol 2:71–78
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Habor Laboratory, New York
Samina M, Baig DN, Jamil F, Weselowski B, Lazarovits G (2009) Characterization of a phenazine and hexanoyl homoserine lactone producing Pseudomonas aurantiaca strain PB-St2, isolated from sugarcane stem. J Microbiol Biotechnol 19:1688–1694
Schnider-Keel U, Seematter A, Maurhofer M, Blumer C, Duffy B, Gigot-Bonnefoy C, Reimmann C, Notz R, Défago G, Haas D, Keel C (2000) Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J Bacteriol 182:1215–1225
Shen J, Huang L, Peng HS, Hu HB, Zhang XH (2009) The reaction mechanism for phenazine-1-carboxylic acid to 2-hydroxyphenazine in Pseudomonas chlororaphis GP72. Abstr J Biosci Bioeng 108:114–134
Smirnov VV, Kiprianova EA (1990) Bacteria of Pseudomonas genus. Naukova Dumka, Kiev, pp 100–111
Whistler CA, Pierson LS III (2003) Repression of phenazine antibiotic production in Pseudomonas aureofaciens strain 30–84 by RpeA. J Bacteriol 185:3718–3725
Veselova MA, Klein Sh, Bass IA, Lipasova VA, Metlitskaya AZ, Ovadis MI, Chernin LS, Khmel IA (2008) Quorum sensing systems of regulation, synthesis of phenazine antibiotics, and antifungal activity in rhizospheric bacterium Pseudomonas chlororaphis 449. Russ J Genet 44:1400–1408
Acknowledgements
This study was supported by the 973 Programs of China (no. 2009CB118906), 863 Programs of China (no. 2006AA10A2009), National Nature Science Foundation of China (NSFC) (nos. 30821005 and 30870075), and Ph.D. Programs Foundation of Ministry of Education of China (no. 20090073110052).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, L., Chen, MM., Wang, W. et al. Enhanced production of 2-hydroxyphenazine in Pseudomonas chlororaphis GP72. Appl Microbiol Biotechnol 89, 169–177 (2011). https://doi.org/10.1007/s00253-010-2863-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2863-1