Abstract
Anaerobic growth of a newly isolated Pseudomonas putida strain WB from an arsenic-contaminated soil in West Bengal, India on glucose, l-lactate, and acetate required the presence of arsenate, which was reduced to arsenite. During aerobic growth in the presence of arsenite arsenate was formed. Anaerobic growth of P. putida WB on glucose was made possible presumably by the non-energy-conserving arsenate reductase ArsC with energy derived only from substrate level phosphorylation. Two moles of acetate were generated intermediarily and the reducing equivalents of glycolysis and pyruvate decarboxylation served for arsenate reduction or were released as H2. Anaerobic growth on acetate and lactate was apparently made possible by arsenate reductase ArrA coupled to respiratory electron chain energy conservation. In the presence of arsenate, both substrates were totally oxidized to CO2 and H2 with part of the H2 serving for respiratory arsenate reduction to deliver energy for growth. The growth yield for anaerobic glucose degradation to acetate was Y Glucose = 20 g/mol, leading to an energy coefficient of Y ATP = 10 g/mol adenosine-5'-triphosphate (ATP), if the Emden–Meyerhof–Parnas pathway with generation of 2 mol ATP/mol glucose was used. During growth on lactate and acetate no substrate chain phosphorylation was possible. The energy gain by reduction of arsenate was Y Arsenate = 6.9 g/mol, which would be little less than one ATP/mol of arsenate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Arsenic is a widespread metalloid compound on earth at an average concentration of 2 mg/kg soil. In some areas of the world, higher concentrations of arsenic in soil and sediments are found, for instance in Bangladesh and West Bengal, India, leading to groundwater concentrations of up to 1 mg/l (Chakraborti et al. 2002). Historically, weathering of arsenic-rich minerals in the Himalaya, transport of the particulate matter by the river Ganges and deposition of the arsenic-containing suspended material were considered to be responsible for the arsenic content of soil and sediments in the alluvial delta of West Bengal. Non-soluble arsenic compounds in soil may be mobilized and transferred as dissolved arsenic compounds into the aquifers (Harvey et al. 2005). Mobilization of arsenic, leading to the observed concentrations in groundwater, might be due to geochemical reactions, but also to microbial metabolism. A large number of bacteria (e.g. Escherichia coli and Staphylococcus aureus) have developed resistance-mechanisms to withstand toxicity of high concentrations of arsenic, but arsenic compounds in potable water are highly toxic for most prokaryotic and all eukaryotic organisms as well and lead to arsenicosis (Chakraborti et al. 2002). Arsenic-resistant bacteria characteristically express arsC-genes which reduce pentavalent arsenate to water-soluble trivalent arsenite, apparently without energy conservation (Rosen 1999). The arsenite is then exported by the microorganisms via an arsenite-specific transporter Arsb, which is located in the cytoplasmic membrane (Mukhopadhyay et al. 2002). On the other hand, there are also a number of bacteria, which are using arsenate for respiration (Oremland and Stolz 2005). These genera are characterized by dissimilatory respiratory arsenate reductase genes (arrA) and comprise a diverse phylogenetic group, including, e.g., Bacillus, Shewanella, and Sulfurospirillum species.
Pseudomonas putida is a Gram-negative, rod-shaped organism that is able to grow on a wide variety of organic substrates under aerobic or anoxic conditions. It is also known that P. putida has a wide spectrum of heavy metal and metalloid resistance genes, e.g., for copper, zinc, and nickel. Two open reading frames for arsenic resistance are encoded in P. putida KT2440 including the arsC-gene (Cánovas et al. 2003). In this study, a P. putida strain was isolated from arsenic-containing groundwater of West Bengal, India, and its arsenic metabolism during aerobic and anaerobic growth on several carbon sources was analyzed. This report shows for the first time the growth of P. putida under anaerobic conditions with arsenate as the only electron acceptor and the formation of hydrogen during complete mineralization of the carbon sources.
Materials and methods
Isolation of bacteria from arsenic-contaminated groundwater
Groundwater was pumped from a 19 ft deep household well in Sahispur (N 23° 04′15.5″, E 88°36′33.5″), district of Nadia and 100 ml of a representative sample was collected from the water stream in a sterilized plastic bottle after several minutes of pumping. The sample contained 285 μg/l total arsenic and a DOC of 1.5 mg/l. The bacterial population was 5 × 105/ml as determined by microscopical counting after ten-fold enrichment by centrifugation. For enrichment of anaerobic, arsenate-resistant bacteria, 1 ml of the water was inoculated into 20 ml R2A-medium (Reasoner and Geldreich 1985) in 100 ml serum bottles that contained 5 mmol/l arsenate. Serum bottles were closed with a rubber stopper that was fixed by an aluminum cap. The air atmosphere was exchanged for nitrogen. The bottles were incubated for 48 h at 27°C on a shaker at 160 rpm. After growth, 100 μl of the enrichment culture were streaked on R2A-agar plates that contained 5 mmol/l arsenate. The agar plates were transferred into an anaerobic jar and incubated at 27°C under a nitrogen atmosphere for 48 h. Several colonies were picked and re-streaked on R2A-agar plates which were subjected to the above mentioned incubation procedure for growth. After the second re-streaking single colonies were picked from the Petri dishes and transferred into serum bottles with 20 ml of R2A medium + 5 mmol/l arsenate. After growth in liquid medium under the same conditions as above, cells from the best growing culture were either harvested for DNA extraction or 1 ml was inoculated into serum bottles with 20 ml of test medium that contained 1 g/l glucose, 0.25 g/l yeast extract, 0.3 g/l K2HPO4 ± 0.695 g/l arsenate under a nitrogen atmosphere. Alternatively, glucose was omitted and 0.2 ml of a 1.5 M stock solution of sodium acetate or a 1 M stock solution of sodium l-lactate was added to 20 ml as the main carbon source to the media. No growth occurred in media without arsenate. For aerobic growth of the isolated culture the test medium was prepared in serum bottles, but instead of sodium-arsenate 0.695 g/l sodium arsenite was supplemented. For an OD of E 578 = 1.2, the cell dry weight was 2.2 mg/20 ml medium. The gas phase for aerobic cultures was synthetic air, which contained 21% oxygen, 79% nitrogen.
Identification of the bacterial isolate WB as a strain of P. putida
From the culture that grew best with arsenate as an electron acceptor in the absence of oxygen genomic DNA was isolated by the method of Murray and Thompson (1980). For identification of the strain universal eubacterial 16S rDNA sequencing primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) (Lane 1991) were used. DNA amplification was performed in a total volume of 25 μl, containing 1 μl dissolved genomic DNA, 1.25 U Taq-polymerase, 10 pmol of each primer, 0.25 μl dNTP-mixture (2.5 mM each), 2.5 μl 10 × polymerase chain reaction (PCR) buffer and 1.5 μl MgCl2. 16S rDNA was amplified in a Biometra Thermocycler T Gradient using following conditions: 95°C for 5 min., followed by 35 cycles with 95°C for 30 s, 52°C for 1 min., 72°C for 2 min. and a final extension of 72°C for 5 min. Amplified DNA was isolated by gel-electrophoresis of aliquots of PCR mixtures (2 μl with 5 μl 6× loading-dye) using 1% agarose in 5 × TBE-buffer and was sequenzed by MWG Biotech (Ebersberg, Germany). Sequences were compared with those in the NCBI database using the BLAST search program to find the most closely related strains. All further experiments were done with this isolate, identified below as P. putida WB (WB derived from “West Bengal”) by its 16S rRNA gene (EMBL accession No. FN6886776).
To validate the classification as P. putida by the 16S rRNA gene strain WB was further characterized by microscopy, Gram staining and by testing for catalase and oxidase. Biomass production during growth in the presence of arsenate and doubling times were also determined. The strain was deposited at DSMZ Braunschweig (DSM 23849).
Detection of the arsC- and arrA-gene
The arsC-gene of P. putida WB was analysed by PCR using primers amlt-42-f (3′-TCGCGTAATACGCTGGAGAT-5′) and amltf-376-r (3′-ACTTTCTCGCCGTCTTCCTT-5′) (Sun et al. 2004). Amplification reactions were performed in a total volume of 25 μl containing 2 μl dissolved genomic DNA, 1.25 U Taq-polymerase, 10 pmol of each primer, 0.25 μl dNTP-mixture (2.5 mM each), 2.5 μl 10× PCR-buffer and 1.5 μl MgCL2. PCR conditions were as follows: 95°C for 3 min, followed by 40 cycles with 95°C for 30 s, 55°C for 30 s, and 72°C for 30 s. The arrA-gene was also detected by PCR using primers arrA for (3′-TTATTCCAGGGAAGATG-5′) and arrArev (3′-TCTTTAAGCGGGGAATTC-5′) (Malasarn et al. 2004). PCR conditions for amplification were as follows: 95°C for 5 min, followed by 40 cycles with 95°C for 30 s, 52°C for 30 s, and 72°C for 30 s. Amplified DNA was verified by gel-electrophoresis of aliqouts of PCR-mixtures (2 μl with 5 μl 6× loading-dye) using 1% agarose in 5× TBE-buffer. As references strains P. putida DSM 291 and Desulfosporosinus auripigmenti DSM 13351 (obtained from Deutsche Sammlung für Mikroorganismen und Zellkulturen GmbH, Braunschweig) were used.
Determination of glucose, acetate, lactate, arsenate, arsenite, hydrogen and cell mass
Glucose was determined by the colorimetric method of Miller (1959) d- and l-lactic acid were determined with the respective test kits of Boheringer (Mannheim). Acetate was determined by gas-chromatography (Model 437, Chrompack, equipped with a FID; Gallert and Winter 1997). Hydrogen and oxygen were also determined by gas-chromatography (Model CP9001, Chrompack, equipped with a TCD) according to Gallert and Winter (1997). Arsenate was determined by ion-exchange-chromatography (ICS90, Dionex) with a 4 × 250 mm IonPac A89-HC column (Dionex Austin, Texas USA). Arsenite in arsenate containing mixtures was also determined by ion-exchange-chromatography by substracting the arsenate amount after complete oxidation of arsenite with 1% H2O2 from the amount of arsenate before arsenite oxidation. Moles of hydrogen or oxygen in headspace gases were calculated with the ideal gas law. Cells from 20 ml cultures were pelleted by centrifugation and the pellet was washed twice with tap water before it was dried to constant weight at 105°C.
Results
Classification of isolate WB as P. putida WB by its 16S rRNA gene sequence and by other features
The 16S rRNA gene sequence of the DNA of isolate WB from groundwater taken in the district Nadia/West Bengal/India was detected with the universal primers 27F and 1492R (Lane 1991) of Pseudomonas putida KT2440 (Tumler and Fraser 2002) using the BLAST search program (Altschul et al. 1990). It was 100% identical with P. putida KT2440. The identity with several other P. putida strains from the NCBI data base was 99% (Table 1). Thus, isolate WB was a P. putida strain and is designated P. putida WB.
As expected, P. putida WB shared the main features of the genus Pseudomonas. It was a Gram-negative, motile, non-spore-forming rod-shaped bacterium, with a size of 1–1.5 × 2–3 μm that reacted positive in oxidase- and catalase-tests. The ideal growth temperature was 27°C. It had a doubling time of 25 h with glucose as a substrate for aerobic growth. In anaerobic 20-ml cultures an optical density of E 578 = 1.2 represented 2.2 mg dry weight of P. putida WB cells.
Arsenate reduction and hydrogen production during anaerobic growth of P. putida WB and P. putida DSM 291 on glucose and acetate
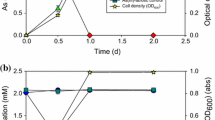
P. putida WB grew exponentially on glucose under anaerobic conditions and formed almost 2 mol acetate per mol glucose, but only when arsenate was provided as an electron acceptor (Fig. 1a, b). Without arsenate P. putida WB did not grow at all. During glucose metabolism arsenate was reduced to arsenite and some hydrogen was released. During anaerobic growth to an optical density of E578 ŋm = 1.2 (Fig. 1a at 100 h), 0.11 mmol glucose and 0.043 mmol arsenate (total amounts per 20-ml assay) were metabolized. An average of 0.2 mmol acetate was generated from 0.11 mmol glucose. All arsenate was reduced to arsenite and about 0.3 mmol hydrogen were liberated. The bacterial dry mass was 2.2 mg/0.11 mmol Glucose, equivalent to 20 g dry mass/mol glucose metabolized anaerobically in the presence of arsenate. The stoichiometry of glucose conversion to acetate in the presence of arsenate (Fig. 1b at 100 h) is given in Eq. 1. It is assumed that release of 1 mmol acetate from pyruvate decarboxylation gives 1 mmol CO2. Biomass formation may have consumed about 10% of the substrate.
a, b, c Anaerobic growth on glucose (filled diamonds) and acetate (filled squares) (a), as well as glucose (b) and acetate metabolism (c) of Pseudomonas putida WB in the presence of arsenate. Arsenate was oxidized to arsenite during glucose conversion to acetate, CO2, and H2. Acetate from glucose (b) or from external sources (c) could only be oxidized to CO2 and H2 when arsenate was present as an electron acceptor. CO2 was not quantified. In the experiment of (c), acetate and arsenate were added at time zero and after 60 and 120 h, acetate once again after 180 h
After complete oxidation of glucose to acetate and reduction of all arsenate to arsenite growth of P. putida WB stopped. No growth at the expense of acetate occurred in the absence of arsenate (Fig. 1a, 70–100 h). However, growth continued with the acetate as carbon source if arsenate was replenished (Fig. 1a, 100 h onwards). During anaerobic metabolism of the acetate that was released during glucose degradation or was supplemented from an external source (Fig. 1b at 140 h) to CO2 and reducing equivalents, the hydrogen served either for arsenate reduction or was released as H2. The replenished 0.3 mol acetate at 140 h (Fig. 1b) were completely oxidized with the formation of 0.043 arsenite from arsenate and additionally 0.5 mmol H2 were generated. Stoichiometrically there was a hydrogen deficiency from acetate oxidation and arsenate reduction, presumably caused by losses through the rubber stopper. Thus, the stoichiometry for anaerobic acetate oxidation by P. putida WB in the presence of arsenate was derived from a separate assay. Acetate was the sole growth substrate and much higher arsenate concentrations for hydrogen consumption were supplied. In addition, serum bottles were incubated bottom-up to avoid hydrogen losses (Fig. 1a, c). Acetate (0.3 mmol) was replenished three times after 60, 120, and 180 h, and arsenate was replenished two times after 60 and 120 h incubation time. Growth, acetate and arsenate reduction as well as arsenite and hydrogen formation were measured during the experiment. Concentrations were summed up and stoichiometries calculated for the whole experiment (Eq. 2; numbers are mmol total; 1 mmol acetate was assumed to give 2 mmol CO2 since yeast extract could be used for biomass formation):
The hydrogen production rate apparently was not affected by accumulating hydrogen in the gas phase, indicating no influence of the H2-partial pressure on hydrogen generation. After 240 h, 4.3 mmol of hydrogen were generated from a total of 1.2 mmol acetate while 0.4 mmol arsenate were apparently reduced to 0.27 mmol arsenite (the difference to arsenate may be due to incomplete oxidation during analysis). Thus, if 1.2 mmol acetate were anaerobically oxidized to 2.4 mmol CO2 and theoretically 4.8 mmol H2, and 0.4 mmol H2 were required for reduction of 0.4 mmol arsenate to arsenite, 4.4 mmol H2 should be generated. This was in good agreement with 4.3 mmol H2 that were actually detected. Growth to a final OD of 1.5 was equivalent to 2.75 mg cells per 20 ml assay. Assuming a molar energy coefficient of Y ATP = 10 g/mol ATP, the 2.75 mg cell dry weight would be equivalent to 0.275 mmol ATP, that must have been generated from reduction of 0.4 mmol arsenate (arsenate added minus used) by arsenate reductase ArrA. Thus the energy conservation from reduction of 0. 4 mmol arsenate to arsenite was Y Arsenate = 6.9 g cells per mol arsenate that was respired.
The same experiments with glucose and acetate as growth substrates were preformed with the reference strain P. putida DSM 291 and similar stoichiometries were obtained (data not shown).
Respiration of arsenate with lactate as carbon source
To test anaerobic growth of P. putida WB with other carbon sources than glucose or acetate, 0.2 mmol of lactate was supplied as the main carbon source in the test medium together with 0.043 mmol of arsenate, which was replenished after 60 h (Fig. 2a, b). P. putida WB grew exponentially to an optical density of 1.2 (Fig. 2a) while the lactate was decarboxylated stoichiometrically to acetate. After 140 h lactate was completely oxidized and totally 0.086 mmol arsenate were reduced to arsenite (Fig. 2b). Acetate utilization apparently started already when little lactate was still left, but growth and acetate utilization stopped after 140 h when arsenate was used up (Fig. 2b). At that time 0.4 mmol hydrogen were produced. The stoichiometry for lactate utilization and arsenate reduction from Fig. 2b can be formulated as follows (Eq. 3):
In a control experiment P. putida DSM 291 was supplied with 0.2 mmol lactate and 0.86 mmol arsenate. The organisms grew exponentially and oxidized the lactate to intermediarily 0.3 mmol acetate which disappeared after lactate depletion. Overall, 0.045 mmol arsenite and 0.4 mmol hydrogen were formed (data not shown).
Aerobic growth with glucose as the only carbon source in the presence of arsenite
To test the influence of arsenite on aerobic growth of P. putida WB 0.079 mmol sodium arsenite was added to the medium. There was an exponential growth for 96 h to a final optical density of 1.6, which was equivalent to 3.3 mg cells in the 20 ml assay (Fig. 3a). Totally, 0.48 mmol of glucose was respired with 3.25 mmol oxygen and 0.22 mmol arsenite were oxidized to 0.22 mmol arsenate (Fig. 3b). The oxygen supply for each portion was calculated for glucose respiration and arsenite oxidation. In a control experiment without glucose, only 0.03 mmol arsenite were oxidized after 96 h, excluding a rapid chemical oxidation. With the data of Fig. 3b the following stoichiometry could be derived (CO2 estimated from glucose respiration; Eq. 4).
P. putida DSM 291 reacted similarly (data not shown). As in the experiment with the P. putida WB, arsenite was completely oxidized to arsenate after 96 h. For complete oxidation of 0.48 mmol glucose and 0.22 mmol arsenite theoretically 3 mmol oxygen were required.
Identification of the arsC- and the arrA-gene
To demonstrate the genetic capacity of P. putida WB for detoxification and/or respiration of arsenate by arsenate reductases arsC- and arrA-genes were isolated. Both genes were detected in P. putida WB. arcC contained 350 bp and arrA 620 bp. As positive controls D. auripigmenti, a sulfur-reducing bacterium that is known to be able to reduce arsenate and P. putida DSM 291 were used. Both control strains and the isolated P. putida strain WB showed the bands for the arsC- and arrA-genes (Fig. 4).
Discussion
This study reports the isolation of a P. putida, strain WB from arsenic-contaminated groundwater in West Bengal and its ability to generate hydrogen during anaerobic mineralization of different carbon sources with arsenate as an electron acceptor. The arsenic concentration in West Bengal can be as high as 1 mg/l (Chakraborti et al. 2002), whereas in the location, where the groundwater samples were taken, about 300 μg/l arsenic was found. A number of studies in this area on geochemical and microbial mobilization of arsenic in soil, contaminating the groundwater are available in the literature (e.g. Nickson et al. 2000; Héry et al. 2010). A new bacterial species that was isolated from an aquifer in West Bengal, Deinococcus indicus, was resistant against arsenic and was able to grow aerobically on different carbon sources such as lactose or arabinose in the presence of 10 mmol/l arsenate (Suresh et al. 2004). However, no accurate turnover of carbon sources and arsenic was determined. There are a number of other studies describing isolates from different arsenic-containing environments that were able to respire arsenate (Huber et al. 2000; Lear et al. 2007; Bachate et al. 2009; Zhou et al. 2010). Newamn et al. (1997) described a sulfur-reducing bacterium, D. auripigmenti that reduced 5 mmol/l of arsenate in 144 h completely to arsenite while growing on 5 mmol/l of lactate, but no growth on acetate or only acetate oxidation was observed. In our study P. putida WB reduced 4.5 mmol/l of arsenate during anaerobic growth on 8.5 mmol/l lactate. In contrast to Newman’s study we added arsenate twice, 2.25 mmol/l each (0.045 mmol total/20 ml assay). The arsenate was reduced completely in 120 h. Srivastava et al. (2009) isolated a Pseudomonas sp. DRBSI that was closely related to Pseudomonas stutzeri which was able to reduce up to 100 mmol/l of arsenate under aerobic conditions in NB-medium. No hydrogen formation was observed, as expected for aerobic conditions. In contrast P. putida WB from our study does not reduce arsenate under aerobic conditions, but it oxidizes arsenite to arsenate. Santini et al. (2002) isolated a strictly anaerobic Bacillus-like strain JMM-4 from arsenic-contaminated mud from a gold-mine in Australia that was able to grow anaerobically on arsenate with lactate as the sole carbon source. This bacterium reduced 5 mmol/l of arsenate completely to arsenite in parallel to the oxidation of 5 mmol/l of lactate. The formation of hydrogen was not investigated. In contrast to D. auripigmenti (Newman et al. 1997) the JMM-4 Bacillus-like strain could use acetate as a sole carbon source. Another well-known arsenate-reducing bacterium, Chrysiogenes arsenatis was also able to grow on acetate as electron donor with arsenate as the terminal electron acceptor. This species reduced 3.9 mmol/l of arsenate while oxidizing 1 mmol/l of acetate (Macy et al. 1996). The formation of hydrogen was again not investigated. Jones et al. (2000) enriched a strain of Clostridium intestinales, an arsenate-reducer from an agricultural soil that contained naturally elevated concentrations of arsenic (840 μmol/kg) and investigated the reduction of arsenate under anaerobic conditions in serum bottles with 50 ml nutrient solution at 25°C and under shaking. As a carbon-source 14C-spiked glucose was used (5 mmol/l) and 0.6–5 mmol/l of As(V) was the sole electron acceptor. Hydrogen production after complete consumption of glucose was equimolar to the released carbon dioxide. Since butyrate was the main fermentation product the authors concluded that their strain was using the butyric acid pathway, generating 1 mol of butyrate, 2 mol of CO2 and 2 mol of H2 per mol glucose. Traces of acetic acid, formic acid, butanol and sec-butyl butyrate were also detected. The formation of hydrogen in these experiments seemed not to be dependent on arsenate reduction, thus C. intestinales did not grow by arsenate respiration.
P. putida WB in contrast had an essential requirement for arsenate to be able to grow at all on glucose, lactate or acetate in the absence of oxygen. Two arsenate reductases were apparently expressed by P. putida WB (Fig. 4), the non-respirative ArsC for detoxification (Rosen 2002) and ArrA for respirative arsenate reduction (Malasarn et al. 2004). Anaerobic glucose degradation to acetate did not require energy from arsenate reduction since 2 mol of ATP were available per mol glucose by substrate level phosphorylation during glycolysis via the EMP-pathway. This was apparently the case since 2.2 mg of cell mass (derived from the OD measurement) was formed from 1.1 mmol of glucose, resulting in YGlucose = 20 g/mol. With a YATP = 10 g/mol this would indicate a specific energy gain of 2 mol ATP. The reducing equivalents generated by glycolysis were partially used for arsenate reduction and partially released as hydrogen. No glucose utilization was possible in the absence of arsenate.
Two moles acetate were generated from 1 mol glucose and remained in the medium when arsenate was used up, and growth stopped. Only when arsenate was replenished (Fig. 1b at 90 h), growth continued and acetate was oxidized under formation of arsenite and hydrogen. The energy for growth on acetate must have come from respiratory arsenate reduction, since acetate oxidation per se would not allow energy conservation. If 1 mol ATP could be generated by reduction of 1 mol arsenate, then the Y ATP was 6.9 g/mol. In Fig. 5 the reactions during anaerobic growth of P. putida WB on glucose, acetate and lactate are summarized. Based on the obtained reaction stoichiometries with the respective substrates and on the growth response, the involvement of ArsC and ArrA was included. Under anaerobic conditions in the absence of oxygen glucose could be converted to acetate with an energy gain from substrate level phosphorylation during glycolysis only in the presence of arsenate when at least some of the hydrogen was removed by non-respiratory ArsC for arsenate reduction. Anaerobic acetate oxidation was coupled to respiratory arsenate reduction and proceded to completion only if enough arsenate was present. Lactate was sequentially oxidized via pyruvate and acetate and was associated with growth of P. putida. Lactate oxidation to pyruvate and the subsequent decarboxylation of pyruvate to acetate as well as the anaerobic oxidation of acetate both seemed to allow growth by arsenate respiration with ArrA.
References
Altschul SF, Gish W, Miller W, Myers EW, Lipmann DJ (1990) Basic local alignment search tool. J Mol Biol 215:403–410
Bachate SP, Cavalca L, Andreoni V (2009) Arsenic-resistant bacteria isolated from agricultural soils of Bangladesh and characterization of arsenate-reducing strains. J Appl Microbiol 107:145–156
Cánovas D, Cases I, de Lorenzo V (2003) Heavy metal tolerance and metal homeostasis in Pseudomonas putida as revealed by complete genome analysis. Environ Microbiol 5(12):1242–1256
Chakraborti D, Rahman MM, Paul K, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, Saha KC, Mukherjee SC (2002) Arsenic calamity in the Indian subcontinent: What lessons have been learnt? Talanta 58:3–22
Gallert C, Winter J (1997) Mesophilic and thermophilic anaerobic digestions of source-sorted organic waste: effect of ammonia on glucose degradation and methane production. Appl Microbiol Biotechnol 48:405–410
Harvey CF, Swartz CH, Badruzzaman ABM, Keon-Blute N, Yu W, Ali MA, Jay J, Beckie R, Niedan V, Brabander D, Oates PM, Ashfaque KN, Islam S, Hemond HF, Ahmed MF (2005) Groundwater arsenic contamination on the Ganges Delta: biogeochemistry, hydrology, human perturbations, and human suffering on a large scale. Geosci 337:285–296
Héry M, Van Dongen BE, Gill F, Mondal D, Vaughan DJ, Pancost RD, Polya DA, Lloyd JR (2010) Arsenic release and attenuation in low organic carbon aquifers from West Bengal. Geobiol 8:155–168
Huber R, Sacher M, Vollmann A, Huber H, Rose D (2000) Respiration of arsenate and selenate by hyperthermophilic Archea. Syst Appl Microbiol 23:305–314
Jones CA, Langner HW, Anderson K, McDermott TR, Inskeep WP (2000) Rates of microbially arsenate reduction and solubilization. Soil Sci Soc Am J 64:600–608
Lane DJ (1991) 16 S/23 S rRNA sequencing. Wiley, Chichester, UK
Lear G, Song B, Gault AG, Polya DA, Lloyd JR (2007) Molecular analysis of arsenate-reducing bacteria within Cambodian sediments following amendment with acetate. Appl Environ Microbiol 73(4):1041–1048
Macy JM, Nunan K, Hagen KD, Dixon DR, Harbour PJ, Cahill M, Sly LI (1996) Chrysiogenes arsenatis gen. nov., sp. nov., a new arsenate-respiring bacterium isolated from gold mine wastewater. Int J Syst Bacteriol 46(4):1153–1157
Malasarn D, Saltikov CW, Campbell KM, Santini JM, Hering JG, Newman DK (2004) arrA is a reliable marker for As(V) respiration. Science 306:455
Miller GL (1959) Use of dinitrosalicylic acid for determination of reducing sugar. Anal Chem 31(3):426–428
Mukhopadhyay R, Rosen BP, Phung LT, Silver S (2002) Microbial arsenic: from geocycles to genes and enzymes. FEMS Microbiol Rev 26:311–325
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8(19):4321–4325
Newman DK, Kennedy EK, Coates JD, Ahmann D, Ellis DJ, Lovley DR, Morel FMM (1997) Disslimilatory arsenate and sulfate reduction in Desulfotomaculum auripigmentum sp. nov. Arch Microbiol 168:380–388
Nickson RT, McArthur JM, Ravencroft P, Burgess WG, Ahmed KM (2000) Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl Geochem 15:403–413
Oremland RS, Stolz JF (2005) Arsenic, microbes and contaminated aquifers. Trends Microbiol 13(2):45–49
Reasoner DJ, Geldreich EE (1985) A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol 49:1–7
Rosen BP (1999) Families of arsenic transporters. Trends Microbiol 7(5):207–212
Rosen BP (2002) Biochemistry of arsenic detoxification. FEBS Lett 529:86–92
Santini JM, Stolz JF, Macy JM (2002) Isolation of a new arsenate-respiring bacterium—physiological and phylogenetic studies. Geomicrobiol J 19:41–52
Srivastava D, Madamwar D, Subramanian RB (2009) Pentavalent arsenate reductase activity in cytosolic fractions of Pseudomonas sp., isolated from arsenic-contaminated sites of Tezpur, Assam. Appl Biochem Biotechnol. doi:10.1007/s1201000988520
Sun Y, Polishuk EA, Radoja U, Cullen WR (2004) Identification and quantification of arsC genes in environmental samples by using real-time PCR. J Microbiol Meth 58:335–349
Suresh K, Reddy GSN, Sengputa S, Shivaji S (2004) Deinococcus indicus sp. nov., an arsenic-resistant bacterium from an aquifer in West Bengal, India. Int J Syst Evol Microbiol 54:457–461
Tumler B, Fraser CM (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4(12):799–808
Zhou Y, Yao J, He M, Choi MMF, Feng L, Chen H, Wang F, Chen K, Zhuang R, Maskow T, Wang G, Zaray G (2010) Reduction in toxicity of arsenic(III) to Halobacillus sp. Y35 by kaolin and their related adsorption studies. J Hazard Mater 176:487–494
Acknowledgement
This research was supported by a grant of the German Research Foundation and the German Federal Ministry of Economic Cooperation and Development (DFG, AZ Wi 524/20-1). We thank the Indian cooperation partners for perfect organization and for their help to take samples on site.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Freikowski, D., Winter, J. & Gallert, C. Hydrogen formation by an arsenate-reducing Pseudomonas putida, isolated from arsenic-contaminated groundwater in West Bengal, India. Appl Microbiol Biotechnol 88, 1363–1371 (2010). https://doi.org/10.1007/s00253-010-2856-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2856-0