Abstract
The aerobic metabolism of monofluorophenols (mono-FPs) by the actinomycete, Pseudonocardia benzenivorans, was studied. This strain was able to grow on 4-fluorophenol (4-FP) and readily transform 2- and 3-fluorophenol to the corresponding metabolites. The detailed mechanism of mono-FPs degradation by P. benzenivorans was elucidated from enzymatic assays and the identification of reaction intermediates by high-performance liquid chromatography (HPLC) and gas chromatography–mass spectrometry. Two types of fluorocatechols (i.e., 3- and 4-fluorocatechol) were identified as the key transformation products. During 4-FP degradation, only 4-fluorocatechol was detected, and a stoichiometric level of fluoride was released. Both fluorocatechols were observed together in cultures containing 3-fluorophenol (3-FP), while only 3-fluorocatechol was found to accumulate in 2-fluorophenol (2-FP)-containing cultures. Whole-cell extracts of P. benzenivorans expressed catechol 1,2-dioxygenase activity, indicating that the transformation of the three tested mono-FPs proceeded via ortho-cleavage pathway. The results presented in this paper provide comprehensive information regarding the metabolism of mono-FPs by a single bacterium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fluorinated compounds are increasingly being used for the commercial production of agrochemicals and pharmaceuticals in recent decades, and have thus become a significant environmental concern (Key et al. 1997). Despite numerous studies on the degradation of chloroaromatic compounds (Haggblom 1990; Mars et al. 1997), relatively little is known about the biological degradation of fluoroaromatic compounds. This could be, in part, because these compounds are rarely found in nature, so existing microorganisms have had comparatively little time to adjust to them as potential substrates. Besides, fluoroaromatic compounds generally tend to be more resistant to microbial degradation. The incorporation of fluorine into a molecule allows a considerable change in the oxidation potential due to its high electronegativity and thus strong electron-withdrawing property. Previous studies have shown that the presence of fluorine in aromatic systems can block the enzymatic oxidation at the specific position (Buhler et al. 1983; Engesser et al. 1988; Thakker et al. 1984). Hence, fluorine substitution has been used in many ways to develop enzyme inhibitors and to reduce the rate of oxidative metabolism (Park et al. 2001). To date, only a few microorganisms have been reported as being capable of using fluorinated compounds as a sole carbon and energy source.
Most of the studies concerning the aerobic metabolism of monofluorophenols (mono-FPs) have focused on co-metabolic transformation by several Rhodococcus (bacteria) and Penicillium (fungi) strains (Boesmna et al. 1998, 2001; Bondar et al. 1998, 1999; Finkelstein et al. 2000; Hofrichter et al. 1994; Marr et al. 1996). Rhodococcus species used in previous researches were originally isolated from the oil-contaminated sites, but they showed a particular ability to degrade phenol (Gorlatov and Golovleva 1992). Oxidative conversion of mono-FPs by Rhodococcus strains gives rise to common metabolic intermediates: two types of fluorocatechols and trihydroxyfluorobenzene derivatives (Boesmna et al. 1998; Finkelstein et al. 2000). Phenol grown cells of the fungus Penicillium frequentans transformed 3- and 4-fluorophenol (3-FP and 4-FP) to the corresponding catechols and 4-carboxymethylenebut-2-en-4-olide in the presence of phenol as co-substrate (Hofrichter et al. 1994). Penicillium simplicissimum was able to completely mineralize 3-and 4-FP under co-metabolic conditions (Marr et al. 1996). Recently, the degradation of mono-FPs by newly isolated bacterial strains has been described (Carvalho et al. 2002; Ferreira et al. 2008). Fluorobenzene-degrading Rhizobiales strain F11 has been shown to effectively utilize 4-FP as a carbon source, but the associated metabolic pathway is not yet known (Carvalho et al. 2002). Arthrobacter sp. IF1 was capable of growing on 4-FP as a carbon substrate. 4-FP was initially oxidized to hydroquinone which was subsequently converted into maleylacetate (Ferreira et al. 2008).
Mycobacteria, streptomycetes, and actinomycetes have been shown to degrade a wide range of halogenated aromatic compounds (Whalen et al. 1993; Zaitsev et al. 1995, Zaitsev and Surovtseva 2000). Since contaminants are typically present in the environment as complex mixtures, the broad metabolic diversities of these bacterial groups make them attractive candidates for use in waste treatment. The actinomycete, Pseudonocardia benzenivorans, was already known to be capable of metabolizing several organic compounds, such as 1,2,3,5-tetrachlorobenzenes and 1,4-dioxane (Kampfer and Kroppenstedt 2004; Mahendra and Alvarez-Cohen 2006), but the comprehensive information on the catabolic pathways and enzymes were not presented in either case. To our knowledge, this is the first report showing that P. benzenivorans can effectively decompose mono-FPs. Few fluorinated compound-degrading microorganisms have been isolated to date, so this bacterium may prove useful in the future remediation of fluorinated compounds. The mechanistic details involved in the mono-FPs degradation have been elucidated using enzyme assay and identification of reaction intermediates.
Materials and methods
Chemicals
2-fluorophenol (2-FP), 3-fluorophenol (3-FP), 4-fluorophenol (4-FP), and 3-fluorocatechol were from Sigma-Aldrich (St. Louis, USA). 4-Fluorocatechol was from TCI America (Tokyo, Japan). Ethyl acetate, acetonitrile, 85% ortho-phosphoric acid and nutrient broth (NB) were from Merck (Darmstadt, Germany). All chemicals and solvents were of the highest grade available.
Bacterial strain and culture conditions
P. benzenivorans was purchased from Deusche Sammlung von Mikroorganismenund Zellkulturen GmbH (DSMZ 44703 T). The cells were grown in 250-ml Erlenmeyer flasks with screw caps containing 50-ml mineral salts medium (Fortnagel et al. 1990) or NB medium with benzene. NB medium had 8 g of Nutrient Broth per liter of distilled water. Cultures were grown in shaking incubator (160 rpm) at 30 °C.
Growth of P. benzenivorans on various substrates
To check the ability of P. benzenivorans to use mono-FPs as sole carbon substrates, we observed the growth of the strain in the presence of 2-FP, 3-FP, and 4-FP with 1 mM. Several probable intermediates, such as phenol, catechol, 3-fluorocatechol, and 4-fluorocatechol, were also tested as growth substrates. Pre-grown cells on benzene were inoculated in 25-ml mineral medium in 125-ml baffled Erlenmeyer flasks with screw caps. All cultures were incubated at 30 °C in rotary shaker. Since the proliferated cells of P. benzenivorans tend to aggregate in the culture medium, which hampers the spectrophotometric measurement of absorbance, the growth was estimated by measuring the dry weight of biomass. Samples were filtered through the dried membrane filters (pore size, 0.2 µm, Advantec MFS, CA). The filters were then dried at 80 °C for 30 min.
Biotransformation of mono-FPs by resting cell suspension
Cells that pre-grown in NB medium or mineral medium with 4-FP were harvested by centrifugation at 13,000×g for 15 min at 4 °C washed three times using 20 mM phosphate buffer (PB, pH 7.2). The cell pellet (3.0 g dry weight/l) was resuspended in sterile 20 mM PB. The biotransformation was monitored by employing 125-ml Erlenmeyer flasks; each bottles contained 20 ml of cell suspension and 2.5 mM of 2-FP, 3-FP, and 4-FP. Heat-killed (70 °C, 40 min) and poisoned (with 10 mM sodium azide) cells were also prepared as controls. All cultures were incubated at 30 °C in rotary shaker. At different time intervals, a set of triplicate flasks was removed, and stored at −70 °C until processing for qualitative and quantitative analysis.
Preparation of washed cell free extracts
Grown cells on 4-FP and induced cells by 2-FP, 3-FP were harvested, respectively, by centrifugation at 13,000×g for 20 min at 4 °C, washed three times with 50 mM Tris–HCl buffer (pH 7.5), and finally resuspended in 10 ml of 33 mM Tris–HCl buffer (pH 8.0). Suspended cells were incubated with lysozyme (1 mg/ml) for 1 h at room temperature, and then disrupted by a chilled homogenizing sonicator (IKA T-25, Germany). The crude cell extracts were centrifuged at 10,000×g for 30 min at 4 °C. The separated supernatant was immediately used for the enzyme activity measurements. Protein concentration was determined using the Bradford reagent.
Enzyme assays
All enzyme assays were done by UV/Vis spectrophotometer (Cary 3-Bio, Varian, Victoria, Australia). Phenol hydroxylase activity (Gurujeyalakshmi and Oriel 1989) was determined at 510 nm. The reaction mixture contained 0.1 mM phenol, 1 mM NADH, 100 mM Na2HPO4 buffer and cell extracts (0.05 mg protein). Catechol 1,2-dioxygenase (Dorn and Knackmuss 1978) and catechol 2,3-dioxygenase (Nozaki 1970) activities were measured at 260 and 375 nm, individually. The reaction samples included 0.1 mM catechol, 33 mM Tris–HCl buffer (pH 8.0), and cell extracts. The activity of 3-fluorocatechol and 4-fluorocatechol 1,2-dioxygenase was measured with 3-fluorocatechol and 4-fluorocatechol in place of catechol. One unit of enzyme activity (U) is defined as that consuming 1 μmol of phenol for phenol hydroxylase or the forming 1 μmol of cis,cis-muconate and 2-hydroxymuconic semialdehyde for catechol 1,2- and 2,3-dioxygenase per minute under the assay conditions. The specific activities are expressed as units per mg of cell protein.
Analytical procedure
The concentrations of mono-FPs and fluorocatechols were determined by reverse-phase high-performance liquid chromatography (HPLC). Samples (1 ml) were withdrawn periodically from the culture medium, centrifuged at 13,000×g for 5 min, and filtered through a 0.45-µm syringe filter (Acrodisc, Pall Corporation, MI). HPLC was equipped with a ZORBAX SB C-18 column and a diode array detector system (Agilent 1100 series, Germany). The mobile phase used was a binary mixture of 0.1% (w/v) phosphoric acid and 20% acetonitrile. Absorption was monitored at 210 nm. The recovery of analytes (mean ± S.D.%) ranged from 94.7 ± 1.5 to 101.7 ± 2.9%.
Identification of fluorocatechols and other polar intermediates was carried out using gas chromatography–mass spectrometry (GC-MS). The GC-MS instrument was composed of Trace GC 2000 coupled with a 60 m DB-5 capillary column (Agilent, Palo Alto, CA) and Polaris Q Ion Trap MS (Thermoquest, San Jose, CA). The initial oven temperature was maintained at 60 °C, held for 5 min, and increased to 300 °C at a rate of 15 °C/min and finally held for 5 min. Before GC-MS analysis, all samples were extracted four times with an equal volume of chilled ethyl acetate and concentrated under reduced pressure, and then derivatized with BSTFA (N,O-bis-(trimethylsilyl) trifluoroacetamide) to form trimethylsilylated compounds by incubation at 60 °C for 40 min. Metabolites were confirmed by comparison of retention time and mass spectra in GC-MS of those standards. The concentration of fluoride in the aqueous phase was determined using an ion selective electrode (model RZ-27504, Cole-Parmer, Vernon Hills, IL).
Results
Determining the catabolic activities of P. benzenivorans
We first tested the ability of P. benzenivorans to use several aromatic compounds, including phenol, mono-FPs, catechol, and fluorocatechols, as growth substrates. Cells pre-grown on benzene were inoculated to mineral medium containing the various compounds at a final concentration of 1 mM each, and growth was monitored over three weeks (actinomycete species tend to grow slowly). After this period, growth was evaluated by measurements of biomass and fluoride release. We found that the tested strain was capable of using phenol, 4-FP, catechol, and 4-fluorocatechol as sole sources of carbon and energy (Table 1). The growth of P. benzenivorans on both catechol and 4-fluorocatechol suggested the presence of a 4-FP-degradation pathway, which is consistent with the observed growth on 4-FP. In contrast, the other FP analogs (2-FP and 3-FP) were not found to be growth substrates for P. benzenivorans.
To assess the extent of mono-FP defluorination by resting cells pre-grown on 4-FP, we measured fluoride levels in cell cultures with various starting concentrations of 2-FP, 3-FP, and 4-FP (1 to 10 mM). As shown in Table 2, fluoride recovery was nearly stoichiometric for initial concentrations of 1 and 2.5 mM 4-FP, but decreased at higher concentrations. Although P. benzenivorans was unable to grow on 2-FP and 3-FP, this strain was capable of transforming these compounds, releasing over 60% of the total fluoride from starting concentrations of 1 and 2.5 mM of 2-FP and 3-FP. This indicates that P. benzenivorans can participate in ring cleavage reactions involving 2-FP and 3-FP.
Growth on 4-FP
We examined the influence of initial 4-FP concentration on the growth of P. benzenivorans by assessing biomass and fluoride liberation in cultures grown with 4-FP at starting concentrations of 1 to 7 mM (Fig. 1a). At 1 and 2 mM, 4-FP was almost completely consumed, stoichiometric amounts of fluoride were released, and there was a linear increase in biomass. This demonstrates that the degradation of 4-FP did not give rise to large quantities of fluorinated dead-end intermediates. However, higher concentrations of 4-FP had toxic effects, with significant cell growth inhibition seen in cultures treated with 4-FP concentrations greater than 5 mM.
In cultures of P. benzenivorans supplied with 1 mM 4-FP, cell growth was accompanied by stoichiometric fluoride formation, biomass increases, and depletion of 4-FP (Fig. 1b). After 3 weeks of growth, the substrate was completely degraded and the maximum dried biomass was 99 mg/L. These results suggest that there was no accumulation of fluorinated by-products that affected the enzymes involved in 4-FP metabolism.
Transformation and mineralization of mono-FPs
In cultures with starting concentration of 2.5 mM of the test compound, 3-FP and 4-FP were completely degraded within 24 h, while 2-FP was consumed more slowly (within 48 h). When suspensions of heat-killed and poisoned cells of P. benzenivorans were incubated with mono-FPs, substrate losses in assays were negligible (Fig. 2). These results indicate that the observed removal of above compounds is due to the enzymatic reactions. The metabolic intermediates detected during the transformations of the mono-FPs are listed in Table 3.
In cultures treated with 2-FP, 3-fluorocatechol (metabolite III) was detected as a major intermediate and metabolite IV was identified as a by-product. A significant amount of 3-fluorocatechol remained detectable at the end of the experiment (Fig. 2a). When formed as an intermediate, 3-fluorocatechol tends to accumulate and inhibit cell growth through its toxic effects (Schreiber et al. 1980). Metabolite ІV had a different retention time than 3-fluorocatechol, but its fragmentation spectrum was similar. This compound was assumed to be dihydroxylated fluorobenzene, but we could not confirm the hydroxylation position in the fluorinated dead-end product due to the lack of an authentic standard for comparison. After 4 days of incubation, this by-product was still present in the medium, indicating that fluoride release was not complete during the metabolism of 2-FP by P. benzenivorans.
The transformation of 3-FP by P. benzenivorans resulted in production of three polar intermediates, of which two (metabolites I and ІІІ) were identified as 4- and 3-fluorocatechol. The other was observed at approximately the same retention time range as metabolite ІV found during 2-FP transformation. On the basis of the possible positions for hydroxylation of 2-FP and 3-FP, we suspect that the by-product compound was 2-fluorohydroquinone. As shown in Fig. 2b, the yield ratio of 3-fluorocatechol to 4-fluorocatechol was almost 2, which can be explained in two ways: Either 3-FP was preferentially hydroxylated at the C2 position versus the C6 position, or the reaction of 4-fluorocatechol with catechol dioxygenase was faster and more successful than that of 3-fluorocatechol with catechol dioxygenase.
During degradation of 4-FP, two metabolites (І and ІІ) were detected in the culture. Metabolite I had the same retention time as 4-fluorocatechol; the appearance of 4-fluorocatechol in the early stage of incubation suggests that P. benzenivorans transforms 4-FP to 4-fluorocatechol during the first metabolic step (Fig. 2c). The 4-fluorocatechol was rapidly degraded thereafter, presumably during subsequent growth reactions, and was thus observed at only very low levels overall. Metabolite ІІ was inferred to be 3-fluoromuconic acid based on its derivatized molecular range, but we were unable to confirm the structure because there was no authentic standard available. This intermediate was detectable for a longer period than 4-fluorocatechol, but it disappeared from the culture medium after 60 h. 4-FP disappearance was concomitant with an equimolar release of fluoride.
Based on these findings, we propose that the degradation of mono-FPs by P. benzenivorans is accompanied by the formation of two types of fluorocatechols and simultaneous release of fluoride.
Enzymatic activities
Crude extracts from cells pre-grown with 4-FP were used to determine the enzymatic activities involved in 4-FP degradation. Specific phenol hydroxylase activity was detected in the presence of NADH, indicating that the hydroxylation of 4-FP to 4-fluorocatechol was the initial step in the 4-FP-degradation pathway. To estimate whether the degradation of 4-fluorocatechol occurred via meta- or ortho-cleavage, we tested for the activities of enzymes involved in ring fission. The cell extracts showed catechol and 4-fluorocatechol 1,2-dioxygenase activities, but no detectable activity was observed for 3-fluorocatechol dioxygenase or catechol 2,3-dioxygenase (Table 4). These results suggested that 4-fluorocatechol, which was the main intermediate in 4-FP metabolism, was degraded to 3-fluoro-cis,cis-muconate by ortho-cleavage. No such activity was found in extracts from cells grown in the presence of glucose, suggesting that these enzymes were induced by the addition of 4-FP.
In addition to the degradation of 4-FP, P. benzenivorans showed the ability to convert 2-FP and 3-FP. To investigate the transformation pathways of these compounds in more detail, 4-FP-grown cells were induced for 24 h in the presence of each of these substrates. Phenol hydroxylase and catechol 1,2-dioxygenase activities were detected in extracts induced with 2-FP and 3-FP, but these activities were very low compared with those observed in extracts from cells grown on 4-FP. Extracts of P. benzenivorans induced with 2-FP had catechol and 3-fluorocatechol 1,2-dioxygenase activities. Similar levels of 3-fluorocatechol and 4-fluorocatechol 1,2-dioxygenase activities were observed in cell extracts induced by 3-FP (data not shown). The results of the enzyme assays were thus comparable to those of the metabolite analyses.
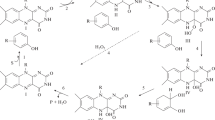
Figure 3 shows a proposed metabolic pathway of mono-FPs by P. benzenivorans. This is based on previous reports (Carvalho et al. 2006; Chaojie et al. 2007; Van Pee and Unversucht 2003) and the present results.
Discussion
As the metabolism of fluorinated compounds has not been well studied, we herein examined the degradation of mono-FPs by P. benzenivorans. This strain was originally obtained from an enrichment based on tetrachlorobenzene (Kampfer and Kroppenstedt 2004). P. benzenivorans was able to grow on 4-FP as a sole carbon source and effectively transform 2-FP and 3-FP to their corresponding metabolic intermediates. The initial step in the degradation of mono-FPs was hydroxylation to form fluorocatechols which were further degraded through an ortho-cleavage pathway.
Many previous reports have suggested that the resistance of fluorinated compounds to bacterial degradation is due to the stability of the C–F bond (Carvalho et al. 2002, 2006; Ferreira et al. 2008; Oltmanns et al. 1989; Schreiber et al. 1980). The high electronegativity of fluorine gives this bond a particularly strong polarity. In addition, the partial charges on the fluorine and carbon contribute to the unusual bond strength of the C–F bond; the bond energy involved is among the highest found in nature (Key et al. 1997). However, the increased oxidative stability of fluorinated compounds has nothing to do with the greater strength of C–F bond. In fact, microbial oxidation does not involve the homolysis of C–F bond (Haggblom and Bossert 2003), so it is necessary for us to consider other factors that could affect the resistance of these compounds.
The catabolism of halogenated aromatic compounds via catechol forms involves two common steps (Gibson 1968; Broderick 1999): (1) introduction of one or two hydroxyl groups that are ortho to each other, and (2) ring cleavage by catechol dioxygenase. The two oxygenation reactions are electrophilic in nature, so the presence of electron-withdrawing substituents deactivates the ring against further reaction (Dorn and Knackmuss 1978). The negative inductive effect of fluorine decreases the electron density of the aromatic nucleus, rendering them less susceptible to electrophilic attack by bacterial oxygenase.
The ring fission of fluorocatechols by catechol 1,2-dioxygenase strongly depends on the position at which fluorine is attached to the benzene ring. Fluorocatechol is stabilized by the action of an enzyme-bound dioxygen species via formation of a positively charged σ-complex (Dorn and Knackmuss 1978). This resonance structure acts as a rate-limiting factor, influencing the conversion rate through its high stability. Theoretically, interference with this transition state is linked to higher enzymatic activity through retardation of the rate-limiting process (Engesser et al. 1988). In the case of 4-fluorocatechol, the positive charge is delocalized in the fluorine-attached carbon (Fig. 4b). The negative inductive effect of fluorine influences the electrons in adjacent p orbitals, thereby destabilizing the reaction intermediate. This hypothesis is consistent with the exceptionally good conversion of 4-fluorocatechol by catechol 1,2-dioxygenase (Fig 2c and enzyme assay). The low turnover rate we observed for 3-fluorocatechol was may be due to the stabilized σ-complex. When fluorine is attached to the C3 position, the positive charge is delocalized in the C2, C4 and C6 positions, thereby maintaining mesomeric stabilization (Fig. 4a).
In the substituted phenols, the negative charge is particularly localized at the C2, C4, and C6 positions, potentially making these sites attractive for electrophilic attack. Depending on the initial mono-FP, we found that P. benzenivorans generated 3-fluorocatechol and/or 4-fluorocatechol. There was a close correlation between the produced metabolites and growth of P. benzenivorans. During the transformation of 2-FP, significant amounts of 3-fluorocatechol was detected, but no catechol was found to accumulate. This indicates that the non-fluorinated C6 position is preferentially hydroxylated relative to the fluorine-substituted C2. Since hydroxylation can occur at unsubstituted C2 and C6 positions in 3-FP, 3-fluorocatechol and 4-fluorocatechol were observed together. Besides fluorocatechols, fluorohydroquinone, the prospective intermediate produced from the hydroxylation at the C4 position of 2-FP and 3-FP, was also detected in the media containing 2-FP and 3-FP. The microbial conversion of mono-FPs to fluorohydroquinone has not been reported yet. The ability of a microorganism to grow on 3-FP may be dependent on the initial hydroxylation reaction, because 3-fluorocatechol is a very poor substrate for catechol 1,2-dioxygenase, and can therefore accumulate and affect enzymatic activities (Bartels et al. 1984). Consistent with this, we found that 3-fluorocatechol had toxicity against catechol 1,2-dioxygenase. 4-Fluorocatechol was consumed rapidly, and a 1,2-dioxygenase activity was nearly as high towards 4-fluorocatechol as catechol. In contrast, 3-fluorocatechol remained detectable for an extended time in the media containing 2-FP and 3-FP. We observed rapid and time-dependent decreases in enzymatic activity in these cultures, indicating that the high concentrations of 3-fluorocatechol had negative effects on the activity of catechol 1,2-dioxygenase. The relative sensitivity of cells to high concentrations of 2-FP and 3-FP (versus 4-FP) can be explained by the turnover of these compounds and the subsequent accumulation of 3-fluorocatechol. 4-FP was always transformed to 4-fluorocatechol regardless of the hydroxylation positions, thereby allowing cells to avoid accumulation of lethal products. That is why 4-FP was always being a better substrate for the growth of P. benzenivorans than either 2-FP or 3-FP.
In conclusion, we suggest that the electron-withdrawing nature of fluorine exhibits a significant impact on the catabolism of fluorinated aromatic compounds. We are currently undertaking mixed-substrate experiments with the halophenols to investigate the effect of halogen substitution on the substrate preference and metabolic pathways of P. benzenivorans.
References
Bartels I, Knackmuss HJ, Reineke W (1984) Suicide inactivation of catechol 2, 3-dioxygenase from Pseudomonas putida mt-2 by 3-halocatechols. Appl Environ Microbiol 47:500–505
Boesmna MG, Dinarieva TY, Middlehoven WJ, Van Berkel WJH, Doran J, Vervoort J, Rietjens IMCM (1998) 19F nuclear magnetic resonance as a tool to investigate microbial degradation of fluorophenols to fluorocatechols and fluoromuconates. Appl Environ Microbiol 64:1256–1263
Boersma MG, Solyanikova IP, Van Berkel WJH, Vervoort J, Golovleva L, Rietjens IMCM (2001) 19F NMR metabolomics for the elucidation of microbial degradation pathways of fluorophenols. J Ind Microbiol Biotechnol 26:22–34
Bondar VS, Boersma MG, Golovlev EL, Vervoort J, Van Berkel WJH, Finkelstein ZI, Solyanikova IP, Golovleva LA, Rietjens IMCM (1998) 19F NMR study on the biodegradation of fluorophenols by various Rhodococcus species. Biodegradation 9:475–486
Bondar VS, Boersma MG, Van Berkel WJH, Finkelstein ZI, Golovlev EL, Baskunov BP, Vervoort Golovleva LA, Rietjens IMCM (1999) Preferential oxidative dehalogenation upon conversion of 2-halophenols by Rhodococcus opacus 1G. FEMS Microbiol Lett 181:73–82
Broderick JB (1999) Catechol dioxygenases. Essays Biochem 34:173–189
Buhler DR, Unlu F, Thakker DR, Slaqa TJ, Conney AH, Wood AW, Chang RL, Levin W, Jerina DM (1983) Effect of a 6-fluoro substituent on the metabolism and biological activity of benzo(a)pyrene. Cancer Res 43:1541–1549
Carvalho MF, Alves CCT, Ferreira MIM, De Marco P, Castro PML (2002) Isolation and initial characterization of a bacterial consortium able to mineralize fluorobenzene. Appl Environ Microbiol 68:102–105
Carvalho MF, Ferreira MIM, Moreira IS, Castro PML, Janssen DB (2006) Degradation of fluorobenzene by Rhizobiales strain F11 via ortho cleavage of 4-fluorocatechol and catechol. Appl Environ Microbiol 72:7413–7417
Chaojie Z, Qi Z, Ling C, Yuan Y, Hui Y (2007) Degradation of mono-fluorophenols by an acclimated activated sludge. Biodegradation 18:51–61
Dorn E, Knackmuss HJ (1978) Chemical structure and biodegradability of halogenated aromatic compounds. Substituent effects on 1, 2 dioxygenation of catechol. Biochem J 174:85–94
Engesser KH, Rubio MA, Ribbons DW (1988) Bacterial metabolism of side chain fluorinated aromatics: cometabolism of 4-trifluoromethyl(TFM)-benzoate by 4-isopropylbenzoate grown Pseudomonas putida JT strains. Arch Microbiol 149:198–206
Ferreira MIM, Marchesi JR, Janssen DB (2008) Degradation of 4-fluorophenol by Arthrobacter sp. strain IF1. Appl Microbiol Biotechnol 78:709–717
Finkelstein ZI, Baskunov BP, Boersma MG, Vervoort J, Golovlev EL, Van Berkel WJH, Golovleva LA, Rietjens IMCM (2000) Identification of fluoropyrogallols as new intermediates in biotransformation of monofluorophenols in Rhodococcus opacus 1cp. Appl Environ Microbiol 66:2148–2153
Fortnagel P, Harms H, Wittich RM, Krohn S, Meyer H, Sinnwell V, Wilkes H, Francke W (1990) Metabolism of dibenzofuran by Pseudomonas sp. strain HH69 and the mixed culture HH27. Appl Environ Microbiol 56:1148–1156
Gibson DT (1968) Microbial degradation of aromatic compounds. Science 161:1093–1097
Gorlatov SN, Golovleva LA (1992) Effect of cosubstrates on the dechlorination of selected chlorophenolic compounds by Rhodococcus erythropolis 1CP. J Basic Microbol 32:177–184
Gurujeyalakshmi G, Oriel P (1989) Isolation of phenol-degrading Bacillus stearothermophilus and partial characterization of the phenol hydroxylase. Appl Environ Microbiol 55:500–502
Haggblom M (1990) Mechanisms of bacterial degradation and transformation of chlorinated monoaromatic compounds. J Basic Microbiol 30:115–141
Haggblom MM, Bossert ID (2003) Dehalogenation: microbial processes and environmental applications, 1st edn. Springer, New York, pp 19–32
Hofrichter M, Bublitz F, Fritsche W (1994) Unspecific degradation of halogenated phenols by the soil fungus Penicillium frequentans Bi 7/2. J Basic Microbiol 34:163–172
Kampfer P, Kroppenstedt RM (2004) Pseudonocardia benzenivorans sp. nov. Int J Syst Evol Microbiol 54:749–751
Key BD, Howell RD, Criddle CS (1997) Fluorinated organics in the biosphere. Environ Sci Technol 31:2445–2454
Mahendra S, Alvarez-Cohen L (2006) Kinetics of 1, 4-dioxane biodegradation by monooxygenase-expressing bacteria. Environ Sci Technol 40:5435–5442
Marr J, Kremer S, Sterner O, Anke H (1996) Transformation and mineralization of halophenols by Penicillium simplicissimum SK9117. Biodegradation 7:165–171
Mars AE, Kasberg T, Kaschabek SR, Van Agteren MH, Janssen DB, Reineke W (1997) Microbial degradation of chloroaromatics: use of the meta-cleavage pathway for mineralization of chlorobenzene. J Bacteriol 179:4530–4537
Nozaki M (1970) Metapyrocatechase (Pseudomonas). Meth Enzymol 17A:522–525
Oltmanns RH, Muller R, Otto MK, Lingens F (1989) Evidence for a new pathway in the bacterial degradation of 4-fluorobenzoate. Appl Environ Microbiol 55:2499–2504
Park BK, Kitteringham NR, O'Neill PM (2001) Metabolism of fluorine-containing drugs. Annu Rev Pharmacol Toxicol 41:443–470
Schreiber A, Hellwiq M, Dorn E, Reineke W, Knackmuss HJ (1980) Critical reactions in fluorobenzoic acid degradation by Pseudomonas sp. B13. Appl Environ Microbiol 39:58–67
Thakker DR, Yagi H, Sayer JM (1984) Effects of a 6-fluoro substituent on the metabolism of benzo(a)pyrene 7, 8-dihydrodiol to bay-region diol epoxides by rat liver enzymes. J Biol Chem 259:11249–11256
Van Pee KH, Unversucht S (2003) Biological dehalogenation and halogenation reactions. Chemosphere 52:299–312
Whalen MY, Armstrong SM, Patel TR (1993) Characterization of a Rhodococcus species that utilizes numerous aromatics. Soil Biol Biochem 25:759–762
Zaitsev GM, Surovtseva EG (2000) Growth of Rhodococcus opacus on mixtures of monohalogenated benzenes and phenols. Microbiology 69:401–405
Zaitsev GM, Uotila JS, Tsitko IV, Lobanok AG, Salkinoja-Salonen MS (1995) Utilization of halogenated benzenes, phenols, and benzoates by Rhodococcus opacus GM-14. Appl Environ Microbiol 61:4191–4201
Acknowledgments
This research was supported by the Korean Science and Engineering Foundation (KOSEF) grant funded by the Korean government (MEST; No. R01-2008-000-20244-0) and “The GAIA project” from the Korea Ministry of Environment.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, EJ., Jeon, JR., Kim, YM. et al. Mineralization and transformation of monofluorophenols by Pseudonocardia benzenivorans . Appl Microbiol Biotechnol 87, 1569–1577 (2010). https://doi.org/10.1007/s00253-010-2647-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2647-7