Abstract
The influence of various carbon anodes; graphite, sponge, paper, cloth, felt, fiber, foam and reticulated vitreous carbon (RVC); on microbial fuel cell (MFC) performance is reported. The feed was brewery wastewater diluted in domestic wastewater. Biofilms were grown at open circuit or under an external load. Microbial diversity was analysed as a function of current and anode material. The bacterial community formed at open circuit was influenced by the anode material. However at closed circuit its role in determining the bacterial consortia formed was less important than the passage of current. The rate and extent of organic matter removal were similar for all materials: over 95% under closed circuit. The biofilm in MFCs working at open circuit and in the control reactors, increased COD removal by up to a factor of nine compared with that for baseline reactors. The average voltage output was 0.6 V at closed circuit, with an external resistor of 300 kΩ and 0.75 V at open circuit for all materials except RVC. The poor performance of this material might be related to the surface area available and concentration polarizations caused by the morphology of the material and the structure of the biofilm. Peak power varied from 1.3 mW m−2 for RVC to 568 mW m−2 for graphite with biofilm grown at closed circuit.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The anode support has been identified as a factor affecting MFC performance. Previous studies have focused on the anode performance and have employed different supports including materials such as stainless steel or graphite (ter Heijne et al. 2008; Dumas et al. 2007). Carbons are generally good candidates for anode materials in microbial fuel cells because of their biocompatibility and low cost (Morozan et al. 2007). A wide range of various modified and unmodified carbon types have been extensively used in microbial fuel cell research attaining high power levels in systems that were optimized for the production of electricity. Materials processed with mechanical and chemical pre-treatments, i.e. ammonia treatment (Cheng and Logan 2007) have been widely tested. Modified materials explored so far include different carbon shapes as woven graphite (Park and Zeikus 2003) or graphite brush (Logan et al. 2007), composite electrodes with metallic layers and conductive polymers such as polyaniline, poly tetrafluoro ethylene or quinone groups, (Qiao et al. 2008; Liu et al. 2007; Zhang et al. 2007; Pietron et al. 2005; Schröder et al. 2004). Most of these modified anodes provided increased energy outputs compared with un-modified materials.
Much of the previous work has attempted to optimize systems for electricity generation; typically using a single electrode material with pure compounds such as acetate (Freguia et al. 2008; Zhang et al. 2006). The surface chemistry of the different carbon materials and their physical properties will influence their ability to act as surfaces for microbial colonization. The surface areas and their cost must be considered to assess them as effective and viable electrodes for MFC fed with wastewaters (Hitchens 1989). On the other hand, in spite of the variety of materials used, the wastewater MFC literature lacks data for different carbon types tested under identical conditions. Systematic work is necessary to distinguish their suitability or not for biofilm support in MFCs: this is the reasoning behind the experimental design to test different carbon materials for MFC anodes. Regarding chemically modified anodes, improvements in performance have frequently not been sustained in the long term and power outputs decay after some time, often to the levels of unmodified equivalent supports (Scott et al. 2008). This is why with the aim of framing the work within the search of materials for sustainable and affordable MFC construction for wastewater treatment, unmodified carbon types were selected for the present research.
Two-chamber MFC systems used in this work had a low ratio of anode area/anolyte volume, with widely spaced electrodes which were not optimal for maximizing energy production. It was not the intention to maximize the power performance of the MFC but to have simple MFC, easy to construct with the available materials, which allowed direct comparison of different treatments in readily reproducible reactors. In all other, respects i.e. inoculum, substrate and reactor configuration, the MFCs were identical.
In this way, it was intended to test the hypothesis that features of the different electrode surfaces would determine the contact area between cells and electrodes and would enhance or limit cell adhesion and therefore specifically select certain organisms in the microbial consortium. This could lead to different rates of electron transfer and water treatment efficiency in the MFCs containing different supports for biofilm growth (Jiang and Li 2007). The materials used were restricted to a selection of carbon materials with relatively high surface areas and different porosities and roughness.
Materials and methods
MFC construction and operation
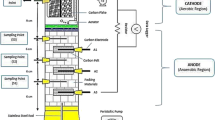
Microbial fuel cells (Fig. 1) were made from two glass chambers, 250 cm3 in volume, separated by a Nafion® 117 cation exchange membrane (DuPont, USA; 6 cm2 area) placed between flanges. The membrane was pre-treated by boiling for 1 h in 0.5 mol dm−3 (M) H2SO4 and 1.0 h in 0.5 M H2O2. The cathode was a platinised titanium mesh 4 cm2, 0.3 mg Pt cm−2 (William Gregor Ltd., UK). The anodes used were: carbon cloth A and carbon felt (Ballard, UK), carbon paper (Toray Carbon Paper TGP60 0WP), carbon sponge, carbon foam, carbon fibre, graphite and reticulated vitreous carbon (RVC) 100 ppi (nominal pores per linear inch) grade (E-teck, UK). Electrodes were sterilised before use by boiling in 1.0 M of HCl for 15 min followed by thorough rinsing in sterile de-ionised water. The cross section area of the anodes was 6 cm2 and they were, depending on material, from 1.5 to 3 mm thick. Before use, all the reactor components were sterilised by autoclaving.
Cathode chambers contained 200 cm3 of potassium phosphate buffer, 50 mM and pH 7. They were neither aerated nor mixed but remained open with the surface of liquid exposed to air. Brewery effluent (Federation Brewery of Newcastle upon Tyne, UK, COD > 50,000 mg/L) was diluted (0.5:100 v/v) into domestic wastewater (Northumbrian Water, Newcastle upon Tyne, UK, COD < 800 mg/L) and used as fuel and the bacterial inoculum. Characteristics of the fuel are depicted in Table 1.
The anode chambers were fed with 200 cm3 of fuel, sealed and purged with oxygen-free nitrogen. A homogeneous distribution of the substrate in the anode was maintained by mixing with magnetic stirrers (Hanna HI-190M, UK). All reactors were operated simultaneously and at room temperature (15 ± 2°C–50% time; day time, 8 ± 2°C–50% time; night time)
For closed circuit experiments each cathode was connected to anode through a 300 kΩ external load. As mentioned above, the aim of this study was not to optimize power generation but to analyze the effect of different carbon materials and the flow of current in the formation of the biofilm in a microbial fuel cell. Indeed such a high external resistance was selected as it represents the “worst-case scenario” and allowed to assess the effect of even very low levels of current (high resistor) on biofilm development. The present study did not aim for high levels of energy generation but for biofilm formation under defined conditions that permitted a controlled comparative analysis of different anode materials.
In addition to the MFCs, control reactors were operated under anaerobic conditions with the carbon materials (6 cm2 cross sectional area) studied in this research. The control reactors allowed comparison of COD removal and microbial diversity between MFCs, and conventional anaerobic reactors operated with biofilm grown on the different supports. One further baseline reactor was maintained under anaerobic conditions with no support material present, in order to determine organic carbon removal efficiency and microbial community composition in reactors without biofilm. All the reactors were monitored for 250 h and samples of 2 cm3 (ml) were withdrawn daily, filtered (0.2 µm) and analysed for COD removal.
In the case of open circuit experiments, cathode and anode were not connected. To test the reproducibility of anode biofilm development and MFC performance at open circuit, six MFCs containing carbon cloth A (E-tek, UK) as anode material were run under similar conditions to the MFC used for comparative analysis of different anode materials.
Experiment were conducted in triplicate and results were consistent and reproducible within an acceptable confidence level (Larrosa-Guerrero et al. 2009); average maximum voltages around 0.6 ± 0.05 V, average final COD removals 95 ± 5%. Values shown correspond to one of the replicates. Two of the three replicates were processed for DNA extraction and microbial community analysis.
Microbial analysis
DNA extraction and PCR-DGGE
At the end of the experiments the biofilms formed in MFCs and control reactors were preserved in sterile saline phosphate buffer (Oxoid, UK) and absolute ethanol, 1:1 v/v for microbial community studies. The entire biofilm was extracted from the anode by shaking it vigorously and the mixed liquor was stored at −20°C until analysis. In the case of the feed and baseline reactor with no carbon support present, water samples were stored in ethanol at a 1:1 v/v. DNA was extracted from 250 µl of the ethanol fixed samples (Fast-DNA Spin Kit, BIO 101 Q-Bio Gene, UK). A combination of P2 and P3 bacterial-specific GC-clamped primers (Muyzer et al. 1993) was used to amplify bacterial 16S rRNA gene fragments corresponding to positions 341 to 534 in the E. coli 16S rRNA sequence. Primer 3 has a 40-nucleotide GC-rich sequence (GC clamp) at the 5′ end.
-
Primer 2:
5′-ATTACCGCGGCTGCTGG-3′
-
Primer 3:
5′-GCCCGCCGCGCGCGGCGGGCGGGGCGGGGGCACGGGGGGCCTACGGGAGGCAGCAG-3′
The polymerase chain reactions (PCRs) of a total volume of 50 μL were prepared with Mega-mix blue (Sigma, USA) containing Taq DNA polymerase, dNTPs, MgCl2, reaction buffer, enzyme stabilizer and blue gel loading dye, 0.2 μM forward primer, 0.2 μM reverse primer, and 1 μL of DNA preparation extracted from samples of biomass from the reactor. They were run on an Omn-E thermal cycler (Hybaid, UK) with initial denaturation at 95°C for 6 min followed by 26 cycles of 95°C for 30 s, 65°C for 30 s and 72°C for 30 s and 15 cycles of 95°C for 30 s, 53°C for 30 s and 72°C for 30 s with a final elongation step of 72°C for 30 s. PCR products were subjected to denaturing gradient gel electrophoresis (DGGE) on 10% w/v polyacrylamide, (37:1 acrylamide: bisacrylamide) The gel was prepared with 10% w/v polyacrylamide, (37:1 acrylamide: bisacrylamide) including a denaturant gradient spanning 30–60% (100% denaturant is 7 M urea plus 40% vol/vol formamide in 1× TAE). From each PCR reaction 11 μL were loaded on the gel and electrophoresis was performed with a d-Gene system (Bio-Rad, Hercules, CA, USA) at 60°C and 200 V constant current for 4.5 h. The separated DNA was stained for 30 min with SYBR green I (Sigma, Poole, UK) diluted 1/10,000 in 1× TAE buffer. Stained gels were viewed and documented under ultraviolet light using a Bio-Rad Fluor-S ® Multi-Imager (Bio-Rad, UK) and recorded for later processing.
Image analysis and statistics
Scanned DGGE gels were processed using the Bionumerics software package (version 3.5, Applied Maths, USA) to analyze the intensity and position of all bands within a single lane in relation to the positions and intensity of the bands in all other lanes. To correct for variations across the gel, a marker sample was run on either side of the samples on all gels. Lanes were defined on the gel and a densitometric curve was recorded for each gel lane. To account for variations in electrophoresis across the gel, the lanes were normalized relative to the marker lanes and bands were assigned. The remaining lanes on the DGGE gel were aligned to the reference band pattern and the band assignments were checked manually.
The banding patterns of the samples were aligned iteratively according to the alignment information provided by the closest neighbouring standard patterns. By aligning the bands from all marker lanes and samples it was possible to compare band patterns from different samples with each other. The patterns were analyzed in two ways:
-
1.
Following gel normalization, DGGE fingerprints were automatically scored for the presence and absence of co-migrating bands without consideration of the band intensity. Pair wise similarities were calculated using the Dice index of similarity, Cs = 2j / (a + b), where j is the number of common bands between samples A and B; a and b are the total number of bands in samples A and B, respectively.
-
2.
Comparison of band patterns between all samples by Pearson correlation of whole lane densitometric curves.
Comparisons of the Dice similarity coefficients used the Mann–Whitney U test to test (software ALGLIB) for significant differences between DGGE profiles from the biofilm communities that colonized the different materials in MFCs and controls under the tested conditions. The Mann–Whitney U test has been described as the nonparametric version of the Student's t test. In the same way as the parametric Student's t test, the nonparametric Mann–Whitney U test is useful to determine significant differences existing between two populations (however population is defined). Unlike the Student's t test, the application of Mann–Whitney U test does not need data to follow a normal distribution to be valid (Conover 1980). The community similarity comparisons done on the basis of Dice similarity coefficients are detailed in Table S1(see Electronic suplementary material).
Wastewater analysis
Volatile suspended solids and chemical oxygen demand from soluble organic carbon were measured according to standard methods (Eaton Andrew 2005). pH was determined by a digital pH meter (Model 20, Denver instruments Ltd.). Volatile fatty acids were quantified by gas chromatography, using a chromatographer (Agilent, 6890N, USA) provided with a flame ionization detector and a 30m × 0.25 mm × 0.25 μm DB-Wax column (Agilent, USA). Further details are provided in the Electronic supplementary material.
Electrochemical measurements
Cell potentials were monitored by a data-logger (ADC-16, Pico Technologies Ltd., UK) connected to a personal computer via a high resolution analogue cable (BS 232 Pico, UK). Electrode potentials are expressed relative to the potential of the standard hydrogen electrode. Values reported for polarisation were not corrected for the internal resistance of the cells since measurement of internal resistance (IRint) indicated that it could be ignored due to the low levels of current produced. Pseudo steady state polarisations were carried out after 50 h and 70 h of operation using a self-made variable resistor box (11 MΩ–10Ω). Electrochemical Impedance Spectroscopy tests were also performed (ACM 946). Connections of the cells to the different instruments and equipment as well as methods of analysis and calculation of the different parameters were done according to previously published literature (Logan et al. 2006)
Characterization of anode materials
All samples used were characterized with a Scanning Electron Microscope (S-3500N, Hitachi) before and after biofilm formation. Samples covered with biofilm were processed by metallization prior to scanning. Metallization consisted of coating with gold for 60 s at 2.2 kV and 15 mA (Sputter coating, Quorum Technologies); the coating was carried out under argon flow and 0.7 bars. Infra-red spectra were collected with a high-performance diamond single bounce attenuated total reflection accessory fitted to a Fourier Transform Infrared Spectrometer (Nexus 5700, Thermo Electron Corporation).
Results
Anodic communities
The level of similarity between anodic bacterial communities formed within the different reactors was statistically analysed by the Mann–Whitney U test using the Dice similarity coefficients calculated in similarity matrices following methods previously described (Prat et al. 2009). Detailed test results of the statistical tests are available in Electronic supplementary material.
Bacterial community profiles from biofilms grown on different materials at closed circuit (Fig. 2) showed that the biofilm communities were highly similar (77.5 ± 24.3%) irrespective of the type of carbon anode.
DGGE results for 16SrRNA analysis of the anodic communities in dummy cells and MFCs supplied with six different anode materials. F feed, C control, M marker, Ci cloth, Fi felt, Gi graphite, Pi paper, Ri RVC, Si sponge, Ve negative. The number 1 or 2 refers to two replications of the same test (under 300 kΩ)
Although the overall similarity of replicate biofilms that developed at closed circuit on all materials was high (77.5 ± 24.3% for replicates 1 and 2), the bacterial consortia that formed at closed circuit on replicate anodes of the same material were more similar to each other (95.3 ± 9.2%). However, the p value obtained for this comparison is on the borderline to of significance at the 95% level (p < 0.05); according to the Mann–Whitney U test the similarity of these DGGE profiles formed on same material was not significantly different from the similarity of DGGE profiles obtained from biofilms from different materials with p = 0.06). Hence, the communities formed at closed circuit on different anode materials were very similar and only subtly distinct.
On the other hand, statistical analysis of the similarities in biofilms bacterial communities formed at open circuit conditions on replicate carbon cloth anodes (Fig. 3a) showed that the communities formed on the carbon cloth anodes from six replicate reactors were highly reproducible (81.0 ± 7.7%). Consequently it was considered that comparison of DGGE profiles from single MFCs with different anode materials would provide representative data.
a DGGE results for 16S rRNA gene analysis of the anodic communities in six MFCs with carbon cloth anodes b DGGE analysis of 16S rRNA gene fragments from the anodic communities in control cells and MFCs fitted with anodes made from eight materials. F feed, B baseline reactor, M marker, Fo foam, S sponge, R RVC, Fe felt, Cl cloth, Fi fibre, P paper, G graphite, Ve negative. No apostrophe indicates samples from MFC at OCP, whilst the apostrophe indicates samples from control reactors
Anode bacterial consortia developed at open circuit in MFCs with different anode materials (Fig. 3b) were distinct from each other (average similarity 53.7 ± 16.1%). Furthermore the biofilm communities in the control reactors and corresponding MFCs with the same carbon materials (Fig. 3b) were also distinct (average similarity 56.4 ± 18.2%). The similarity between the bacterial communities that developed on different materials in MFCs was significantly less than the similarity within communities from replicate MFC with the same anode material \( \left( {p = {2} \times {1}{0^{ - {6}}}} \right) \).
The DGGE fingerprint obtained from the carbon cloth anode in Fig. 3a was different from the profiles obtained from the six replicate carbon cloth anodes obtained in a parallel experiment (Fig. 3b). This is because the 6 replicate MFCs with carbon cloth anodes were run in a different batch using wastewater sampled on a different date. To ensure comparability, only data from the same gel were used for statistical comparisons.
The previously analysed communities developed at closed circuit on anodes of six of these materials used here were highly similar (77.5 ± 24.3% similarity). This similarity was significantly higher \( \left( {p = {1}.{8} \times {1}{0^{ - {4}}}} \right) \) than the similarity between the communities that developed on the same materials under open circuit conditions (53.7 ± 16.1%).
There was no significant difference (p = 0.69) in the mean similarity of biofilm communities of the MFCs containing different anode materials at open circuit voltage (OCP) and the biofilm communities in the corresponding control reactors.
Efficiency of the anodic biofilms for wastewater treatment
The consumption of organic matter was monitored daily by measurement of soluble COD. The extents of COD removal achieved at the end of the test for closed circuit MFCs, controls and baselines are depicted in Table 2. Final COD removal was over 95% for all MFCs whilst the control chamber, in which there was no electrical circuit, showed just 73% COD removal. The baseline reactor, in turn, only removed 37% of the COD.
Substrate concentration was enough to maintain steady voltage output until COD removal >90% was achieved, at around 200 h (Fig. 4). Voltage and current production are proportional and current is a measure of rate of electrochemical processes. Thus the velocity of electrochemical processes in the MFCs increased gradually during removal of the first 40% of the organic matter corresponding to the initial rise in potential (and current) in Fig. 4. This took place over the first 48–72 h of operation. The initial increase in velocity is likely related to the breakdown of long chain and complex organic matter by bacteria to more readily degraded monomers and fermentation products such as fatty acids and hydrogen. Initially biomass growth and attachment as well as supply of monomers and fermentation products will limit electricity production until the rate of supply and the rate of oxidation by bioelectrochemical processes at the anode are in equilibrium, resulting in a constant flux of electrons to the anode. At that point, the electrochemical reactions inside the reactors remained at constant rate until the concentration of substrate became limiting. This occurred when COD removal was greater than 90%, which occurred at around 200 h (Fig. 4).
The final pH was lower than the initial pH (7.2) for the MFCs with felt, paper and RVC (6.9). However, the pH slightly increased (between 7.5 and 7.7) in those containing graphite, cloth and sponge anodes. VFAs in these three cells were not detectable at the end of the experiment (Table 3). The average final pH of the control reactors was somewhat lower than for the MFCs (6.78) and the pH of the baseline reactors was considerably lower than that of all other reactors (5.22)
The differences in final %COD removal for MFCs were below 5% in all cases (Table 2). Studies have indicated that a standard deviation of 12% of COD removal must be assumed when working at open circuit voltage (OCV) with two replicates with a confidence level of 95% (Larrosa-Guerrero et al. 2009).
Electrochemical performance of biofilms grown at closed circuit
The potential of MFCs under a 300 kΩ external load as a function of time is shown in Fig. 5. There are three different stages evident, relating to growth of the microbial community; and hence a function of biofilm state and its corresponding kinetics. Over the first 50 h, cell potentials increased approximately linearly to around 0.6 V (3.33 mA m−2), for all MFCs except with the RVC anode. With RVC, the potential rose over 75 h attaining a maximum of 0.4 V (2.22 mA m−2). Subsequently, all cells maintained a constant cell potential for around 150 h. Following this and coinciding with the very low levels of organic matter concentration remaining, cell voltages decreased. With the exception of the RVC anode the plateau voltage was similar for all cells (0.65 V paper; 0.60 V felt; 0.62 V cloth; 0.65 V graphite; 0.65 V sponge) and its duration was also very similar for all anode materials.
Voltage losses due to internal resistance (IRlosses). would be negligible due to the small current which flowed through the system (3.33 mA m−2), i.e. for R int of 700 Ω for the two chambered MFCs, having 6 cm2 of anode, at the voltage losss would be 1.4 mV.
At open circuit, the maximum voltages obtained (Electronic supplementary material, Fig. S1) with carbon foam (0.83 V), felt (0.83 V) and graphite (0.79 V) anodes were slightly higher than with sponge (0.75 V), fibre (0.72 V) and paper (0.71 V) anodes which were, in turn, greater than the carbon cloth anode (0.64 V). Electrochemical measurements of the replicate MFC systems containing carbon cloth anodes demonstrated that the plateau voltages varied by 0.10 V. Statistical analysis of these data revealed that, with three replicates of each reactor, a standard deviation of 0.31 V working at OCP would be required to observe differences in performance between different anode materials at a confidence level of 95% (Larrosa-Guerrero et al. 2009). In the case of the eight carbon materials tested, the difference between voltages for cells with different anodes was less than 0.19 V in all cases, except for RVC. Therefore, none of the anodes studied, apart from RVC, could be differentiated from the others in terms of maximum OCP or voltage generated.
As described above, Coulombic efficiencies during sustained operation in cells at closed circuit were consistently below 2% for all materials tested. These low values, as previously discussed is directly related to the high external resistance used which did not allow higher current to flow. On the other hand low coulombic efficiencies are typical for two chambered cells and non pre-enriched or pre-selected inocula and fuel (Lu et al. 2009), as was apparent in the present study which used raw domestic sewage and beer wastewater as fuel and inoculum.
SEM images of all anodes from MFCs at closed circuit were taken to observe the presence of attached biofilm and to provide information about surface properties of the materials and to relate the extent of biofilm coverage to the characteristics of the anodes and the different levels of sustained output given by the different MFCs. This was also assessed in relation to the performance of the MFC during polarization in terms of peak power and maximum current produced. Figures 6 and 7 show the surface of RVC and graphite at different levels of magnification, allowing the different surfaces and structures (open, smooth and vitreous for RVC and rough and flat for graphite) and the presence of a biofilm.
Pseudo steady state polarizations were performed for all cells (those maintained at open and closed circuit) after 70 h of operation. In each polarisation, each resistance value was maintained for 2 min and the pseudo-steady-state voltage recorded. Figure 8 depicts voltage-current curves on two different current density scales (a) and (c); as well as power output curves on different current density scales (b) and (d), from MFCs sustained under closed circuit.
Open circuit potentials of the MFCs operated at closed circuit at the beginning of the polarizations were over 0.7 V except for the MFC with an RVC anode which was slightly higher than 0.4 V. Potentials for cells operated at open circuit were between 0.6 and 0.7 V except for the MFCs with cloth (0.36 V), fibre (0.3 V) and RVC (0.15 V) anodes. In all cases voltage constantly decayed with the increase of current flowing through the cells (Fig. 8a, b). In all cases, after each test, OCP returned to the original values experienced before polarization. The current and power outputs during polarization of the MFCs with different materials with biofilms grown at open and closed circuit were divided into two ranges (Table 4).
For open circuit MFCs, the cell polarisations obtained with the eight anode materials were significantly different (Electronic supplementary material, Fig. S2) and maximum power decreased in the order; graphite, foam, sponge, cloth, fibre, felt, paper and RVC. Values of peak power densities varied from 0.34 mW m−2 for RVC to 30 mW m−2 for graphite. Power densities in all cases were lower than those obtained for biofilms grown on the same selection of materials at closed circuit (Table 4). Moreover, RVC, carbon paper and carbon felt exhibited, as in the closed circuit MFCs case, the lowest power.
Regarding results from closed circuit cells, graphite, cloth and sponge achieved power densities of around 500 mW m−2 and current densities over 1,500 mA m−2. However felt anodes barely surpassed 20 mW m-2 and produced a maximum current density less than 200 mA m−2; whilst RVC and carbon paper did not even attain 3 mW m−2 nor reached 50 mA m−2 (Fig. 8c, d). These three materials which had the poorest performance during polarization were also those that showed a higher level of VFA accumulation at the end of the test (Table 3).
MFCs with graphite, cloth and sponge gave limiting current densities; 1,600, 1,400 and 1,100 mA m−2, respectively. The cathode (platinised titanium mesh) did not exhibit significant polarisation (see Fig. 9), the internal resistance did not contribute significantly to the cell polarisation and the data collected can be directly related to the behaviour of the anode materials.
To compare the relative activity of the three anodes performing in the higher current density range, graphite, carbon cloth and carbon sponge; Tafel slopes were determined from the overpotential vs. logarithm of current density plots. Values obtained were similar to those reported in other studies with modified anode materials in microbial fuel cells, i.e. 140 mV per decade (Scott et al. 2007; Lowy et al. 2006). Graphite had the lowest Tafel slope, followed by cloth and sponge which had very similar values (Table 4).
With MFCs run at open circuit, a first polarisation was carried out after 50 h and the second after 70 h. On the second polarization the peak cell voltage and power were greater than those obtained with the initial polarisation for all reactors. In the case of MFCs with a graphite anode the power almost tripled (from 13 to 30 mW m−3) from the first to the second polarization (Fig. 9a). This effect of cell polarisation on the maximum power output of the MFCs was consistently seen in repeated cell tests.
Figure 9b shows the cell voltage, anode and cathode potentials obtained on the second polarisation of the MFC with a graphite anode. The cathode voltage remained reasonably constant during the whole test whilst the anode voltage increased with current density, decreasing the overall voltage of the cell. This trend was seen in the data for all MFCs tested. Therefore the anode was the limiting factor for these MFCs.
Electrochemical impedance spectroscopy (EIS) was performed on the MFCs at the start of the experiments and after 72 h of operation. The impedance values (reported in Table 5) obtained at different frequencies decreased by up to 300 Ω from the initial test to the test after 72 h; values corresponded to the sum of the resistance of the solution and the anode biofilms. The main difference in the systems between the two tests was the presence of a stable anodic biofilm after 72 h; the conductivity changes due to any COD variation were negligible. Details of SEM and EIS measurement are provided in the ESI section.
Discussion
Anodic communities
The microbial community data formed at closed circuit show a clear effect of current flow as factor in selection for the biofilm consortia and suggest that there may be a small effect of the anode material on biofilm community composition. On the other hand the data at open circuit clearly showed that the nature of the anode material influenced selection of biofilm micro-organisms, as had been previously suggested (data under load). Although the communities that developed on anodes of the same material under electrical load were more similar to each other than those developed on different anode materials, the difference was statistically marginal (p = 0.06). However, the differences in communities that developed on different anode materials at open circuit were significant. While anode material influences the bacterial community that develops on MFC anodes, when the biofilms are formed at closed circuit this effect of anode material is overridden by the effect of the flow of even a small amount current. This is in agreement with previous results (Kim et al. 2006; Rabaey et al. 2004; Scott et al. 2008; Scott et al. 2007). Thus, statistically the dominating or most important factor in deciding communities formed on anodes is the passage of current and less so the anode material. Although under certain circumstances the anode material may have a role in selecting the anode community. Higher current is likely to provide a stronger selection for electroactive bacteria and hence result in a more enriched efficient biofilm in MFCs (Rabaey et al. 2004)
Consortia from biofilms grown in the control reactors were akin to those from the MFC provided with the same material (Figs. 2 and 3) Thus polarization and connection of the MFC circuit for short periods of time had little effect on the selection of the anodic bacteria.
Efficiency of the biofilms for wastewater treatment
The COD removal efficiency of biofilms formed on the different anode materials under closed circuit was statistically indistinguishable. Thus, the choice between the six anode materials for a MFC system would be based on material fabrication cost and power output rather than COD removal efficiency.
The extent and rate of wastewater treatment was expected to be higher in MFCs than in control reactors; treatment efficiencies of MFCs were actually 22 to 26% better than the control reactors. Anaerobic digestion using supported biofilms (control reactor) was improved by withdrawing current from the bacteria (MFCs) which even in the absence of usable electricity generation is a potential advantage of MFC over other treatment systems (Du et al. 2007; Logan 2004); however, due to the high internal resistance of the systems used in this study and the high external load applied under closed circuit (chosen, as previously specified, to have the worst case scenario to study current as a factor for bacteria selection on anodic communities), the flow of current obtained, typically around 3 mA m-2, was not enough to account for the 26% difference in COD removal between MFC and systems with an anode biofilm that was not connected to an MFC circuit. Controls were sealed reactors whilst MFCs had an aerobic chamber, connected through a proton exchange membrane to the anaerobic anode chamber. It has been demonstrated that PEMs can allow gas diffusion from the cathode to the anode chamber (Rozendal et al. 2008; Rozendal et al. 2007), so there was probably a small amount of aerobic COD removal taking place in the anode compartments of MFCs which could not occur in controls. This might at least partially explained the differences in COD removal between controls and MFCs at low current levels.
Another factor involved in the differences obtained from MFCs and controls is the presence of electrochemical processes related to COD removal. The rate of organic matter consumption in MFC is meant to be faster than that in conventional anaerobic digesters and the extent of improvement in COD removal would be increased as greater current was withdrawn from the bacteria (Jadhav and Ghangrekar 2009). The increase of current may be at the expense of reduced power, thus there would be a trade-off between power generation and treatment efficiency in wastewater MFCs. Hence, working with flow of current and under particular conditions of feed concentration, pH, and temperature, the MFC configuration potentially provides advantages over other wastewater treatment systems (Du et al. 2007; Logan 2004)
The baseline reactor (no anode material), gave significantly lower COD removal than both MFCs and controls: 36% (in relation to control) to 62% (compared with MFCs) lower. The presence of the biofilm enhanced the water treatment process regarding COD removal, by between 100% and 150%, compared with anaerobic digestion with suspended microorganisms (baseline reactor). It confirmed previous finding and illustrates that growth in biofilms increased the resistance of bacteria to physical and chemical stresses and thus they perform better than planktonic cells in the wastewater treatment (Mitchell et al. 2008; Stewart and Costerton 2001; Watnick and Kolter 2000).
The Coulombic efficiencies in these MFCs were very low (<2%) mainly due to the high external resistance/low current used, chosen, as indicated in Materials and methods, to have the worst case scenario to assess the role of current in selection of microorganisms on the biofilm. The proportion of electrogenic bacteria is then likely to be lower than at greater current levels because their growth using the electrogenic metabolic pathway to the maximum extent is impeded due to the 300 kΩ resistor used. If electrons are produced by facultative electrogens at a higher rate than the anode is able to accept then these microbes are expected to combine electrogenesis and other anaerobic metabolisms using different final acceptors. However, this does not negate the findings regarding the effect of the flow of current on selection bacterial communities. Whilst some of the electrogens may have been below the detection limit of the molecular community analysis used, data reported clearly show that anodic communities formed under current were significantly similar on all materials; and for anodic communities formed under current on different materials the level of similarity increased. While the direct effects on the electrogenic bacteria in the anodic biofilms may not be observed, the differences/similarities in anodic bacterial community composition are likely to result from differences in competition between the electrogens and non-electrogens.
Polarization of the cells did not noticeably affect the COD removal at the scale measured.
Electrochemical efficiency of the biofilms
The first initial rise in potential for the MFC corresponded to the microbial settlement and growth; after which the anodic bacterial community drove the device to a current and potential level where growth and maintenance of the biofilm, together with the electrical activity, were sustainable. Then the reactors remained in a stationary period with respect to cell potential (Fig. 5). The biofilm microbial community therefore has the ability to self regulate in response to changing conditions in an MFC. This regulation is based either on changes in microbial community composition with changes in conditions or on physiological regulation in individual organisms (Wang et al. 2009; Freguia et al. 2007; Rabaey et al. 2007). It has also been previously reported that voltage in MFC is closely related to the state of the microbial community, the level of COD and physicochemical properties of the anode surface (Zhan et al. 2008; Rabaey et al. 2007).
When the cells were at closed circuit, the similarities in the duration of the steady period for the carbon materials was better than that obtained with the MFCs at open circuit and also greater than in similar studies at OCV (Scott et al. 2007).
Maximum voltages obtained for all materials were within the same range as others using chemically modified carbon materials (Scott et al. 2007). The variation in voltage between the different anode materials was not sufficiently significant to recommend one particular material for sustained operation, although RVC clearly appears un-suitable. Based on the polarisation response graphite, carbon sponge and carbon cloth can be recommended as the anode support rather than carbon felt, carbon paper and especially not RVC. Regarding RVC, it has been suggested as a good candidate anode material in fuel cells, because of its three-dimensional structure and high surface area (Antonio Rinaldi et al. 2008; Logan et al. 2006; Friedrich et al. 2004). As shown in SEM images (Fig. 6) the RVC surface was vitreous and smooth and presumably difficult for bacteria to attach to, which might partially explain the poor performance of this material. However, SEM images of other materials such as graphite show that there is not complete coverage with biofilm (Fig. 7) casting doubt on this suggestion. Other studies on different materials have shown an increase in power output with an increase of anode surface area (Chaudhuri and Lovley 2003). In this study RVC, due to its open structure, had a real area of electrode surface available for bacteria to colonize lower than the other materials. This might be the reason behind the consistently the lowest power output of the MFC containing this electrode. Moreover, all the surface area of this material may have not been effectively available for bacteria. In this case, it would therefore seem that having such a high surface area, macro and micro porous material does not benefit MFC performance as bacteria do not penetrate significantly inside the internal structure because of the very small pore sizes. Even if there is biological growth internally, this is likely to be limited by a slow rate of internal diffusion of dissolved COD/BOD, leading to poor potential and current distribution inside the electrode and due to pores becoming blocked with bacteria and their exudates (Picioreanu et al. 2008; Marcus et al. 2007; Picioreanu et al. 2007; Rabaey et al. 2007). The macroscopic appearance of the colonized RVC anode (Fig. S11 in ESI) indicates that the anode supported a large amount of biomass. However most of the biomass filled the macroscopic recesses in the RVC with extensive exo-polysaccharides (EPS) which may have limited diffusion to the active surfaces of the anode. The EPS was eliminated or partially removed during the sample preparation for scanning electron microscopy. On the other hand, the actual area of electrode surface available for bacteria to colonize in RVC, due its very open structure is simply smaller than in the rest of the cases.
Polarization results (Fig. 8 and Table 4) show a different peak power for each reactor. According to the similarity of the microbial communities and the close pattern of COD removal rate, power output levels would have been expected to be in the same range for all MFCs. However, there were two clearly different groups of materials in terms of power output, from MFCs run at closed circuit as well as for MFCs run at open circuit. Graphite, sponge and cloth, performed much better than paper, felt and RVC. In the case of biofilms formed at closed circuit, polarization results of these last three anodes correlate to VFAs accumulation and the pH at the end of the test, when these materials presented the most acidic environment. The relationship of these parameters to polarization results is still not clear. Since DGGE analysis showed a significant similarity of the anode communities, differences in energy generation might be related to the surface charge and other properties of each anode since these have a strong effect on bacterial adhesion (Hitchens 1989; Wang et al. 2009; Scott et al. 2007). In addition there were different concentrations of biocatalyst on each anode, and the DGGE analysis performed only provides information on differences of the relative abundance of different members of the community in the biofilms but not the absolute amount of biofilm biomass.
Taking current as a potential factor in selection of the anode bacterial community, biofilms formed at closed circuit are expected to contain more electro-active organisms than those grown under open circuit conditions. It has been observed that during polarisation, biofilms developed with current flowing had a higher power than those grown in the absence of current and this reinforces the hypothesis that, beyond the effect of the anode support material, flow of current is a major factor in selection for electro active bacteria; in agreement to previously reported findings (Rabaey et al. 2004).
Overall energy generation was far from the maximum values reported in the field, close to 3 W m−2 using pure substrate, a pure culture and an air cathode (Xing et al. 2008). This is explained by the high external resistance used; under higher flow of current during biofilm formation and sustained operation, the anodic community would have developed a higher capability to produce peak power in polarisation (Rabaey et al. 2004). Besides, two chambered H-type MFCs have a high internal resistance and also measurements were taken from the system with the methodology of no feeding cycles to acclimatize the microflora but direct monitoring of the performance. This methodology of single feeding cycle was used for all experiments reported and it is a procedure validated by earlier works (Kim et al. 2006); it produces a higher amount of valid results per time unit, although it will not be a suitable choice when attempting to optimize power generation (which was not the case here). Values obtained were in the range reported from other studies using two-chamber systems (Manohar et al. 2008a, b; Lee et al. 2008).
Tafel slopes (Table 5) suggest that graphite had more favourable electrochemical characteristics compared to sponge or carbon cloth, which had very similar Tafel slopes. The value obtained for carbon sponge is higher than in previously reported works, where 100 mV decade−1 put this material ahead of graphite as a candidate anode material for marine sediment microbial fuel cells (Scott et al. 2008)
Polarisation appeared to benefit cell performance. The temporary imposition of higher anodic potentials appeared to enhance the activity of the electroactive bacteria at the anode as previously observed (Wang et al. 2009). Repeated polarisation does not negatively affect electrode behaviour (Manohar et al. 2008a) and the effect of polarisation on the performance of MFCs and the metabolism of electrogenic bacteria warrants further investigation under controlled conditions of COD and initial potential. The results of the cell polarisation studies suggest that performance may well depend on the state of the biofilm, an observation consistent with others reports (Fan et al. 2008; Manohar et al. 2008a; Ramasamy et al. 2008).
The almost constant cathode potential depicted in Fig. 9b suggests that the anode limited the MFC system. Platinum is an excellent catalyst for oxygen reduction, therefore it was expected that the use of platinised titanium electrodes would ensure that the cathode would not be a limiting factor in cell performance, even without oxygen sparging. Limitation of the system by the anode when the reaction in the cathode compartment is facilitated by a good catalyst is in agreement with earlier studies (Manohar et al. 2008a; Ramasamy et al. 2008)
Biofilm development was found to contribute positively to the impedance response of the MFCs (Table 6). The decrease in resistance at different frequencies observed in the test performed at 0 and 72 h may be attributed to the development of the biofilm containing electro-active bacteria enhancing electrochemical activity at the anode (Wang et al. 2009; Ramasamy et al. 2008; Scully et al. 1993). Other researchers have previously found a functional relationship between the resistance and cell voltage in MFCs (Manohar et al. 2008b) or have reported a increase in the power output with the increase of bacterial density in the biofilm (Rabaey et al. 2007).
Overall, the primary hypothesis that the anodic material selects for different bacterial communities and the flow of even a small current is enough to have an effect on the nature of the biofilm formed hold and are defensible as conclusions based on the data that present. COD removal was enhanced by the electrons being drawn from the bacteria by the MFC anode. Nevertheless, there was a high percentage of organic matter oxidized by conventional anaerobic digestion rather than anodic processes due partially to the high external resistor used to setup the suitable scenario for the study. The proportion of COD removed electrogenically would increase if a higher flow of current was allowed (use of lower external resistance) during development of the biofilm and later. Anode materials affected power generation during polarization, possibly due to differences in their physical and chemical surface characteristics. Materials with macrostructures likely to get blocked by exudates of bacteria, such as RVC, should be avoided for the anode support and the electrochemical and surface properties of the anode material must be accounted for in addition to the physical structure of the material. Temporary imposition of high anodic potential (during polarization) was also shown to benefit the electrical activity of the biofilm which is in agreement with previous results (Wang et al. 2009; Aelterman et al. 2008); therefore such treatments may in future allow the performance of MFC to be enhanced.
References
Aelterman P, Freguia S, Keller J, Verstraete W, Rabaey K (2008) The anode potential regulates bacterial activity in microbial fuel cells. Appl Microbiol Biotechnol 78(3):409–418
Antonio Rinaldi, B. M., Garavaglia V, Licoccia S, Di Nardo P, Traversa E (2008) Engineering materials and biology to boost performance of microbial fuel cells: a critical review. Energy and Environmental Science (In Press)
Chaudhuri SK, Lovley DR (2003) Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat Biotechnol 21(10):1229–1232
Cheng S, Logan BE (2007) Ammonia treatment of carbon cloth anodes to enhance power generation of microbial fuel cells. Electrochem Commun 9(3):492–496
Conover WJ (1980) Practical Nonparametric Statistics. Wiley, New York
Du Z, Li H, Gu T (2007) A state of the art review on microbial fuel cells: a promising technology for wastewater treatment and bioenergy. Biotechnol Adv 25(5):464–482
Dumas C, Mollica A, Féron D, Basséguy R, Etcheverry L, Bergel A (2007) Marine microbial fuel cell: use of stainless steel electrodes as anode and cathode materials. Electrochim Acta 53(2):468–473
Eaton Andrew DC (2005) Standard Methods for the Examination of Water and Wastewater. American Public Health Association, American Water Works Association, Water Environment Federation. Washington, DC
Fan MZ, Liang P, Cao XX, Huang X (2008) Effect of the initial anode potential on electricity generation in microbial fuel cell. Huanjing Kexue/Environmental Science 29(1):263–267
Freguia S, Rabaey K, Yuan Z, Keller J (2007) Electron and carbon balances in microbial fuel cells reveal temporary bacterial storage behavior during electricity generation. Environ Sci Technol 41(8):2915–2921
Freguia S, Rabaey K, Yuan Z, Keller J (2008) Syntrophic processes drive the conversion of glucose in microbial fuel cell anodes. Environ Sci Technol 42(21):7937–7943
Friedrich JM, Ponce-de-Leon C, Reade GW, Walsh FC (2004) Reticulated vitreous carbon as an electrode material. J Electroanal Chem 561:203–217
Hitchens GD (1989) Electrode surface microstructures in studies of biological electron transfer. Trends Biochem Sci 14(4):152–155
Jadhav GS, Ghangrekar MM (2009) Performance of microbial fuel cell subjected to variation in pH, temperature, external load and substrate concentration. Bioresour Technol 100(2):717–723
Jiang D, Li B (2007) ACS National Meeting Book of Abstracts. Boston, MA
Kim GT, Webster G, Wimpenny JWT, Kim BH, Kim HJ, Weightman AJ (2006) Bacterial community structure, compartmentalization and activity in a microbial fuel cell. J Appl Microbiol 101(3):698–710
Larrosa-Guerrero A, Lozano LJ, Katuri KP, Head I, Scott K, Godinez C (2009) On the repeatability and reproducibility of experimental two-chambered microbial fuel cells. Fuel 88: 1852–1857
Lee HS, Parameswaran P, Kato-Markus A, Torres CI, Rittmann BE (2008) Evaluation of energy-conversion efficiencies in microbial fuel cells (MFCs) utilizing fermentable and non-fermentable substrates. Water Res 42:1501–1510
Liu JL, Lowy DA, Baumann RG, Tender LM (2007) Influence of anode pretreatment on its microbial colonization. J Appl Microbiol 102(1):177–183
Logan B, Cheng S, Watson V, Estadt G (2007) Graphite fiber brush anodes for increased power production in air-cathode microbial fuel cells. Environ Sci Technol 41(9):3341–3346
Logan BE (2004) Penn State cuts cost of waste water fuel cell. Ind Bioprocess 26(7):2–3
Logan BE, Hamelers B, Rozendal R, Schröder U, Keller J, Freguia S, Aelterman P, Verstraete W, Rabaey K (2006) Microbial fuel cells: methodology and technology. Environ Sci Technol 40(17):5181–5192
Lowy DA, Tender LM, Zeikus JG, Park DH, Lovley DR (2006) Harvesting energy from the marine sediment-water interface II. Kinetic activity of anode materials. Biosens Bioelectron 21(11):2058–2063
Lu N, Zhou SG, Zhang JT, Ni JR (2009) Electricity generation from corn steepwater using microbial fuel cell technology. Huanjing Kexue/Environmental Science 30(2):563–567
Manohar AK, Bretschger O, Nealson KH, Mansfeld F (2008a) The polarization behavior of the anode in a microbial fuel cell. Electrochim Acta 53(9):3508–3513
Manohar AK, Bretschger O, Nealson KH, Mansfeld F (2008b) The use of electrochemical impedance spectroscopy (EIS) in the evaluation of the electrochemical properties of a microbial fuel cell. Bioelectrochemistry 72(2):149–154
Marcus AK, Torres CI, Rittmann BE (2007) Conduction-based modeling of the biofilm anode of a microbial fuel cell. Biotechnol Bioeng 98(6):1171–1182
Mitchell AC, Phillips AJ, Hamilton MA, Gerlach R, Hollis WK, Kaszuba JP, Cunningham AB (2008) Resilience of planktonic and biofilm cultures to supercritical CO2. J Supercrit Fluids 47(2):318–325
Morozan A, Stamatin I, Stamatin L, Dumitru A, Scott K (2007) Carbon electrodes for microbial fuel cells. J Optoelectron Adv Mater 9(1):221–224
Muyzer G, De Waal EC, Uitterlinden AG, (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 165 vRNA. Appl Environ Microbiol 59:695–700
Park DH, Zeikus JG (2003) Improved fuel cell and electrode designs for producing electricity from microbial degradation. Biotechnol Bioeng 81(3):348–355
Picioreanu C, Head IM, Katuri KP, van Loosdrecht MCM, Scott K (2007) A computational model for biofilm-based microbial fuel cells. Water Res 41(13):2921–2940
Picioreanu C, Katuri KP, Head IM, Van Loosdrecht MCM, Scott K (2008) Mathematical model for microbial fuel cells with anodic biofilms and anaerobic digestion. Water Sci Technol 57:965–971
Pietron JJ, Jones-Meehan J, Little B, Ray R, Ringeisen BR (2005) ACS National Meeting Book of Abstracts. Washington, DC
Prat C, Ruiz-Rueda O, Trias R, Anticó E, Capone D, Sefton M, Bañeras L (2009) Molecular fingerprinting by PCR-denaturing gradient gel electrophoresis reveals differences in the levels of microbial diversity for musty-earthy tainted corks. Appl Environ Microbiol 75(7):1922–1931
Qiao Y, Bao SJ, Li CM, Cui XQ, Lu ZS, Guo J (2008) Nanostructured polyaniline/titanium dioxide composite anode for microbial fuel cells. ACS Nano 2(1):113–119
Rabaey K, Boon N, Siciliano SD, Verhaege M, Verstraete W (2004) Biofuel cells select for microbial consortia that self-mediate electron transfer. Appl Environ Microbiol 70(9):5373–5382
Rabaey K, Rodriguez J, Blackall LL, Keller J, Gross P, Batstone D, Verstraete W, Nealson KH (2007) Microbial ecology meets electrochemistry: electricity-driven and driving communities. ISME J 1(1):9–18
Ramasamy RP, Ren Z, Mench MM, Regan JM (2008) Impact of initial biofilm growth on the anode impedance of microbial fuel cells. Biotechnol Bioeng 101(1):101–108
Rinaldi A, Mercheri B, Garavaglia V, Licoccia S, Di Nardo P, Traversa E (2008) Engineering materials and biology to boost performance of microbial fuel cells: a critical review. Energy and Environmental Sciences 1:417–429
Rozendal RA, Hamelers HVM, Molenkamp RJ, Buisman CJN (2007) Performance of single chamber biocatalyzed electrolysis with different types of ion exchange membranes. Water Res 41(9):1984–1994
Rozendal RA, Hamelers HVM, Rabaey K, Keller J, Buisman CJN (2008) Towards practical implementation of bioelectrochemical wastewater treatment. Trends Biotechnol 26(8):450–459
Schröder U, Nießen J, Scholz F (2004) ACS, Division of Environmental Chemistry—Preprints of Extended Abstracts, Philadelphia, PA, 17–21 August 2008
Scott K, Cotlarciuc I, Hall D, Lakeman JB, Browning D (2008) Power from marine sediment fuel cells: the influence of anode material. J Appl Electrochem 38: 1–7
Scott K, Rimbu GA, Katuri KP, Prasad KK, Head IM (2007) Application of modified carbon anodes in microbial fuel cells. Process Saf Environ Prot 85(5 B):481–488
Scully JR, Silverman David C, Kendig Martin W (1993) Electrochemical Impedance: analysis and Interpretation. ASTM, Philadelphia
Stewart PS, Costerton JW (2001) Antibiotic resistance of bacteria in biofilms. Lancet 358(9276):135–138
ter Heijne A, Hamelers HVM, Saakes M, Buisman CJN (2008) Performance of non-porous graphite and titanium-based anodes in microbial fuel cells. Electrochim Acta 53(18):5697–5703
Wang X, Feng Y, Ren N, Wang H, Lee H, Li N, Zhao Q (2009) Accelerated start-up of two-chambered microbial fuel cells: Effect of anodic positive poised potential. Electrochim Acta 54(3):1109–1114
Watnick P, Kolter R (2000) Biofilm, city of microbes. J Bacteriol 182(10):2675–2679
Xing D, Zou Y, Cheng S, Regan JM Logan BE (2008) Electricity generation by Rhodopseudomonas palustris DX-1. Environ Science & Tech 42(11):4146–4151
Zhan YL, Wang Q, Zhang PP, Yan GX, Guo SH (2008) Investigation on influence factors and mechanism of microbial fuel cell. Gaodeng Xuexiao Huaxue Xuebao/Chemical Journal of Chinese Universities 29(1):144–148
Zhang E, Xu W, Diao G, Shuang C (2006) Electricity generation from acetate and glucose by sedimentary bacterium attached to electrode in microbial-anode fuel cells. J Power Sources 161(2):820–825
Zhang T, Zeng Y, Chen S, Ai X, Yang H (2007) Improved performances of E. coli-catalyzed microbial fuel cells with composite graphite/PTFE anodes. Electrochem Commun 9(3):349–353
Acknowledgments
The support of the European Union for Transfer of Knowledge award (MTKD-CT-2004-517215) for biological fuel cells, the EPSRC and the Spanish Ministry of Science and Innovation (MICINN ENE2006-09395) are acknowledged. Mrs. Fiona L. Read and Mr. Alberto Alcolea are acknowledged as well for their support in microbial analysis and SEM.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 8379 kb)
Rights and permissions
About this article
Cite this article
Larrosa-Guerrero, A., Scott, K., Katuri, K.P. et al. Open circuit versus closed circuit enrichment of anodic biofilms in MFC: effect on performance and anodic communities. Appl Microbiol Biotechnol 87, 1699–1713 (2010). https://doi.org/10.1007/s00253-010-2624-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2624-1