Abstract
The baculovirus expression vector system is one of the most powerful and versatile eukaryotic expression systems available. However, as the recombinant baculovirus is usually generated by replacing the foreign gene into the polyhedrin locus, the resulting polyhedrin-negative virus is less infectious to the host larvae when administered via oral ingestion. This limits the large-scale production of the recombinant protein, as the host larvae can only be inoculated through dorsal injection, which is a laborious task. In this paper, we describe a new Bombyx mori nucleopolyhedrovirus polyhedrin-plus Bac-to-Bac baculovirus expression system for application in silkworm, B. mori. In this system, the foreign gene and the polyhedrin are co-expressed, and polyhedra are produced as in the wild-type virus, and thus the recombinant baculovirus can be used directly via oral infection. It effectively improves the efficiency of the baculovirus expression system and also widens the application of baculovirus in other fields, such as the development of new biological insecticides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Baculoviruses exist as two phenotypes in their biphasic life cycle, budded virions (BV) and occlusion-derived virus (ODV), which have a common nucleocapsid structure and carry the same genetic information (Blissard 1996; Rohrmann 1992; Rohrmann 2008). ODVs are highly infectious to the midgut epithelial cells and mediate animal-to-animal transmission, but have very low infectivity when injected directly into the hemocoel of insects, or when used to infect cultured insect cells. BVs on the other hand are highly infectious for hemocoel tissues and for cultured cells. The infection discrepancy between BV and ODV is mostly due to the specificity of the nucleocapsids and envelope proteins (Faulkner et al. 1997; Haas-Stapleton et al. 2004; Gutiérrez et al. 2005), for example P74 is specific to ODV and plays an important role in oral infection (Faulkner et al. 1997; Braunagel and Summers 2007).
The baculovirus expression vector system is proven to be one of the most powerful and versatile eukaryotic expression systems available (O’Reilly et al. 1992; Kidd and Emery 1993). Currently there are two types of baculovirus-based expression systems used for the production of recombinant proteins. Besides the Autographa californica multiple nucleopolyhedrovirus system, the Bombyx mori nucleopolyhedrovirus (BmNPV) expression system is also extensively applied and has some advantages: Recombinant proteins can be massively produced at lower cost in silkworm larvae, which are large bodied and easily reared (Lee et al. 2006; Zhao et al. 2008). However, the recombinant baculovirus is usually generated through inserting the foreign gene into the polyhedrin locus, resulting in a polyhedrin-negative virus, i.e., BV phenotype. Such polh-negative baculovirus is less infectious to host larvae via oral ingestion (Liu et al. 2007; Jinn et al. 2009). This limits the large-scale production of recombinant protein, as host larvae can only be inoculated through dorsal injection; this work is laborious and, moreover, requires skill. It has become a bottleneck for large-scale production of recombinant proteins in the silkworm system. However, it is possible to generate recombinant baculoviruses that retain a polyhedrin gene. Such viruses form occlusion bodies and are therefore infectious to the insect larvae, enabling the production of the heterologous protein within the infected insect. A system was described for the rapid generation of BmNPV-based expression vectors (Je et al. 2001). In their work, the BmNPV genomes are garnished with a mini-F replicon for maintenance in Escherichia coli but lack a portion of the essential ORF1629 gene and therefore cannot replicate independently in insect cells. However, they can serve as parental genomes for the generation of expression vectors by cotransfection into B. mori cells with a transfer plasmid that includes an intact ORF1629. Only recombinant viruses that have acquired the ORF1629 gene from the transfer vector can replicate after cotransfection. Transfer vectors are constructed in such an intelligent way that a foreign gene of interest for expression is also acquired by the recombination of ORF1629 into the formerly defective viral genome. However, the system still needs cotransfection and subsequent screening of the recombinants in B. mori cells.
In this study, we constructed a novel BmNPV polyhedrin-plus (Polh +) Bac-to-Bac baculovirus expression system, based on our BmNPV Bac-to-Bac system (polyhedrin-minus) previously constructed especially for application in silkworm (Motohashi et al. 2005; Cao et al. 2006). The BmNPV polyhedrin ORF sequence was inserted downstream of the p10 promoter through homologous recombination. A gene of interest can subsequently be inserted into the new BmNPV Polh + construct from a suitable donor plasmid by transposition occurring in E. coli resulting in a single construct with all gene functions for infection of B. mori cells. In this system, in infected B. mori cells, the foreign gene and the polyhedrin gene are co-expressed, and thus the recombinant baculovirus could be directly inoculated to the host larvae via oral infection. It effectively improves the efficiency of the baculovirus expression system and also widens the application of baculovirus in other fields, such as the development of new biological insecticides.
Materials and methods
Materials
The major components of the BmNPV Bac-to-Bac (polh −) expression system (Cao et al. 2006) include donor plasmids that allow the generation of an expression construct containing the gene of interest (where expression of the gene of interest is controlled by a baculovirus-specific promoter), E. coli host strain DH10BmBacP (competent cells) containing a BmNPV bacmid (BmBacmid), and a helper plasmid that allows the generation of a recombinant bacmid following transposition of the donor expression construct in E. coli. The donor plasmid pFastBacHTs were purchased from Invitrogen (USA). Fetal calf serum and culture medium TC-100 for cultured cells were purchased from GibcoBRL (USA). Cellfectin reagent for transfection was purchased from Invitrogen, and EGFP antibody was available from Cell Signal (USA).
B. mori BmN cells were maintained at 27°C in TC-100 medium supplemented with 10% fetal calf serum. BmNPV was stored in our laboratory. A hybrid strain of silkworm (commercial name: Jingsong × Haoyue) was used for the infection test. The larvae were reared on mulberry leaves at 23–25°C.
Construction of the recombinant transfer vector
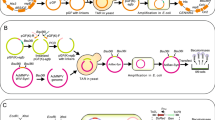
In order to insert the polyhedrin ORF sequence (polh) into the p10 locus of BmBacmid through homologous recombination, a recombinant transfer plasmid was constructed as shown in Fig. 1. The upstream and downstream fragments of the p10 gene (named p10-up and p10-down, about 2.0 kb) were cloned from the BmNPV genome. The p10-up fragment included the p10 promoter. The primers were designed as listed in Table 1. The PCR was run using the following conditions: denaturing at 94°C for 50 s, annealing at 55°C for 30 s, and extension at 72°C for 2 min; the reaction was run for 30 cycles. Subsequently, the amplified fragments p10-up and p10-down were digested with HindIII/PstI and PstI/BamHI, respectively, and cloned into the corresponding sites of pUC19. This construct was named pUC19-p10-up–down.
A 780-bp polyhedrin ORF sequence was PCR-amplified from the BmNPV genome by using the primers PolhF/PolhB displayed in Table 1. It was digested with PstI and ligated into the PstI site of pUC19-p10-up–down to generate the final recombinant plasmid pUC19-p10-up-polh-down.
Screening of the Polh + BmBacmid
The flowchart for screening of the Polh + BmBacmid is displayed in Fig. 2. The pUC19-p10-up–polh-down (1 μg/μL) and BmNPV bacmid DNA (1 μg/μL) were cotransfected into BmN monolayer cells. For 15 min at room temperature, 10 μL of plasmid, 1 μL of BmNPV bacmid DNA, 14 μL of lipofectin, and 15 μL of MilliQ H2O (final volume 40 μL) were gently mixed and incubated and added to the cell medium. The cells were allowed to grow for 5 days, the polyhedra were collected, and virus genomic DNA was extracted (actually the Polh + BmBacmid). The Polh + BmBacmid was then electrotransformed into DH10β-competent cells. Blue colonies were selected from plates containing kanamycin (50 μg/mL), X-gal (100 μg/mL), and IPTG (40 μg/mL). Finally, selected colonies were cultured in LB medium containing kanamycin, and the Polh + BmBacmid was extracted. The correct recombination of polyhedrin ORF sequence into p10 locus was identified by PCR.
Construction of the BmNPV Polh + Bac-to-Bac expression system
The Polh + BmBacmid was transformed into E. coli DH10β-competent cells, named as DH10BmBac (Polh +). A helper plasmid (13.2 kb, pMON7124), encoding the transposase and conferring resistance to tetracycline, was transformed into the DH10BmBac (Polh +) cells. Colonies were screened on plates containing kanamycin and tetracycline (7 μg/mL). Thus, in addition to the donor plasmids that are already commercially available, this novel polyhedrin-plus (Polh +) Bac-to-Bac baculovirus expression system was developed for specific application in silkworm, named as BmNPV Polh + Bac-to-Bac baculovirus expression system.
Application of the BmNPV Polh + Bac-to-Bac expression system using a reporter gene
Construction of the recombinant virus vBmBac(polh +)-EGFP
To test the efficiency of the constructed BmNPV Polh + Bac-to-Bac system in silkworm, the reporter gene EGFP (enhanced green fluorescent protein; Zhang et al. 1996) was used for the generation of recombinant baculovirus and expression. The 720-bp EGFP gene was cloned into EcoRI sites of the donor plasmid pFastBacHTc. Approximately 1 ng of the thus-obtained recombinant donor plasmid was transformed into 100 μL of DH10BmBac(Polh +)-competent cells, incubated at 37°C for 4 h, serially diluted using SOC medium, and spread evenly on plates containing kanamycin, tetracycline, and X-gal. After a 48-h incubation at 37°C, the white colonies expected to represent recombinant BmNPV bacmids containing a transposed donor expression construct [vBmBac(polh +)-EGFP] were picked and cultured overnight in medium containing kanamycin. The Bacmid DNA was subsequently extracted and transfected into BmN cells to generate the recombinant baculoviruses, designated as vBmBac(polh +)-EGFP. After 5 days, the medium supernatant was collected and used to infect the silkworm larvae. For evaluating the EGFP expression in cells infected with vBmBac(polh +)-EGFP, a positive control, vBmBac(polh −)-EGFP (without polyhedrin gene) was established through transforming the above donor plasmid pFastBacHTc-EGFP into DH10BmBac(Polh −)-competent cells (Cao et al. 2006) and subsequently extracting the bacmid and transfection.
SDS-PAGE and Western blot analysis of the expressed protein
Protein samples were electrophoresed in 10% SDS-PAGE gels and transferred to a PVDF membrane with a Bio-Rad liquid transfer apparatus, according to the manufacturers' recommended protocols. The primary antibody was mouse monoclonal EGFP antibody. The secondary antibody was goat monoclonal mouse antibody marked with horseradish peroxidase; TMB was used to display the color.
Oral infection of silkworm larvae using the polyhedra of vBmBac(polh +)-EGFP
BmN cells were inoculated using the recombinant viruses, and polyhedra were collected 96 h post-infection. Purified polyhedra were suspended in PBS buffer and counted. The newly molted silkworm larvae of the third, fourth, and fifth instars were used for investigation of per oral infection efficiency. A 3.0 × 108 polyhedron/mL solution was smeared on the mulberry leaves and administrated for feeding. The infection symptoms were observed 120 h later.
Results
Identification of the Polh + BmBacmid and its infection in BmN cells
Recombinant baculovirus was successfully generated through cotransfection of plasmid pUC19-p10-up–polh-down and BmBacmid (polh −) DNA in BmN cells that subsequently expressed polyhedrins and formed polyhedra. The Polh + BmBacmid obtained, plus the helper plasmid and donor vectors, was developed as a novel BmNPV Polh + Bac-to-Bac baculovirus expression system. Using this system, a reporter gene (EGFP) was applied for generation in E. coli, a recombinant polyhedron-plus virus, vBmBac(polh +)-EGFP. Large amounts of polyhedra were observed after infection in the BmN cells, similar to the wild-type BmNPV (Fig. 3a, b). The size and shape of polyhedra were almost the same as in the control. These results indicated that the polyhedra could be normally produced in the case of deletion of p10, implying that the p10 gene in BmNPV will not affect the formation of polyhedra, confirming the work of Vlak et al. (1988). Furthermore, the virions in the polyhedra were checked, and we found that these polyhedra could also embed the virions, like the wild-type BmNPV (Fig. 3c, d).
Expression of reporter gene in BmNPV polh + Bac-to-Bac system
In the BmN cells infected by vBmBac(polh +)-EGFP, strong green fluorescence excited by blue light was observed (Fig. 4a, b). The expression of EGFP was further identified by SDS-PAGE and Western Blot analysis; the predicted band (around 30 kDa) was clearly displayed on the electrophoresis pattern (Fig. 4c). For comparison, BmN cells were also infected by BmNPV (lane 2 in Fig. 4c) as positive control for polyhedrin and negative control for EGFP and by vBmBac(polh −)-EGFP (lane 3 in Fig. 4c) as negative control for polyhedrin and positive control for EGFP. The results showed that the expression level of EGFP in cells infected by vBmBac(polh +)-EGFP was almost the same as in the positive control. It was further confirmed by Western Blot analysis by using EGFP antibody (Fig. 4d). The polyhedron protein is almost of the same size as EGFP and is detected as to be expected only in SDS-PAGE of BmN cells infected by wild-type BmNPV and of BmN cells infected by vBmBac(polh +)-EGFP (Fig. 4c). The results clearly indicate that the novel system established in this study could not only produce polyhedra but also expresses well the foreign gene.
Expression of EGFP in BmN cells infected by vBmBac(polh +)-EGFP. BmN cells infected by vBmBac(polh +)-EGFP as viewed under a visible light; b blue excitation light; c SDS-PAGE of BmN cells infected by wild-type BmNPV (lane 2, negative control), vBmBac(polh -)-EGFP (lane 3, positive control), and vBmBac(polh +)-EGFP (lane 4). The marker of molecular weight is shown in lane 1 with sizes listed at the left. Arrows indicate the polyhedron protein (purple) and EGFP (blue). d The proteins of the SDS-PAGE were transferred to a PVDF membrane and probed with antibodies against EGFP. Positive EGFP signals are marked by blue arrows
Per oral infection of silkworm larvae using the polyhedra of vBmBac(polh +)-EGFP
Purified polyhedra were used for per oral infection. The results are summarized in Table 2. Polyhedra of vBmBac(polh +)-EGFP infected the silkworm larvae as effectively as the wild type. The larvae presented typical infectious symptoms after per oral feeding, with 100% of third and fourth instar, nearly 90% of fifth instar larvae infected. These results demonstrate that the polyhedra produced by Polh + Bac-to-Bac system was almost as effective for per oral infection as the wild-type virus.
Discussion
The baculovirus expression system was developed more than 20 years ago and is recognized as one of the most efficient expression systems. However, with the exception of expression in cultured cells, large-scale production using host larvae was so far difficult because of a bottleneck, dorsal injection, which is relatively tedious and difficult (Je et al. 2001; Wang et al. 2007; Jinn et al. 2009). In this study, a novel BmNPV Polh + Bac-to-Bac baculovirus expression system specifically for silkworm was successfully developed. It has two advantages, one being that like previous Bac-to-Bac systems, it could quickly generate recombinant baculovirus through transposition in bacteria. The second is that it can express the foreign gene and the polyhedrin simultaneously. The polyhedra produced by this system embed recombinant virus and consequently can be used via oral infection of host larvae. Hence, this system greatly improves the production efficiency of the baculovirus system, especially in the case of large-scale industrial production of recombinant protein using silkworms as bioreactors.
In this novel system, two very late and strong promoters, p10 and polh, were used for driving the expression of the polyhedrin and the foreign gene respectively. Neither is essential for viral replication and infection (Vlak et al. 1988). The polyhedrin Orf is designed to be driven by relatively weaker p10 promoter to produce polyhedra (to some extent), and the gene of interest will be driven by powerful polyhedrin promoter to guarantee high-level expression of foreign protein. The results of this experiment showed that the reporter gene EGFP was well expressed (Fig. 4c, d); moreover, lots of polyhedra were produced when the polyhedrin ORF sequence was driven by the p10 promoter, and knock-out of p10 did not affect the formation of polyhedra (Fig. 2b). Interestingly, we observed a delay in the lysis of nuclei and release of polyhedra from the cells (not shown). Previous studies reported that p10 was associated with the formation of the polyhedra envelopes (Vlak et al. 1988; Williams et al. 1989) and that polyhedra without p10 were “naked” and therefore relatively unstable, but with an infective ability increased by 2-fold (Vlak et al. 1988). In this study, the recombinant virus without the p10 gene was more stable and well infectious. Therefore the Polh + Bac-to-Bac baculovirus expression system established in this study will be more suitable for industrial production of recombinant protein in the silkworm. Thus, we established an important technique platform for large-scale protein production in silkworm. It will benefit the development of biotechnological industry in those countries, particularly in developing countries where silkworm rearing and sericulture is an important agricultural activity, to exploit the silkworm as efficient biofactory for production of useful protein. Besides being used for recombinant protein production, this system could also be exploited for the development of new biological insecticides by constructing recombinant virus expressing the toxins.
References
Blissard GW (1996) Baculovirus–insect cell interactions. Cytotechnol 20:73–93
Braunagel SC, Summers MD (2007) Molecular biology of the baculovirus occlusion-derived virus envelope. Curr Drug Targets 8:1084–1095
Cao C, Wu X, Lu X, Zhao N, Yao H (2006) Development of a rapid and efficient BmNPV baculovirus expression system for application in mulberry silkworm, Bombyx mori. Curr Sci 91(12):1692–1697
Faulkner P, Kuzio J, Williams GV, Wilson JA (1997) Analysis of p74, a PDV envelope protein of Autographa californica nucleopolyhedrovirus required for occlusion body infectivity in vivo. J Gen Virol 78(12):3091–3100
Gutiérrez S, Mutuel D, Grard N (2005) The deletion of the pif gene improves the biosafety of the baculovirus-based technologies. J Biotechnol 116(2):135–143
Haas-Stapleton EJ, Washburn JO, Volkman LE (2004) P74 mediates specific binding of Autographa californica nucleopolyhedrovirus occlusion-derived virus to primary cellular targets in the midgut epithelia of Heliothis virescens larvae. J Virol 78(13):6786–6789
Je YH, Chang JH, Kim MH, Roh JY, Jin BR, O’Reilly DR (2001) The use of defective Bombyx mori nucleopolyhedrovirus genomes maintained in Escherichia coli for the rapid generation of occlusion-positive and occlusion-negative expression vectors. Biotechnol Lett 23:1809–1817
Jinn TR, Kao SS, Tseng YC, Chen YJ, Wu TY (2009) Aerosol infectivity of a baculovirus to Trichoplusia ni larvae: an alternative larval inoculation strategy for recombinant protein production. Biotechnol Prog 25(2):384–389
Kidd IM, Emery VC (1993) The use of baculoviruses as expression vectors. Applied Biochem Biotechnol 42:137–159
Lee KS, Je YH, Woo SD, Sohn HD, Jin BR (2006) Production of a cellulase in silkworm larvae using a recombinant BmNPV nucleopolyhedrovirus lacking the virus-encoded chitinase and cathepsin genes. Biotechnol Lett 28:645–650
Liu Y, DeCarolis N, Beek N (2007) Protein production with recombinant baculoviruses in lepidopteran larvae. Methods Mol Biol 388:267–280
Motohashi T, Shimojima T, Fukagawa T, Maenaka K, Park EY (2005) Efficient large-scale protein production of larvae and pupae of silkworm by Bombyx mori nucleopolyhedrosis virus bacmid system. Biochem Biophys Res Commun 326(3):564–569
O’Reilly DR, Miller LK, Luckow VA (1992) Baculovirus expression vectors: A laboratory manual. Freeman, New York
Rohrmann G (1992) Baculovirus structural proteins. J Gen Virol 73:749–761
Rohrmann G (2008) Baculovirus molecular biology. National Library of Medicine (US), National Center for Biotechnology Information, Bethesda
Vlak JM, Klinkenberg FA, Zaal KJM, Geervliet JBF, Roosien J, Van Lent JWM (1988) Functional studies on the p10 gene of Autographa californica nucleopolyhedrosis virus using a recombinant expressing a p10-galactosidase fusion gene. J Gen Virol 69:765–777
Wang B, Shang J, Liu X, Cui W, Wu X, Zhao N (2007) Enhanced effect of fluorescent whitening agent on peroral infection for recombinant baculovirus in the host Bombyx mori L. Curr Microbiol 54(1):5–8
Williams GV, Rohel DZ, Kuzio J (1989) A cytopathological investigation of Autographa californica nucleopolyhedrosis virus p10 gene function using insertion/deletion mutants. J Gen Virol 70:187–202
Zhang G, Gurtu V, Kain SR (1996) An enhanced green fluorescent protein allows sensitive detection of gene transfer in mammalian cells. Biochem Biophys Res Commun 227(3):707–711
Zhao N, Yao H, Lan L, Cao C, Wu X (2008) Efficient production of canine interferon-α in silkworm Bombyx mori by using BmNPV/Bac-to-Bac expression system. Appl Microbiol Biotechnol 78:221–226
Acknowledgment
This work was supported by the National Natural Science Foundation of China (30871827), Zhejiang Provincial Program (2009C14G2010086).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Xiang, X., Yang, R., Yu, S. et al. Construction of a BmNPV polyhedrin-plus Bac-to-Bac baculovirus expression system for application in silkworm, Bombyx mori . Appl Microbiol Biotechnol 87, 289–295 (2010). https://doi.org/10.1007/s00253-010-2476-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2476-8