Abstract
Aerobic sludge granules are compact, strong microbial aggregates that have excellent settling ability and capability to efficiently treat high-strength and toxic wastewaters. Aerobic granules disintegrate under high organic loading rates (OLR). This study cultivated aerobic granules using acetate as the sole carbon and energy source in three identical sequencing batch reactors operated under OLR of 9–21.3 kg chemical oxygen demand (COD) m−3 day−1. The cultivated granules removed 94–96% of fed COD at OLR up to 9–19.5 kg COD m−3 day−1, and disintegrated at OLR of 21.3 kg COD m−3 day−1. Most tested isolates did not grow in the medium at >3,000 mg COD l−1; additionally, these strains lost capability for auto-aggregation and protein or polysaccharide productivity. This critical COD regime correlates strongly with the OLR range in which granules started disintegrating. Reduced protein quantity secreted by isolates was associated with the noted poor granule integrity under high OLR. This work identified a potential cause of biological nature for aerobic granules breakdown.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The aerobic sludge granulation process is superior to the conventional activated sludge processes in terms of its great granule settleability (Morgenroth et al. 1997; Tay et al. 2001; Yang et al. 2003; Liu and Tay 2004; Adav et al. 2007a), high biomass retention (Adav et al. 2008a), and ability to withstand high organic loading rates (OLR) (Moy et al. 2002; Tay et al. 2002) and to resist inhibitory and toxic compounds (Adav et al. 2007a, b). State-of-the-art granulation processes have been commented (Liu and Tay 2004; Maximova and Dahl 2006; de Kreuk et al. 2007; Adav et al. 2008a).
The OLR is essential to determining granule stability (Arrojo et al. 2004; McSwain et al. 2004; de Kreuk et al. 2005). Tay et al. (2004a) was unable to cultivate aerobic granules at <4 kg chemical oxygen demand (COD) m−3 day−1 using glucose and peptone as substrates. Conversely, granules subjected to high OLR would disintegrate (Moy et al. 2002; Liu and Liu 2006; Zheng et al. 2006; Thanh et al. 2008). Moy et al. (2002) noted that acetate-fed granules did not withstand an OLR >9 kg COD m−3 day−1. Zheng et al. (2006), who cultivated sucrose-fed aerobic granules at an OLR of 6 kg COD m−3 day−1, observed that the granules converted from bacteria-dominated to filamentous granules prior to disintegration. Chen et al. (2008) demonstrated that aeration air velocity and OLR affect aerobic granulation. Kim et al. (2008), who cultivated aerobic granules at different OLR, identified the optimal COD loading for granulation processes.
Liu and Liu (2006) demonstrated that overgrowth of filamentous microorganisms could result in reactor failure when the amount of dissolved oxygen is inadequate, which commonly occurs in reactors with high OLR. Zheng et al. (2006) proposed that the mass transfer limitation of large granules cultivated under high OLR lead to anaerobe activity inside granules; hence, granules disintegrate. Adav et al. (2009) experimentally confirmed the existence of proteolytic bacteria belonging to genera Pseudomonas, Raoultella, Acinetobacter, Pandoraea, Klebsiella, Bacillus, and uncultured bacterium inside aerobic granules. These bacteria were demonstrated as responsible for granule core deterioration, resulting in granule disintegration. To the authors’ best knowledge, no study has identified the biological causes of granule failure under high OLR.
This work identified the biological causes of granule breakdown under high OLR. Aerobic granules were cultivated in three geometrically identical sequencing batch reactors (SBRs). The bacterial strains were isolated and identified. The auto-aggregation indices and the capability of isolates to secrete extracellular polymeric substances (EPS) were determined under high OLR to demonstrate the biological breakdown of granules.
Materials and methods
Experimental setup and seeding
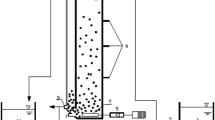
Activated sludge acquired from a local municipal wastewater treatment plant in Taipei, Taiwan, was used as the seed inoculum. This study used three geometrically identical column-type SBRs (R1–3), each 120 cm high and 6 cm in diameter, with 2-l working volume. Fine air bubbles for aeration and mixing were supplied through a dispenser at the reactor bottom at superficial velocity of 3.4 cm s−1. All reactors were operated in 4-h cycles, each cycle consisting of 5-min settling, 10-min effluent withdrawal, 10-min filling, and 215 min of reaction. The volumetric exchange ratio, defined as the fraction of supernatant that was withdrawn, was 50%.
Medium
The synthetic wastewater consisted of acetate as the sole carbon and energy source. The composition of wastewater for initial sludge acclimation is as follows: acetate, 0.4 g l−1; (NH4)2SO4, 1.0 g l−1; NaCl, 0.2 g l−1; MgSO4·7H2O, 0.2 g l−1; FeCl3, 0.02 g l−1; CaCl2·2H2O, 0.01 g l−1; buffer (K2HPO4, 1.65 g l−1; KH2PO4, 1.35 g l−1); and micronutrients, 1.0 ml l−1 (Adav et al. 2007b). The feed OLR were altered by proportionally altering concentrations of acetate and all ingredients, except for those of the buffer and FeCl3. Following acclimation, the sludge was fed into R1–3 at an OLR of 9.0 kg COD m−3 day−1 (COD of 2,990 mg l−1) for 1 week. The OLR for R2 and R3 were step increased to 12.6 and 16.7 kg COD m−3 day−1 (COD of 4,185 and 5,550 mg l−1), respectively. After 60 days of operation, the OLR for R3 was increased to 19.5 kg COD m−3 day−1 (COD of 6,470 mg l−1), and operated until it reached COD removal performance of 94–95%; then the OLR was further increased to 21.3 kg COD m−3 day−1 (COD of 7,070 mg l−1) for a period of only 2 weeks, as reactor operation failed due to excessive foaming and disintegration of granules.
Strain isolation and identification
Granules were cultivated in R1–3 for a 60-day period of feeding at an OLR of 9.0 kg COD m−3 day−1. Granules were collected and were aseptically broken in sterilized tubes containing sterile reactor-fed medium. Supernatant was serially diluted (101–109-fold dilutions), and 1 ml of each 105–109 dilution was spread onto an agar plate containing reactor-fed medium. The plates were placed in an incubator at 37 °C, and 52 morphologically identified colonies were isolated via several cycles of replating on reactor-fed agar medium.
The DNA from the isolated strain was extracted, and polymerase chain reaction (PCR)-amplified using primers 27f (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1,492r (5′-GGTTACCTTGTTACGACTT-3′). Amplification was conducted in an Eppendorf mastercycler (Eppendorf AG, Hamburg, Germany) via denaturation at 94 °C for 3 min, 25 cycles at 94 °C for 20 s, 54 °C for 30 s, 72 °C for 45 s, and final extension at 72 °C for 7 min. The PCR-amplified 16S rRNA was sequenced using the ABI Prism model 3730 (version 3.2) DNA sequencer. The 16S rDNA sequences of isolates were compared with 16S rDNA sequences obtained via BLAST searches of the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov). Multiple sequence alignments were performed using Clustal W version 1.8, and phylogenetic trees were generated using the neighbor-joining method. The gene sequences of the isolates deposited in GenBank with accession numbers listed in Table 1.
The isolates noted were cultivated individually in medium (“Medium” section) at 400 mg l−1 acetate (equivalent to 232 mg COD l−1) for 18 h. One representative isolate from one operational taxonomic unit was tested under COD of up to of 3,966 mg l−1.
The auto-aggregation index of individual tested isolates was determined (Adav et al. 2008b). The OD (OD(initial)) of cultures grown for 18 h was measured at 600 nm. The test solutions were left to stand at 25 °C for 30 min. In total, 1 ml supernatant was carefully drawn and placed in a microcuvette. The optical density of the suspension was measured at 600 nm. The auto-aggregation index for isolates was calculated by comparing the initial OD (OD(initial)) and OD value after 30 min (OD(30)) as follows: \( {\text{auto - aggregation}}\;{\text{index}}\;\left( \% \right) = \frac{{{\text{O}}{{\text{D}}_{\left( {\text{initial}} \right)}} - {\text{O}}{{\text{D}}_{\left( {\text{30}} \right)}}}}{{{\text{O}}{{\text{D}}_{\left( {\text{initial}} \right)}}}} \times 100 \).
Analytical methods
The dry weight of granules and volatile suspended solids and the sludge volume index (SVI) were measured using standard methods (APHA 1998). The size of sludge granules was determined by a particle sizer (Mastersizer Series 2600; Malvern Instruments, Worcestershire, UK). Washed granules were prepared for scanning electron microscopy (SEM) by fixing with 2.5% glutaraldehyde for 2 h, and dehydrated via successive passages through 30%, 50%, 75%, 85%, 90%, 95%, and 100% ethanol before being subjected to critical drying.
Cultures were centrifuged at 14 °C at 6,000×g for 10 min. The supernatant containing soluble EPS was separated from the pellet. To extract the bound EPS, the pellet phase was resuspended in 5 ml water and incubated for 1 h in a shaking water bath at 50 °C. Following centrifugation at 6,000×g for 10 min, the obtained supernatant contained bound EPS. The carbohydrate content in EPS was measured using the anthrone method (Gaudy 1962) with glucose as the standard. The protein content in EPS was measured using the modified Lowry method (Frolund et al. 1995) with bovine serum albumin as the standard.
Results
Granulation process
At the end of week 2, tiny aggregates with clear, spherical shapes were developed in R1–3 operated at OLR of 9, 12.6, and 16.7 kg COD m−3 day−1, respectively. Figure 1 shows the biomass, SVI and R3 performance under high OLR. The SVI was 30 ml g−1 on day 60, increased to 38 ml g−1 due to increase in OLR, and stabilized at 34 ml g−1 with reactor stabilization (Fig. 1). The SVI values fluctuated immediately after further increase in OLR and improved with column operation time. The size of granules increased to 3–4 mm following cultivation for 60 days (data not shown). On day 60, the granules in all reactors were compact and removed influent COD at a rate of 94–96%. At an OLR of 19.5 kg COD m−3 day−1, filamentous outgrowth existed in R3. Further increasing the OLR to 21.3 kg COD m−3 day−1 caused the granules to start disintegrating (Fig. 2) and decreased the COD removal rate.
Through SEM, bacteria were identified as the predominant microbial component in the granular sludge (images not shown). The bacteria have varied morphologically as rods, cocci, and filaments, and were embedded in a thick EPS matrix as single cells or dense clusters. Cocci frequently occurred in spherical clusters in large cell groups. Colonization of fungal filaments and ciliate stalks was observed; ciliated outgrowth was also observed. The composition of the bacterial community and distribution of distinct morphotypes differed with OLR. At a high OLR, bacterial cells were embedded in thick EPS, while at low OLR, filamentous bacteria were observed.
Microbial diversity and phylogenetic analysis of isolates
Of the 52 bacterial isolates sequenced (accession numbers in GenBank listed in Table 1), 27 belonged to Gammaproteobacteria (Enterobacteriaceae, 1; Xanthomonadaceae, 1; Pseudomonadaceae, 2; Moraxellaceae, 22); 20 were Proteobacteria (Rhodocyclaceae, 4; Comamonadaceae, 2; Neisseriaceae, 10); and four were Bacteroidetes (Flavobacteriaceae, 3; Flexibacteriaceae, 1). Isolates AOLR42, AOLR39, AOLR32, AOLR44, AOLR52, AOLR51, and AOLR41 had 16S rRNA nucleotide sequences identical to the sequence from Acinetobacter sp. and grouped together with an 80–100% bootstrap confidence level (Fig. 3). Isolates AOLR57, AOLR31, and AOLR01 clustered with Chryseobacterium gleum with bootstrap values of 68–100%. The 16S rRNA sequences of isolates AOLR48 and AOLR14 were similar to that of Comamonas sp. Isolates AOLR19 and AOLR13 clustered with members of the Pseudomonadaceae family with 100% bootstrap values. Isolate AOLR24 exhibited a 16S rRNA nucleotide sequence nearly identical to that from iron-reducing bacteria. Isolates AOLR30 and AOLR28 clustered with bootstrap values of 72–100% and contain a 16S rRNA nucleotide sequence identical to that of Aquitalea sp. The 16S rRNA nucleotide sequences of some isolates were identical to that of Rubrivivax gelatinosus.
Auto-aggregation and EPS secretion by isolates
All tested isolates grew in the medium at up to 2,556 mg COD l−1, whereas Acinetobacter sp., Pseudomonas nitroreducens, iron-reducing bacterium, Ideonella sp. Flexibacteraceae bacterium, and Zoogloea oryzae tolerated 2,964 mg COD l−1. Only strain P. nitroreducens and iron-reducing bacterium tolerated a COD of 3,966 mg l−1.
The tested isolates had a low auto-aggregation index at low COD; however, the auto-aggregation index increased as COD increased, achieving maximal aggregation at certain COD level, and then decreased as COD further increased (Fig. 4). The auto-aggregation index for both Aquitalea magnusonii and Flexibacteraceae bacterium peaked at a COD of 1,929 mg l−1, whereas that for Enterobacter aerogenes peaked at a COD of 292 mgl−1. Strains Chryseobacterium sp., Acinetobacter sp., P. nitroreducens, and Flexibacteraceae had higher auto-aggregation activity than other isolates. The auto-aggregation indices of these strains were 65–72% at a COD of 1,929 mg l−1. At a COD of 3,966 mg l−1, only strains P. nitroreducens and iron-reducing bacterium had limited auto-aggregation capability.
The quantities of proteins and polysaccharides secreted by Flexibacteraceae bacterium peaked at a COD of 1,929 g l−1, whereas those by A. magnusonii peaked at a COD of 2,556 mg l−1 (Tables 2 and 3).
Discussion
Response of isolates under high OLR
The cultivated aerobic granules could keep their structural integrity and high COD degradation rates at an OLR of 19.5 kg m−3 day−1, and started disintegrating at an OLR of 21.3 kg m−3 day−1, corresponding to feed COD of 6,470 and 7,070 mgl−1, respectively. As the volumetric exchange ratio in this study was 50%, the feed concentration was diluted by 50% when it was fed into the reactor. For example, at OLR of 19.5 and 21.3 kg m−3 l−1, the effective COD concentration experienced by strains in granules would be roughly 3,240 and 3,530 mg l−1, respectively. This COD regime corresponds to the critical COD values noted for granule disintegration (3,000–4,000 mg l−1). In other words, this work demonstrates that failure of constituent cells in aerobic granules may be associated with the noted granule disintegration under high OLR.
The proposed breakdown mechanism under high OLR differs from those proposed in literature, including overgrowth of filamentous microorganisms (Liu and Liu 2006) and intracellular protein hydrolysis and degradation at the anaerobic granule core (Zheng et al. 2006; Adav et al. 2009). This study demonstrates that under a high OLR, most constituent members do not aggregate, which runs counter to the common assumption that cells typically agglomerate into clusters in harsh environments (Bossier and Vertraete 1996). The change in aggregation tendency led to granule disintegration in this study.
Secreted proteins and biological breakdown
Aerobic granules are enriched with proteins compared with conventional activated sludge flocs that are not enriched (Adav et al. 2007a). Extracellular enzymes can hydrolyze proteins and carbohydrates in sludge flocs (Frolund et al. 1995; Nielsen et al. 1996). Adav et al. (2007a) determined that large vacuoles exist in stored granules prior to breakdown. Adav et al. (2009) isolated and identified proteolytic bacteria in aerobic granules responding to protein hydrolysis inside stored granules. Li et al. (2008) combined immunohistochemical staining and a multicolor fluorescent scheme to confirm internal hydrolysis within the granule core; thus, both protein and polysaccharide content should correspond in principle to the loss of granule stability.
A strong correlation (Fig. 5c, d) exists between secreted protein quantity (Table 2) and auto-aggregation indices of individual isolates (Fig. 4). No such correlation was noted between polysaccharide quantity and auto-aggregation indices (data not shown). Additionally, the quantity of polysaccharides was much lower than that of proteins. Hence, the role of polysaccharides on granule stability should be minimal. The quantity of proteins secreted by isolates likely contributes to granule stability under high OLR. As Fig. 5c and d reveals, Chryseobacterium sp. and Acinetobacter sp. aggregate and then secrete protein to strengthen their aggregation. The decrease in protein productivity by isolates under high OLR indicates that the EPS structure of granules was weak, thereby leading to granule breakdown.
Auto-aggregation index and protein or polysaccharide production quantities by individual isolates cultivated in 400 mg l−1 acetate medium for 18 h at 37 °C. a Polysaccharides by Chryseobacterium sp. MN13; b proteins by Chryseobacterium sp. MN13; c polysaccharides by Acinetobacter sp.; d proteins by Acinetobacter sp.
References
Adav SS, Chen MY, Lee DJ, Ren NQ (2007a) Degradation of phenol by aerobic granules and isolated yeast Candida tropicalis. Biotechnol Bioeng 96:844–852
Adav SS, Lee DJ, Ren NQ (2007b) Biodegradation of pyridine using aerobic granules in the presence of phenol. Water Res 41:2903–2910
Adav SS, Lee DJ, Show KY, Tay JH (2008a) Granular sludge: recent advances. Biotechnol Adv 26:411–423
Adav SS, Lee DJ, Lai JY (2008b) Intergeneric coaggregation of strains isolated from phenol degrading aerobic granules. Appl Microbiol Biotechnol 79:657–661
Adav SS, Lee DJ, Wang AJ, Ren NQ (2009) Functional consortium for hydrogen production from cellobiose: concentration-to-extinction approach. Bioresour Technol 100:3465–3470
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Arrojo B, Mosquera-Corral A, Garrido JM, Mendez R (2004) Aerobic granulation with industrial wastewater in sequencing batch reactors. Water Res 38:3389–3399
Bossier P, Vertraete W (1996) Triggers for microbial aggregation in activated sludge? Appl Microbiol Biotechnol 45:1–6
Chen Y, Jiang WJ, Liang DT, Tay JH (2008) Aerobic granulation under the combined hydraulic and loading selection pressures. Bioresour Technol 99:7444–7449
de Kreuk MK, Pronk M, van Loosdrecht MCM (2005) Formation of aerobic granules and conversion processes in an aerobic granular sludge reactor at moderate and low temperatures. Water Res 39:4476–4484
de Kreuk MK, Kishida N, van Loosdrecht MCM (2007) Aerobic granular sludge—state of the art. Water Sci Technol 55:75–81
Frolund B, Keiding K, Nielsen PH (1995) Enzymatic activity in the activated sludge flocs matrix. Appl Microbiol Biotechnol 43:755–761
Gaudy AF (1962) Colorimetric determination of protein and carbohydrate. Ind Water Wastes 7:17–22
Kim IS, Kim SM, Jang A (2008) Characterization of aerobic granules by microbial density at different COD loading rates. Bioresour Technol 99:18–25
Li AJ, Yang SF, Li XY, Gu JD (2008) Microbial population dynamics during aerobic sludge granulation at different organic loading rates. Water Res 42:3553–3560
Liu Y, Liu QS (2006) Causes and control of filamentous growth in aerobic granular sludge sequencing batch reactors. Biotechnol Adv 24:115–127
Liu Y, Tay JH (2004) State of the art of biogranulation technology for wastewater treatment. Biotechnol Adv 22:533–563
Maximova N, Dahl O (2006) Environmental implications of aggregation phenomena: current understanding. Curr Opin Colloid Interf Sci 11:246–266
McSwain BS, Irvine RL, Wilderer PA (2004) The effect of intermittent feeding on aerobic granule structure. Water Sci Technol 49:19–25
Morgenroth E, Sherden T, van Loosdrecht MCM, Heijnen JJ, Wilderer PA (1997) Aerobic granular sludge in a sequencing batch reactor. Water Res 31:3191–3194
Moy BY, Tay JH, Toh SK, Liu Y, Tay STL (2002) High organic loading influences the physical characteristics of aerobic sludge granules. Lett Appl Microbiol 34:407–412
Nielsen PH, Frolund B, Keiding K (1996) Changes in the composition of extracellular polymeric substances in activated sludge during anaerobic storage. Appl Microbiol Biotechnol 44:823–830
Tay JH, Liu QS, Liu Y (2001) Microscopic observation of aerobic granulation in sequential aerobic sludge blanket reactor. J Appl Microbiol 91:168–175
Tay JH, Liu QS, Liu Y (2002) Aerobic granulation in sequential sludge blanket reactor. Water Sci Technol 46:41–48
Tay JH, Pan S, He Y, Tay STL (2004) Effect of organic loading rate on aerobic granulation. I: Reactor performance. J Environ Eng 130:1094–1101
Thanh BX, Visvanathan C, Ben Aim R (2008) Characterization of aerobic granular sludge at various organic loading rates. Process Biochem. doi:10.1016/j.procbio.2008.10.018
Yang SF, Liu Y, Tay JH (2003) A novel granular sludge sequencing batch reactor for removal of organic and nitrogen from wastewater. J Biotechnol 106:77–86
Zheng YM, Yu HQ, Liu SH, Liu XZ (2006) Formation and instability of aerobic granules under high organic loading conditions. Chemosphere 63:1791–1800
Acknowledgement
This project is financially supported by the State Key Laboratory of Water Resource and Environment (SKLWRE), Harbin Institute of Technology, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adav, S.S., Lee, DJ. & Lai, JY. Potential cause of aerobic granular sludge breakdown at high organic loading rates. Appl Microbiol Biotechnol 85, 1601–1610 (2010). https://doi.org/10.1007/s00253-009-2317-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2317-9