Abstract
A marine actinobacterium isolated from the Bay of Bengal, India and previously found to be producing an antimicrobial and cytotoxic terpenoid was further investigated for antimicrobial metabolites. The bacterium was preliminarily identified as a new species of the genus Streptomyces (strain MS1/7). The cell-free culture broth was extracted with n-butanol and purified using silica gel column chromatography and high-performance liquid chromatography. Molecular characterization was done using ESI mass, IR and 1H and 13C NMR spectrometry. 2-Allyloxyphenol (MW 150; C9H10O2), a synthetic drug and chemical intermediate, was obtained as a natural product for the first time. Serendipitous natural occurrence provided new insights into the synthetic molecule. 2-Allyloxyphenol was found to be inhibitory to 21 bacteria and three fungi in the minimum range 0.2–1.75 mg mL−1 determined by agar dilution method. 2-Allyoxyphenol possesses strong antioxidant property (IC50 22 μg mL−1, measured by 1, 1-diphenyl-2-picryl hydrazyl scavenging activity). Hydroxyl and allyloxy groups in 2-allyloxyphenol were responsible for antimicrobial and antioxidant activities. 2-Allyloxyphenol has marked resemblance to smoky aroma and is two to three times more active as an antimicrobial than some commercial smoke-flavour compounds. Absence of hemolytic toxicity, potential carcinogenicity, cytotoxicity and reports of toxic reactions in literature suggest possible application of 2-allyloxyphenol as a food preservative and an oral disinfectant.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine actinomycetes are a prolific but underexploited source for the discovery of novel secondary metabolites. There is a tremendous diversity and novelty among the marine actinomycetes present in marine environments (Lam 2006). The Bay of Bengal has recently been targeted as a potential source of marine-derived bacterial bioactive compounds by several investigators. Screening of marine sediment samples near the coast of the Andaman Islands in the Bay of Bengal resulted in the isolation of numerous marine actinomycetes. The isolates belonged largely to the genus Streptomyces and were found to be active against bacteria, fungi as well as multiple drug-resistant bacteria (Peela et al. 2005). Lately, Japanese investigators (Ara et al. 2007) isolated a novel strain belonging to the genus Nonomuraea, being most closely related to Nonomuraea kuesteri and a novel species of the genus Actinomadura (Ara et al. 2008) from Maheshkhali, Cox’s Bazar, an unexplored region of the southern coast of Bangladesh. The Sundarbans, the world’s largest tidal mangrove forest, lies on the delta of the Ganges, Brahmaputra and Meghna rivers off the Bay of Bengal. The forest covers 10,000 km2 of which about 6,000 km2 is in Bangladesh and the rest in India. Mukherjee and co-workers (Mitra et al. 2008 and related references therein) were the first to explore the biotechnological applications of the microbial biodiversity of the Indian Sundarbans.
Earlier, we described the isolation of an active terpenoid compound (MW 300.2, predicted molecular formula C20H28O2) from a marine Actinobacterium (Strain MS1/7, isolated from the Bay of Bengal), inhibitory to three Gram-positive and three Gram-negative multiple drug-resistant bacteria, seven non-clinical Gram-positive, four Gram-negative bacteria and five fungi. Fifty-four percent of human leukaemia (HL-60) cells were also killed by the compound (Saha et al. 2006). During this study we had observed that at least three other column-eluted fractions showed antimicrobial activity, though in lower yields. The present study was thus performed with the objective to characterise one of these active fractions that were being obtained along with the terpenoid compound.
Materials and methods
Identification of MS1/7 and growth of the microorganism
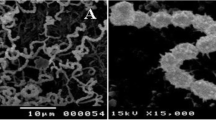
The marine isolate MS1/7 (acc. no. MTCC 5272) was isolated from the sediments of the Sundarbans, Bay of Bengal and reported as a Gram-positive spore-forming bacterium, which produces white aerial and substrate mycelium (Saha et al. 2006). It has no obligate requirement of NaCl for growth but can tolerate up to 200 g L−1 NaCl, and the biomass increase displayed a graded response to variations in NaCl concentration (upto 100 g L−1 NaCl) indicating its intertidal habitat. Further identification was done by the German Collection of Microorganisms and Cell Cultures (DSMZ), Braunschweig, Germany. The fatty acids were extracted, methylated and estimated by GC using the standard Sherlock MIDI (Microbial Identification) system (Kämpfer and Kroppenstedt 1996). For species identification, the 16S rDNA of the strain was sequenced (Rainey et al. 1996) and compared with all the sequences available till date (Maidak et al. 1996) and the DSMZ database. Additional International Streptomyces Project (ISP) criteria following Shirling and Gottlieb (1972) and Küster (1972) were also employed. The spore-chain morphology and spore-surface ornamentation of strain MS1/7 were studied by examining gold–palladium-coated dehydrated specimens of 10- to 14-day cultures grown on ISP 2 agar by scanning electron microscopy in a FEI Quanta 200-MK2 electron microscope.
The isolate was grown (250 rpm at 30°C for 96 h) in a modified Marine Difco 2216 medium (all units g L−1): starch 2.0, glucose 2.0, FeCl3 0.1, NaCl 19.45, MgCl2. 6H20 8.8, Na2SO4 3.2, CaCl2. 6H2O 1.8, KCl 0.55, NaHCO3 0.16, KBr 0.08, SrCl2.6H2O 0.034, H3BO3 0.022, Na2SiO3.9H2O 0.004, NaF 0.0024, NH4NO3 0.0016, NaHPO4. 7H2O 0.008, distilled water 1,000 mL, pH 7.2.
Isolation and purification of active compound
The cell-free supernatant was extracted by n-butanol, the organic phase concentrated to dryness; the residue dissolved in methanol and was stored at 0–4°C. The crude extract was adsorbed onto a silica gel (60/120 mesh) column and eluted with chloroform/petroleum ether (5:95 v/v). The active fractions were run on reverse phase high-performance liquid chromatography (HPLC; Shimadzu SPD 20A, Japan) C18 column (250 × 4.60 mm, 5 μm), mobile phase 100% acetonitrile, flow rate 1.0 mL min−1 UV detection at 275 nm. The final product was checked for purity by HPLC and GC (Perkin Elmer Autosystem XL), PE-5 column (25 m × 0.2 mm ID), carrier gas helium, flow rate 0.7 mL min−1, split ratio 25:1. The oven temperature was 120°C, the injection temperature was 175°C and a FID detector was employed. The solvents used for extraction and running the column were periodically monitored for the inadvertent contamination by 2-allyloxyphenol by analysing solvent samples in the HPLC. Density of the final product was determined by weighing 500 μL of the sample in a sensitive five-decimal-place Sartorius CP 225D balance, while boiling point was obtained by the distillation method. Mass spectrometry (ESI, negative mode) was done using Q-TOF Micro mass (LCMS) spectrometer, IR spectrum was recorded on a JASCO-FT-IR Model 410 using samples on NaCl cells and 1H NMR and 13C NMR (300 MHz) spectrum was recorded on a Bruker DPX 300 NMR instrument.
Representative procedure for synthesis of 2-allyloxyphenol and analogues
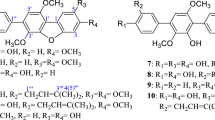
Catechol (5.5 g) and 6.9 g of potassium carbonate was mixed in 10.0 mL of acetone. Allyl bromide (6.05 g) was added slowly with constant stirring and refluxing. After cooling the solid material was filtered off and washed with 50 mL of acetone. The brown oil was collected and stored at 4°C (Charles et al. 1930). The same procedure was followed to synthesise 2-allyloxyphenol analogues (2-propenoxy-4-nitro phenol, 2-propenoxy-4-methyl phenol, 2-propenoxy-4-methoxy phenol, 2-propenoxy-4-tertiary butyl phenol, 1, 2-diallyloxybenzene, allyloxy benzene and 2-allyloxynaphthalene) where equimolar quantities were taken as reactants (Table 1). The products were purified by column chromatography using Silica Gel (60/125 mesh), and the compounds were identified by Q-TOF Micro mass (LCMS) spectrometer.
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) of the purified 2-allyloxyphenol, phenol, smoke-flavour compounds (eugenol, guaiacol and 2-ethoxyphenol, purchased from Acros Organics, Belgium) and analogues were determined by the agar dilution method (EUCAST Definitive Document, E.Def. 3.1, 2000). About 104 CFU per spot of the bacteria (see Table 2) were spotted onto Mueller–Hinton agar plates against different concentrations (0.0625, 0.125, 0.25, 0.5, 1.0 and 2.0 mg mL−1, dissolved in dimethylsulfoxide) of each compound and incubated overnight at 37°C (Saha et al. 2006). Further concentrations within the intervals were tested to determine the exact MIC value. The MIC values against fungi (see Table 2) were determined following the method of Liu et al. (2002). Freshly made fungal suspensions (104 cells mL−1) were inoculated onto the potato–dextrose–agar plates and incubated at 26°C for 48–72 h. All determinations were performed thrice in duplicate sets and the average of the values is reported.
Determination of antioxidant activity
The antioxidant activity of the 2-allyloxyphenol, phenol, smoke-flavour compounds and analogues (Table 1), dissolved in dimethylsulfoxide, were assessed based on the scavenging activity of the stable 1, 1-diphenyl-2-picryl hydrazyl (DPPH) free radical (Hwang et al. 2001; Soldera et al. 2008). Ten microliters of each test compound (concentration ranging from 21 mg mL−1 to 21 μg mL−1) was added to 200 µL of DPPH in methanol (100 µM) in the wells of a 96-well microtitre plate. After incubation at 37°C for 30 min, the absorbance of each solution was determined at 490 nm in an ELISA microplate reader (BioRad Laboratories Inc., CA, USA, Model 550). The corresponding control readings were also taken and the remaining DPPH was calculated. IC50 value is the concentration of the sample required to scavenge 50% DPPH free radicals and was obtained by plotting the percentage of free radicals scavenged versus the putative antioxidant concentration. All determinations were performed thrice in duplicate sets, and the average of the values is reported.
Determination of toxicity
Hemolytic activity of 2-allyloxyphenol towards murine and human erythrocytes was tested; 3.0 mL of 1% washed erythrocyte suspension was incubated with 2-allyloxyphenol at concentrations ten times the MIC values at 37°C for 45 min. Distilled water and normal saline were taken as positive and negative controls, respectively. After incubation, the erythrocytes were separated, and the amount of haemoglobin released was determined by measuring the absorbance of the supernatant at 540 nm (Saha et al. 2005).
Determination of potential carcinogenicity
Bacterial reverse mutation assay was performed following Ames et al. (1973). Salmonella typhimurium TA98 (MTCC 1251) was used as the tester strain. The tester strain was freshly prepared by growing the bacterium overnight at 37°C in nutrient broth. Top agar was prepared by dissolving 0.6 g of agar and sodium chloride in 100 mL of distilled water and sterilising the solution for 15 min at 121°C. The top agar was stored between 40°C and 45°C. A sterile 10 mL mixture of l-histidine (0.5 mM) and d-biotin (0.5 mM) was added to the top agar medium just before use. Pour plates were made by adding 100 μL of the tester strain culture to a small sterile test tube that contained 2 mL of molten top agar. An appropriate volume of 2-allyloxyphenol dissolved in dimethylsulfoxide (the maximum concentration was below the MIC, 0.65 mg mL−1, against S. typhimurium TA98) was added to the tube, mixed well and poured onto the surface of a minimal agar plate [1.5% (w/v) agar, 2% (w/v) glucose]. Sodium azide (2.0 μg plate−1) was used as the positive control, while plates without any added chemicals were used to check for spontaneous revertants. All plates are incubated at 37°C for 2 days, after which the numbers of revertant colonies appearing were counted. All determinations were performed thrice in duplicate sets, and the average of the values is reported.
Determination of cytotoxicity
Human hepatocellular carcinoma (Hep G2) cell lines were obtained from National Centre for Cell Science, Pune, India. Stock cultures of these cell lines were cultured in Dulbecco modified essential medium (DMEM) supplemented with 10% inactivated foetal bovine serum (FBS), penicillin (100 U mL−1), streptomycin (100 mg mL−1) and amphoterecin B (5 mg mL−1) in a humidified atmosphere of 5% CO2 at 37°C until confluent. The cells were dissociated with 0.2% trypsin and 0.02% EDTA in phosphate buffer saline solution. The stock cultures were grown in 25-cm2 tissue culture flasks, and all cytotoxicity experiments were carried out in 96-well microtitre plates (Tarsons, India). Cell lines in exponential growth phase were washed, trypsinised and resuspended in complete culture media. Cells were plated at 10,000 cells per well in 96-well microtitre plate and incubated for 24 h during which a partial monolayer forms. The cells were then exposed to various concentrations of 2-allyloxyphenol, its analogues (Table 1), eugenol, guaiacol, 2-ethoxyphenol and phenol in quadruplicate. Control wells received only maintenance medium (2% FBS in DMEM). The plates were incubated at 37°C in a humidified incubator with 5% CO2 for a period of 72 h. Morphological changes of drug treated cells were examined using an inverted microscope at different time intervals and compared with the cells serving as control. At the end of 72 h, cell viability was determined by MTT assay (Skehan et al. 1990).
Results
Identification of the producing microorganism (MS1/7)
The microorganism formed an extensively branched grey to olive green substrate mycelia and the reverse side of the colony appeared brown. Aerial hyphae carried smooth surfaced spores (Dietz and Mathews 1971) in a recti-flexibilis arrangement. The profile of the cellular fatty acids was typical of the genus Streptomyces. The first 500 bp of the 16S rDNA sequence (which is the variable region of the complete 16S rRNA gene) of MS1/7 showed highest sequence similarity with only 97.9% to the type strains of Streptomyces puniceus DSM 40083T, Streptomyces floridae DSM 40938T and Streptomyces californicus DSM 40058T. On the basis of the available sequence information, a clear assignment to one of these species was not possible. Melanoid pigments were not observed in peptone–iron and tyrosine media. Among the ISP sugars tested as carbon sources, growth was observed with glucose, d-xylose, mannitol and d-fructose, while no growth was recorded with l-arabinose, sucrose, inositol, rhamnose and raffinose. Presently, it is not possible to identify the strain MS1/7 clearly with one of the species within the genus Streptomyces. Probably the strain is a member of a new species.
Isolation and characterization of the antimicrobial compound
In this study, we cultivated our isolate in a modified Marine Difco 2216 medium, compared to natural seawater and used butanol in place of butyl acetate (Saha et al. 2006) for the extraction process because butanol evaporates more easily compared to butyl acetate. These two procedural differences might account for the recovery of 2-alloxyphenol. In contrast to the column purification described in Saha et al. (2006), 2-allyloxyphenol was found in the earlier fractions, which was effectively separated by further column and HPLC purification. A single peak in the HPLC as well as the GC chromatogram indicated the purity of the product. The average yield of the compound during the cultivation of eight batches was 1.5 mg L−1. The product is a deep brown oil (density 1.1 g mL−1, b.p. 247.0°C, slightly soluble in water) and has a strong smoky aroma.
The IR spectrum of the active principle showed the presence of a hydroxyl group at 3,532 cm−1, C═C (olefinic) and aromatic C═C strong stretching frequency found at 1,596 and 1,501 cm−1, respectively. Mass spectrum showed a molecular ion peak at m/z 148.93 [M–H]− and indicated a molecular formula of C9H10O2. The proton NMR spectrum of the compound (DMSO-d 6) exhibited signals at δ 5.99–6.06 (m, 1H) for olefinic –CH. A two-proton doublet of doublets at δ 5.35 (dd,J = 17.29 Hz, 3.75 Hz, 2H) indicative of allylic methylene with coupling constant 17.29 and 3.75 Hz for geminal and vicinal coupling, respectively, and another two proton doublets at δ 4.54(d,J = 3.67 Hz,2H) represented O–CH2– group in the molecule. The aromatic signals appeared at δ 6.67–6.92 (m, 4H), and one phenolic OH singlet proton, which showed at δ 8.91 (s, 1H) but disappeared when treated with D2O. The 13C NMR (CDCl3) spectrum exhibited signals at δ 146.35 (C2), 145.98 (C1), 133.90 (C 8), 122.17 (C5), 120.47 (C4), 118.68 (C6), 115.18 (C3), 112.67 (C 7) and 70 (C9). DEPT 135 analysis 13C NMR confirmed the presence of two CH2 carbons by showing down peaks at δ 112.6 and 70.23. The molecular formula was deduced to be C9H10O2 and molecular weight 150 (see Table 1 for molecular structure).
Antimicrobial activity
2-Allyloxyphenol inhibited the growth of Gram-positive and Gram-negative bacteria (Table 2) as well as fungi, although at a higher concentration. The MIC values were about 2–3-folds lower than phenol and other smoke-flavour compounds tested (eugenol, guaiacol and 2-ethoxyphenol). The MIC values of the analogues (nos. 2, 3, 4 and 5 in Table 1) were comparable to the values obtained for 2-allyloxyphenol. However, the analogues (nos. 6, 7 and 8 in Table 1) failed to exhibit any antibacterial or antifungal activity at concentrations ten times more than the MICs reported. The results indicate that addition of nitro, methyl, methoxy or tertiary butyl group to 2-allyloxyphenol does not enhance the antimicrobial activity. However, removal of phenolic hydroxyl group results in complete loss of activity, indicating the key role of the –OH group in the antimicrobial activity. Again, addition of the allyloxy group to the phenolic –OH enhances the antimicrobial activity, compared to phenol showing the synergistic effect of these groups. In contrast to the other smoke-flavour compounds (Table 2), it appears that the allyloxy group is more active than propene, methoxy or ethoxy groups.
Antioxidant activity
Table 3 shows that 2-allyloxyphenol possesses strong antioxidant property. Among the smoke-flavour compounds tested, eugenol is a better antioxidant than 2-allyloxyphenol. Addition of a nitro group to 2-allyloxyphenol completely destroys the antioxidant activity, while addition of a methyl group enhances the activity by about 6-fold. Addition of methoxy or tertiary butyl group does not produce any significant change to the activity. Results also indicate that phenolic –OH group or allyloxy group alone does not yield activity, but the presence of both in 2-allyloxyphenol creates strong antioxidant activity.
Toxicity and carcinogenicity
2-Allyloxyphenol lysed only 1.5% murine erythrocytes and 1.4% human erythrocytes at concentrations ten times the MIC values of 2-allyloxyphenol. The compound, therefore, is not a bacterial hemolytic toxin (Tateno and Goldstein 2003). The dose–response relationship (Table 4) between the number of revertants and added 2-allyoxyphenol indicates that 2-allyloxyphenol is not a potential carcinogen. All determinations were performed thrice in duplicate sets, and the reported average values varied within 10% deviation from the mean. 2-Allyloxyphenol, its analogues (Table 1), eugenol, guaiacol, 2-ethoxyphenol and phenol were not toxic to Hep G2 cells up to 2.0 mg mL−1 as determined by the MTT assay.
Discussion
Propenyl- and allyl-phenols have gained importance as flavouring agents and also as putative precursors in the biosynthesis of 9, 9-deoxygenated lignans in higher plants. In spite of several decades of investigation, the complete biosynthetic pathway to a propenyl/allylphenol had not yet been reported until recently. Koeduka et al. (2006) showed that glandular trichomes of sweet basil possess an enzyme that can use coniferyl acetate and NADPH to form eugenol. Petunia flowers, too, possess an enzyme homologous to the basil eugenol-forming enzyme that also uses coniferyl acetate and NADPH as substrates but catalyses the formation of isoeugenol. 2-Allyloxyphenol however was not detected or isolated by the investigators. This compound is an important intermediate in several drug and chemical syntheses, few examples being azaheterocyclymethyl derivatives (Gary 1999), glycidyl ether (Norbert 2007) and snap cure epoxy adhesives (Quinn and Rose 1998). Till date, literature relating to the chemical synthesis of 2-allyloxyphenol is abundant but the biosynthesis of the same in any living organism has not yet been discovered. As marine environmental conditions are different from terrestrial ones, it is surmised that marine bacteria possess characteristics distinct from those of terrestrial bacteria and therefore may produce different types of bioactive compounds. Xue et al. (2006) reported the biosynthesis of petrochemical products, 4-hydroxybenzoate together with its butyl, heptyl and nonyl esters from a marine bacterium belonging to the Microbulbifer genus. The yield of 2-allyloxyphenol obtained in the present study is albeit low to qualify the producer actinobacterium as a biological source of 2-allyloxyphenol.
Results from the study on the antimicrobial properties of 2-allyloxyphenol analogues show that the hydroxyl and allyloxy group in 2-allyloxyphenol are responsible for the antimicrobial activity. We believe that this study is the first description of the antimicrobial properties of 2-allyloxyphenol. Interestingly, the antioxidant activity of the compound can also be attributed to the hydroxyl and allyloxy groups. A high MIC value limits the application of this compound as a drug. Nevertheless, reviewing the scientific literature (including patent literature) we propose the possible application of 2-allyloxyphenol as a food preservative and in oral mouthwash preparations.
During the course of our investigations we observed that 2-allyloxyphenol had a strong resemblance to smoky aroma. Smoke application has historically been used successfully for food preservation. Generated by controlled smouldering of wood in the absence of or at reduced oxygen levels, it is a suspension of vapours, solid particles and liquid droplets. Recently, the chemical analysis of natural wood smoke has been disclosed (Soldera et al. 2008). The phenolic compounds, considered to be important contributors to smoke aroma and to the antimicrobial and antioxidant activities in smoked foods, consist mainly of phenol, 2-methoxyphenol (guaiacol), 2, 6-dimethoxyphenol (syringol) and their derivatives and of dihydroxybenzenes originating from the pyrolysis of lignin. Although 2-allyloxyphenol was not detected by the authors, this compound is structurally closely related to broad classes of phenolics mentioned. That 2-allyloxyphenol is a likely component of the natural smoke flavour is supported by a Canadian patent granted to Brown and Webb (1976) of Bush Boake Allen Ltd. (UK). The inventors discovered that 2-allyloxyphenol and other alkoxyphenols are particularly effective in providing a flavour resembling natural smoke. The antimicrobial or antioxidant properties were not investigated.
Antimicrobial activity of seven commercial smoke preparations available in the European and North American markets was studied by Sunen (1998). The MIC was determined against a selection of food spoilage and pathogenic microorganisms and was found to be between 0.2% and 1.5%. The present study reports the MIC of 2-allyoxyphenol being at least five to ten times lower than the values reported for the natural smoke flavours by Sunen (1998), although according to our experiments the MIC is about two to three times lower. Similarly the antioxidant activity of 2-allyloxyphenol was approximately 150 times higher than the commercial smoke-flavour preparations reported by Soldera et al. (2008), which was, however, not supported by our experiments. Another patent (Wu et al. 2007) describes the application of smoke flavour for the control of oral microflora. The inventors describe the application of 2.5 mg mL−1 of the smoke flavour for the inhibition of Streptococcus mutans, Fusobacterium nucleatum and Porphyromonas gingivalis. Our results show that S. mutans, the causative agent of the most common oral infection, dental caries can be effectively inhibited by 2-allyloxyphenol at concentrations five times lower than that described by the authors. These results along with the absence of hemolytic toxicity, potential carcinogenicity and cytotoxicity (also, reports of toxic effects of 2-allyloxyphenol were not found in literature) would make 2-allyoxyphenol a probable candidate for use as a food preservative and oral disinfectant. The limitations of the Ames test should, however, be considered. As the metabolism of a mammalian cell is distinct from that of a bacterial cell, compounds which are activated in a mammalian cell may therefore show no activity in a bacterial cell. Many carcinogens have an affinity for a specific sequence of nucleotides. As the number of sequences that are shared between mammals and bacteria is very small, there may be many chemicals which do not cause mutations in bacteria but have drastic effect on mammals. The Ames test, therefore, has value as an initial screening test, but only as long as its limitations are acknowledged.
Although our aim was not to study the antimicrobial effects of smoke flavours, the constraint of the use of 2-allyloxyphenol as a drug prompted us to explore alternative applications. Serendipitous natural occurrence of the compound provided us new insights into the synthetic molecule. Results of the analogue study indicate that among the myriad chemicals that make up the smoke flavour, a handful may actually be responsible for the organoleptic, antimicrobial and antioxidant effects. Further, any given antimicrobial compound may not be a good antioxidant. A systematic “structure-activity” study would therefore be helpful in the design of a better smoke flavour and an oral disinfectant where many unnecessary chemicals may be eliminated.
In conclusion, we have established 2-allyloxyphenol, a known synthetic compound as a new natural product occurring in a putatively novel Streptomyces. Molecular cloning of the biosynthetic genes may allow economically viable production of this important drug and chemical intermediate. Comprehensive investigations on the antimicrobial, antioxidant activity and toxicity studies suggest the application of this compound as a food preservative and oral disinfectant.
References
Ames BN, Lee FD, Durston WE (1973) An improved bacterial test system for the detection and classification of mutagens and carcinogens. Proc Nat Acad Sci USA 70:782–786
Ara I, Kudo T, Matsumoto A, Takahashi Y, Omura S (2007) Nonomuraea maheshkhaliensis sp. nov., a novel actinomycete isolated from mangrove rhizosphere mud. J Gen Appl Microbiol 53:159–166
Ara I, Matsumoto A, Bakir MA, Kudo T, Omura S, Takahashi Y (2008) Actinomadura maheshkhaliensis sp nov a novel actinomycete isolated from mangrove rhizosphere soil of Maheshkhali, Bangladesh. J Gen Appl Microbiol 54:335–342
Brown KG, Webb D (1976) Synthetic smoke flavours. Canadian Patent No. CA 987539
Charles DH, Harry G, Forest DP (1930) The behaviour of allyl derivatives of catechol and resorcinol towards heat. J Amer Chem Soc 52:1700–1706
Dietz A, Mathews J (1971) Classification of Streptomyces spore surfaces into five groups. Appl Microbiol 21:527–533
EUCAST-European Committee for Antimicrobial Susceptibility Testing of the European Society of Clinical Microbiology, Infectious Diseases (2000) Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by agar dilution. Clin Microbiol Infect 6:509–515
Gary PS (1999) Azaheterocyclymethyl derivatives of 2, 3, 8, 9-tetrahydro-7h-1,4-dioxino (2,3-e) indol-8-one. US Patent No. 5869490
Hwang BY, Kim HS, Lee JH, Hong YS, Ro JS, Lee KS, Lee JJ (2001) Antioxidant benzoylated flavan-3-ol glycoside from Celastrus orbiculatus. J Nat Prod 64:82–84
Kämpfer P, Kroppenstedt RM (1996) Numerical analysis of fatty acid patterns of coryneform bacteria and related taxa. Can J Microbiol 42:989–1005
Koeduka T, Fridman E, Gang DR, Vassao DG, Jackson BL, Kish CM, Orlova I, Spassova SM, Lewis NG, Noel JP, Baiga TJ, Dudareva N, Pichersky E (2006) Eugenol and isoeugenol, characteristic aromatic constituents of spices, are biosynthesized via reduction of a coniferyl alcohol ester. Proc Nat Acad Sci USA 103:10128–10133
Küster E (1972) Simple working key for the classification and identification of named taxa included in the International Streptomyces Project. Int J Syst Bacteriol 22:139–148
Lam KS (2006) Discovery of novel metabolites from marine actinomycetes. Curr Opin Microbiol 9:245–251
Liu Y, Tortora G, Ryan ME, Lee HM, Golub LM (2002) Potato dextrose agar antifungal susceptibility testing for yeasts and molds: evaluation of phosphate effect on antifungal activity of CMT-3. Antimicrob Agents Chemother 46:1455–1461
Maidak BL, Olsen GJ, Larsen N, Overbeek R, McCaughey MJ, Woese CR (1996) The Ribosomal Database Project (RDP). Nucleic Acids Res 24:82–85
Mitra A, Santra SC, Mukherjee J (2008) Distribution of actinomycetes, their antagonistic behaviour and the physico-chemical characteristics of the world’s largest tidal mangrove forest. Appl Microbiol Biotechnol 80:685–695
Norbert A (2007) Method for producing halosilanes by impinging microwave energy. US Patent No. 7265235
Peela S, Kurada VVSNB, Terli R (2005) Studies on antagonistic marine actinomycetes from the Bay of Bengal. World J Microbiol Biotechnol 21:583–585
Quinn KT, Rose AS (1998) Snap-cure epoxy adhesives. US Patent No. 5770706
Rainey FA, Ward-Rainey N, Kroppenstedt RM, Stackebrandt E (1996) The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol 46:1088–1092
Saha M, Ghosh D Jr, Ghosh D, Garai D, Jaisankar P, Sarkar KK, Dutta PK, Das S, Jha T, Mukherjee J (2005) Studies on the production and purification of an antimicrobial compound and taxonomy of the producer isolated from the marine environment of the Sundarbans. Appl Microbiol Biotechnol 66:497–505
Saha M, Jaisankar P, Das S, Sarkar KK, Roy S, Besra SE, Vedasiromani JR, Ghosh D, Sana B, Mukherjee J (2006) Production and purification of a bioactive substance inhibiting multiple drug resistant bacteria and human leukemia cells from a salt-tolerant marine actinobacterium isolated from the Bay of Bengal. Biotechnol Lett 28:1083–1088
Shirling EB, Gottlieb D (1972) Cooperative description of type strains of streptomyces V. Additional descriptions. Int J Syst Bacteriol 22:265–394
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anti cancer drug screening. J Natl Cancer Inst 82:1107–1112
Soldera S, Sebastianutto N, Bortolomeazzi R (2008) Composition of phenolic compounds and antioxidant activity of commercial aqueous smoke flavorings. J Agric Food Chem 56:2727–2734
Sunen E (1998) Minimum inhibitory concentration of smoke wood extracts against spoilage and pathogenic microorganisms associated with foods. Lett Appl Microbiol 27:45–48
Tateno H, Goldstein IJ (2003) Molecular cloning, expression, and characterization of novel hemolytic lectins from the mushroom Laetiporus sulphureus, which show homology to bacterial toxins. J Biol Chem 278:40455–40463
Wu Y, Bedford J, Riley K (2007) Antimicrobial smoke flavour for oral microflora control. International Patent No. WO/2007/095374 A2
Xue P, Kyoko A, Choryu C, Hiroaki K, Kaneo K, Yoshikazu S, Norihiko M (2006) Discovery of a marine bacterium producing 4-hydroxybenzoate and its alkyl esters, parabens. Appl Environ Microbiol 72:5556–5561
Acknowledgements
Financial support through CSIR Grant No. 60(0070)/05/EMR-II to J. Mukherjee is thankfully acknowledged. The authors wish to thank the anonymous reviewers for their valuable comments.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Prof. Saroj Kanti Majumdar, the pioneer of actinomycetes research in India.
Rights and permissions
About this article
Cite this article
Arumugam, M., Mitra, A., Jaisankar, P. et al. Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl Microbiol Biotechnol 86, 109–117 (2010). https://doi.org/10.1007/s00253-009-2311-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2311-2