Abstract
Fluorescent-labeled invertase, a hyperglycosylated mannoprotein from Saccharomyces cerevisiae, was found to bind to Lactococcus lactis IL1403 at acidic pH. Proteins on the cell wall of the bacterium affinity-purified using invertase as a ligand were identified to be heat shock proteins such as DnaK and GroEL and glycolytic enzymes such as pyruvate kinase and glyceraldehyde-3-phosphate dehydrogenase. DnaK bound to both the bacterium and yeast at pH 4 and aggregated them at above 0.1 mg/ml, whereas no significant difference between the circular dichroism spectra of DnaK at pH 4 and 7 was observed. These results indicate that the cytosolic proteins, including DnaK displayed on the cell wall, cause the lactic acid bacterium to adhere to the yeast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fermented foods offer various benefits (Murooka and Yamashita 2008). It is known that most fermented foods are the result of the symbiosis between microorganisms, particularly lactic acid bacteria (LAB) and yeasts. They play important roles together in various fermented foods such as bread, alcoholic drinks, dairy products, and seasonings (Gobbetti 1998; Corsetti et al. 2001; Narvhusa and Gadagab 2003; Kawarai et al. 2007), and it is difficult to produce these fermented foods in the absence of one of the partners.
The interaction between LAB and yeasts has been considered to occur through the exchange of substances such as nutrients (Gobbetti 1998; Corsetti et al. 2001; Narvhusa and Gadagab 2003; Kawarai et al. 2007). For example, LAB metabolizes carbohydrates such as starch and oligosaccharides, and it provides lactic acid as a substrate to yeast which is incapable of assimilating such carbohydrates. At the same time, yeast assimilates lactic acid that inhibits LAB at high concentrations or provides some amino acids as nutrients to LAB (Gobbetti 1998). This symbiosis can be found in many fermented foods, including kefir, a fermented milk that originated from Caucasia.
We have reported that the production of an exopolysaccharide called kefiran by Lactobacillus kefiranofaciens is enhanced by a mixed culture with Saccharomyces cerevisiae (Cheirsilp et al. 2001, 2003a, b, 2007; Tada et al. 2007). In addition, the physical contact between LAB and yeast was shown to be important to stimulate the production of kefiran. It was found that the presence of yeast enhances kefiran production by LAB even if the yeast cells were inactivated by autoclaving or glass beads disruption, whereas no significant enhancement was observed when the active yeast cells were encapsulated in alginate beads (Cheirsilp et al. 2003b).
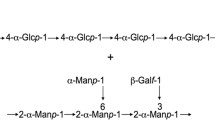
This finding led to two questions: What is it in LAB that recognizes the yeast and how is the signal transduced? Since yeast cells are covered with mannan proteins and Gusils et al. (1999) reported that some LAB have lectin-like proteins that agglutinate yeast cells and animal erythrocytes, it is expected that there exist proteins that recognize yeast mannan on the surface of LAB.
To answer the first question, in this study, cell-wall-associated proteins of Lactococcus lactis IL1403 were affinity-purified using yeast mannan as a ligand, separated by two-dimensional electrophoresis and identified by peptide mass fingerprinting. One of the identified proteins, DnaK, was shown to adhere LAB to the yeast.
Materials and methods
Strains and culture conditions
S. cerevisiae IFO0216 was cultivated in YPD medium (1% yeast extract, 2% peptone, 2% glucose, pH 5.5) at 30°C with shaking. L. kefiranofaciens JCM6985 and L. lactis subsp. lactis IL1403 were cultivated statically and anaerobically in MRS broth (Difco, pH 5.5 adjusted with H2SO4) at 37°C. Escherichia coli DH5α and BL21(DE3) were cultivated aerobically in Luria–Bertani (LB) medium (0.5% yeast extract, 1% bactotryptone, 0.5% NaCl, pH 7.5) at 37°C.
Preparation of DnaK
A DNA fragment encoding DnaK (gene ID: 1114585) was amplified from the chromosome of L. lactis IL1403 using 5′-ctgtaggatccatgtctaaaattatcggtat and 5′-gtattctcgagttatttgctttcttcaaagt as primers (BamHI and XhoI sites are underlined, respectively). The resultant fragment digested with BamHI and XhoI was ligated with pET-21a(+) (Novagen) digested with the same restriction enzymes and introduced into E. coli DH5α. After verification of the sequence, the plasmid was introduced into E. coli BL21 (DE3), and the transformant was cultivated in LB medium containing 100 μg/ml ampicillin at 37°C to an OD600 of 1.0. Isopropyl-β-d-thiogalactopyranoside (0.5 mM) was added and cultivation was continued for 3 h. The cells were collected by centrifugation and resuspended in a phosphate buffer (20 mM sodium phosphate, 500 mM NaCl, pH 7.4). After ultrasonication, the recombinant protein was purified using Ni SepharoseTM 6 Fast Flow (GE Healthcare) following the manufacturer’s instruction. After extensive dialysis, the protein concentration was determined on the basis of the absorbance at 280 nm using an extinction coefficient of 0.20 l g−1 cm−1 which was determined by the method of Gill and von Hippel (1989) on the basis of the amino acid composition of DnaK.
Immobilization of DnaK on magnet beads
Tosyl-activated magnet beads (Dynabeads M-280, 3 × 109 particles) were washed twice with 0.1 M sodium phosphate buffer (pH 7.4). Dialyzed DnaK solution (0.21 mg in 0.3 ml of the same buffer) was added to the beads and incubated for 2 h at 37°C. After washing the beads twice with the buffer, active tosyl groups were blocked by incubating the beads in 0.2 M Tris–HCl (pH 7.5). The resultant beads were washed twice with the buffer containing 0.1% bovine serum albumin followed by washing with 0.1 M sodium acetate (pH 4.0).
Fluorescent labeling of proteins
Invertase (Code 14504, Sigma-Aldrich) or the purified DnaK was dialyzed against 0.1 M sodium phosphate buffer (pH 7.0) and incubated with 2 mol excess fluorescein isothiocyanate (FITC; Sigma-Aldrich) for 1 h at 37°C. After adding 1/10 volume of 1 M Tris–HCl (pH 8.0) to quench the reaction, the sample was dialyzed against 10 mM sodium phosphate buffer (pH 7.0).
Binding assay of the proteins
The cells (one OD660 unit) were washed with 0.1 M sodium citrate–phosphate buffer at various pH and resuspended in the same buffer (150 μl). The FITC-labeled protein (0.3 mg) dissolved in the buffer was added and incubated for 1 h at 37°C. After washing the cells with the same buffer three times, the bound protein was solubilized by adding 0.1 M sodium phosphate buffer (pH 7.0) containing 8 M urea. The fluorescence intensity of the solution obtained by centrifugation was measured at 30°C (λ ex 490 nm, λ em 535 nm). For the competitive assay, yeast mannan (Sigma-Aldrich, M7504) was added to 0.1 M sodium citrate–phosphate buffer (pH 3).
Extraction and affinity purification of cell surface proteins
IL1403 cells (200 OD660 units harvested at OD660 of 2.0) were digested in a buffer (20 mM Tris–HCl, 0.73 M sucrose, 0.5 M NaCl, 2 mM EDTA, pH 8.0) containing hen egg lysozyme (Seikagaku Co., 1.6 mg/ml), mutalolysin (Sigma-Aldrich, 20 units/ml), and bovine pancreatic ribonuclease A (Sigma-Aldrich, 0.16 mg/ml) for 3 h at 37°C. After centrifugation at 10,000×g for 10 min, solid glycine was added to the supernatant to give a final concentration of 0.1 M. After adjusting the pH to 3.0 using HCl, insoluble materials were removed by centrifugation at 10,000×g for 10 min.
For the preparation of invertase–Sepharose, invertase (480 mg, dialyzed against 0.1 M sodium hydrogen carbonate (pH 8.3) containing 0.5 M NaCl) was reacted with 2 g of CNBr-activated Sepharose 4B (GE Healthcare) following the manufacturer’s instruction. The extract was loaded onto the invertase–Sepharose column (1.5 cmϕ × 4 cm, 30 mg invertase per milliliter gel bed) equilibrated with a buffer (0.1 M glycine, 0.5 M NaCl, and 2 mM EDTA, pH 3.0). After washing the column, the bound proteins were eluted with a pH gradient from 3.0 to 8.0 using 50 mM sodium citrate–phosphate buffer (total 100 ml). Proteins eluted at pH above 7 were concentrated and desalted using an ultrafiltration cartridge (cutoff 10 kDa, Millipore).
Separation and identification of the affinity-purified proteins
The affinity-purified proteins (30 μg) were separated by two-dimensional electrophoresis (Multiphor II, GE Healthcare) using an Immobiline DryStrip with a pH range of 4–7 following the manufacturer’s instruction and stained with Coomassie Brilliant Blue R-250. The major spots were cut and destained with 40 mM ammonium bicarbonate containing 50% methanol for 30 min with vigorous shaking followed by drying at 60°C in vacuo. The gel piece was reswelled in 10–20 µl of trypsin solution (4 nM, proteomic-grade trypsin (Sigma-Aldrich) in 40 mM ammonium bicarbonate) for 45 min on ice. The enzyme solution was replaced with 10% acetonitrile solution in 40 mM ammonium bicarbonate followed by incubation at 37°C for 14–16 h. The digested peptides in the gel were extracted with 0.1% trifluoroacetate (TFA), then with acetonitrile containing 0.1% TFA. Each extracted sample was combined in a tube and dried in vacuo. The extracted peptides were purified with ZipTip C18 (Millipore) tips following the manufacturer’s protocol. The m/z values of the peptides were analyzed by matrix-assisted laser desorption/ionization mass spectrometry (Autoflex, Bruker Daltonics Inc.) in the measurement range of m/z 800–4,600 using cyano-4-hydroxycinnamic acid as a matrix. The obtained peptide mass fingerprints were analyzed using Mascot 2.0 (Matrix Science Inc.) by searching the NCBInr database for L. lactis subsp. lactis IL1403.
Lactate dehydrogenase assay
After the IL1403 cells were treated with the lytic enzymes as described above, the cell suspension was sonicated for 1 min. The clear supernatant (0.1 ml) obtained by centrifugation (10,000×g, 15 min) was mixed with 0.5 ml of 0.2 M Tris–HCl (pH 7.8), 0.2 ml of 50 mM fructose-1,6-bisphosphate, 0.1 ml of 3 mM NADH, and 0.1 ml of sodium pyruvate. The enzyme activity was calculated from the rate of decrease in the absorbance at 340 nm using the molecular extinction coefficient of 6.3 × 103 M−1 cm−1. One unit is defined as the amount of enzyme that oxidizes 1 μmol of NADH in 1 min.
Results
Binding of invertase to LAB
We reported that kefiran production by L. kefiranofaciens is stimulated by physical contact with yeast cells (Cheirsilp et al. 2003b). Since the surface of yeast cells is covered with mannan proteins, we assumed the presence of proteins on the surface of LAB that recognize mannan. On the basis of this assumption, yeast invertase was selected as an affinity ligand to detect and purify the cell surface proteins. Yeast invertase is a hyperglycosylated protein localized on the cell wall (Colonna et al. 1975; Lehle et al. 1979) that was reported to contain 270 mol of mannose per mole of subunit (Williams et al. 1985). As expected, the FITC-labeled invertase bound to L. kefiranofaciens at acidic pH, and the binding was strongest at pH 3 (Fig. 1a). Since the binding was inhibited by the addition of mannan prepared from yeast in a dose-dependent manner (Fig. 1b), the FITC-labeled invertase was shown to bind to L. kefiranofaciens via its mannose chain. Since similar results were obtained in the case of L. lactis subsp. lactis IL1403, this strain was used in the subsequent experiments because its complete genome sequence has already been reported (Bolotin et al. 2001).
Identification of cell surface proteins
To solubilize the proteins that recognize mannan, IL1403 cells (200 OD660 units) were treated with a mixture of lytic enzymes in the presence of osmolytes to prevent cell bursting. After digestion at 37°C for 3 h, the lactate dehydrogenase (LDH) activity in the supernatant of the cell suspension was measured to confirm the leakage of cytosolic proteins from the cells. The supernatant contained only 0.006 units/ml LDH, whereas it contained 7.9 units/ml when the cells in the suspension were disrupted by sonication. These results showed that the leakage of cytosolic proteins during the treatment is negligible.
After adjusting the pH of the supernatant to 3, at which the binding of the FITC-labeled invertase to LAB was strongest, the crude extract was loaded onto the invertase–Sepharose column. The bound proteins eluted at pH above 7 were separated by two-dimensional gel electrophoresis (Fig. 2) and identified by peptide mass fingerprinting. The proteins with MOWSE scores of more than 70 are listed in Table 1. Fifteen of the 16 spots were identified to be cytosolic proteins including molecular chaperones DnaK, GroEL and GroE, and enzymes in the central metabolic pathway such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH), pyruvate kinase (PK), and 6-phosphofructokinase. Spot no. 16, hypothetical protein L142733, was deduced to be a cytosolic protein by SOSUIsignal, a program for the prediction of signal peptide (Gomi et al. 2004).
Binding of DnaK to LAB and yeast
DnaK gene of IL1403 was cloned into pET-21a(+) by PCR, and His-tagged DnaK was expressed in E. coli BL21(DE3). DnaK purified by nickel chelate chromatography (Fig. 3, lane 2) was labeled with FITC. Figure 4 shows the binding of the FITC-labeled DnaK to the microorganisms at various pH. DnaK bound to not only the yeast but also both L. kefiranofaciens and L. lactis at pH 4.
To examine the target site of DnaK on the surface of LAB, substances that bind to DnaK immobilized on the magnet beads were analyzed. The pH of the cell surface extract described above was adjusted to 4.0, and the extract was incubated with the DnaK beads for 30 min at 37°C. After extensive washing with 0.1 M sodium acetate buffer (pH 4.0), the bound substances were eluted by adding sodium phosphate buffer (pH 7.0). The eluent contained a protein (Fig. 3, lane 3), and the protein was identified to be lysozyme that was used for the digestion of LAB. Together with the result that the eluent contained a significant amount of carbohydrate (tested positive by anthrone sulfate reaction, data not shown), the target site of DnaK on LAB was deduced to be a peptidoglycan.
Aggregation of LAB and yeast by DnaK
The yeast and IL1403 cells were harvested at mid-log phase and washed twice with 50 mM sodium citrate–phosphate buffer (pH 4). These cells were mixed in the same buffer in the presence of various concentrations of DnaK and incubated for 1 h at 37°C. Aggregation of the yeast cells was observed when the yeast cells (1 × 108 cfu/ml) were mixed with LAB cells (5 × 107 cfu/ml) at DnaK concentrations above 0.1 mg/ml (Table 2 and Fig. 5). However, when an excess of LAB cells (5 × 109 cfu/ml) was added to the yeast cells or in the absence of LAB cells, no aggregation of the yeast cells was observed even in the presence of DnaK at 1.0 mg/ml (since the diameters of S. cerevisiae and L. lactis subsp. lactis cells are about 5 and 0.7 μm (Do et al. 2001), respectively, one yeast cell is surrounded by 50 LAB cells). In addition, in the absence of the yeast cells, no aggregation of the LAB cells was observed regardless of DnaK concentration.
Discussion
Identified proteins were cytosolic
To investigate the interaction between LAB and yeast cells, proteins localized on the surfaces of IL1403 cells were solubilized by lytic enzyme treatment and were affinity-purified using the invertase–Sepharose column. The purified proteins are considered to have an affinity to both the cell wall of LAB and yeast mannan, whereas some of the proteins might have affinity to these dual functional proteins but have no affinity to either the cell wall or yeast mannan. Contrary to expectations, all the proteins purified from the surface of LAB were identified to be cytosolic. Although it is suspected that cytosolic proteins might leak following cell lysis, the LDH activity in the supernatant of the cell suspension after the lytic enzyme treatment was less than 0.1% of the total LDH activity contained in the cell suspension. These results showed that the cytosolic proteins were localized on the surface of LAB by a regulated mechanism, but not by cell lysis. The observed molecular weights and isoelectric points of these proteins were almost in accordance with their theoretical values (Table 1), indicating that these proteins were not degraded significantly by proteases.
In various microorganisms, it has been reported that cytosolic proteins localize on their cell surface and act as adhesion molecules to their host cells, whereas the mechanism of the localization is still unclear. For example, DnaKs (hsp70) of E. coli (Jesus et al. 2005) and Helicobacter pylori (Huesca et al. 1996, 1998) mediate gastric colonization, and GroELs (hsp60) of Legionella pneumophila (Garduno et al. 1988), Clostridium difficile (Hennequin et al. 2001), Actinobacillus actinomycetemcomitans (Goulhen et al. 1998), and Salmonella typhimurium (Ensgraber and Loos 1992) are involved in adhesion or invasion of their target cells or tissues. Furthermore, GAPDH of Paracoccidioides brasiliensis that causes pulmonary mycosis binds to host extracellular matrix components (Barbosa et al. 2006). Moreover, in cases of LAB, Kinoshita et al. (2008) reported that GAPDH on the cell surface of Lactobacillus plantarum adheres to human colonic mucin. Furthermore, GroEL and PK (Bergonzelli et al. 2006) and elongation factor Tu (EF-Tu; Granato et al. 2004) of Lactobacillus johnsonii are reported to be cell-surface-associated and involved in interactions with mucin and intestinal cells. Kelly et al. (2005) reported that 84-kDa proteins associated with the cell wall of Lactobacillus salivarius were identified to be DnaK, PK, and EF-Ts. Considering these reports, our finding that cytosolic proteins are localized on the cell surface is not exceptional. However, it is notable that most of the adhesion proteins described above were identified in this study.
Specificity and interaction mode of adhesion proteins
In the previous studies, some cell surface proteins were reported to recognize glycoproteins on their host cells, such as mucin at neutral pH. In this study, in addition to these proteins, many proteins on L. lactis cells involving central metabolism and protein synthesis were found to interact with yeast mannan at acidic pH. Although the specificities of the identified proteins against carbohydrates should be elucidated in detail, some of them are expected to bind to both intestinal cells and yeast. Adlerberth et al. (1996) and Tallon et al. (2007) reported that the adhesion of L. plantarum to human cells is protease-sensitive, and the adhesions were inhibited by methyl-α-d-mannoside. Moreover, Peng et al. (2001) reported that the agglutination of Pediococcus damnosus is mannose-sensitive. These adhesions are considered to be mediated by proteins localized on the surface of LAB, and it is also considered that these proteins would recognize mannan.
Most of the previous studies focused on the adhesion of a microorganism to human intestinal cells from the viewpoints of probiotics or infection. Therefore, binding experiments were carried out at neutral pH, whereas a few experiments were carried out at pH 5. In contrast, our binding experiments were conducted at acidic pH where LAB grow in natural environments. Kinoshita et al. (2008) reported that GAPDH of L. plantarum dissociates from the cells by washing with phosphate-buffered saline. Moreover, the binding of GroEL, PK, and EF-Tu to human cells was pH-dependent and higher at pH 5.0 than at pH 7.2 (Bergonzelli et al. 2006). These results agree with our results that the binding of invertase to LAB and yeast was pH-dependent and strongest at pH 3 (Fig. 1a).
Fluorescent-labeled invertase associated with LAB at pH 3−4 (Fig. 1a) even in the presence of 2 M NaCl (data not shown) but dissociated in the presence of 8 M urea. Together with the fact that peptidoglycan and mannan are rich in hydroxyl groups, these results suggest that the interaction is mediated significantly by hydrogen bonds between the polysaccharides and protonated carboxyl groups of the side chains of glutamate and aspartate residues of the proteins.
DnaK adheres LAB to yeast
After washing IL1403 cells, the proteins retained on the cell surface were affinity-purified using an invertase–Sepharose column. This indicates that the recovered proteins can bind to both the cells and invertase, suggesting that these proteins act as adhesion molecules between the LAB and yeast. To confirm the adhesive function, DnaK that gave the densest spot in Fig. 2 was selected as the representative protein and recombinant DnaK was prepared. As expected, the fluorescent-labeled DnaK bound to both LAB and yeast at pH 4 (Fig. 4). These results show that DnaK from IL1403 has three functions, namely, molecular chaperone, ligand for cell surface of LAB, and ligand for yeast mannan. Since DnaK localizes originally in the cytosol where the pH is approximately 7, the conformation of DnaK might be different at acidic pH. However, no significant difference between the circular dichroism spectra of DnaK at pH 4 and 7 was observed (data not shown). With the same conformation, therefore, DnaK was considered to act both as a molecular chaperone in the cytosol and as an adhesion molecule between LAB and yeast on the cell surface.
To confirm the adhesive function of DnaK, LAB and yeast cells were mixed at pH 4 in the presence of DnaK. When almost the same number of LAB cells and yeast cells were mixed (5 × 107 and 1 × 108 cfu/ml, respectively), aggregation of the yeast cells was observed in the presence of the recombinant DnaK at above 0.1 mg/ml (Table 2 and Fig. 5). In contrast, in the absence of one of the partners, the other did not aggregate even in the presence of 1.0 mg/ml DnaK. Furthermore, when an excess number of LAB cells was added to yeast cells, the yeast cells did not aggregate because all the yeast cells would be surrounded by LAB cells. These results show that DnaK has two independent binding sites, one is specific for LAB and the other is specific for yeast.
Significance of adherence of LAB to yeast
On the basis of the results described above, we propose a working hypothesis; that is, when LAB are subjected to stresses such as accumulation of lactic acid, they cause yeast cells to help reduce the stresses by displaying these proteins on their cell surface.
LAB produce lactic acid for their growth, but the accumulation of lactic acid debilitates and kills LAB even when the pH of the culture is maintained by adding an alkaline. The debilitation of LAB by the accumulation of lactic acid has bothered researchers who attempt to let LAB produce useful materials such as nisin and extracellular polysaccharides (Shimizu et al. 1999; Cheirsilp et al. 2003a). In the natural environment, including traditional fermented foods, incidentally, LAB often coexist with yeast that are capable of assimilating lactic acid aerobically (Murooka and Yamashita 2008; Cheirsilp et al. 2003a). Thus, we co-cultured L. kefiranofaciens with S. cerevisiae to remove lactic acid and succeeded to improve the productivity of kefiran, an extrapolysaccharide (Cheirsilp et al. 2003a, b, 2007; Tada et al. 2007).
In growth environments of LAB and yeast, a concentration gradient of lactic acid is formed. The concentration of lactic acid increases near LAB cells and decreases near yeast cells. This distribution becomes notable in natural environments such as fallen fruits and cereals that are unstirred and viscous, whereas artificial media in laboratories are usually stirred and not viscous owing to their high water contents. Under these conditions wherein the diffusion of substances is slow, the lactic acid concentration around an LAB cell increases rapidly and the LAB cell is subjected to stress. However, if the LAB cell adheres to the yeast cell that assimilates lactic acid, the stress would be relieved. Similarly, when LAB cells that do not possess catalase activity are exposed to oxidative stress caused by hydrogen peroxide, the stress would also be relieved by the adhesion of the LAB cells to yeast that has catalase activity.
On the basis of the present results and considerations described above, it would be possible to formulate a working hypothesis; that is, when LAB are subjected to lactic acid and oxidative stresses, they cause yeast to help reduce the stresses by displaying the adhesion proteins on their cell surface. If a protein displayed on LAB surface had a significant level of affinity to mannan and allowed the LAB cell to approach yeast cell, the stresses would be relieved. As a result, the adhesive function of the protein would evolve because the rapid growth of LAB would allow to produce progeny.
Although it is necessary to clarify the mechanisms of the localization of adhesion proteins on the cell surface and the interactions between the adhesion proteins and yeast, this working hypothesis will contribute to understanding the symbioses between LAB and yeasts in natural environments and fermented foods.
References
Adlerberth I, Ahrne S, Johansson ML, Molin G, Hanson LA, Wold AE (1996) A mannose-specific adherence mechanism in Lactobacillus plantarum conferring binding to the human colonic cell line HT-29. Appl Environ Microbiol 62:2244–2251
Barbosa MS, Bao SN, Andreotti PF, de Faria FP, Felipe MS, dos Santos Feitosa L, Mendes-Giannini MJ, Soares CM (2006) Glyceraldehyde-3-phosphate dehydrogenase of Paracoccidioides brasiliensis is a cell surface protein involved in fungal adhesion to extracellular matrix proteins and interaction with cells. Infect Immun 74:382–389
Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthesy-Theulaz IE (2006) GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun 74:425–434
Bolotin A, Wincker P, Mauger S, Jaillon O, Malarme K, Weissenbach J, Ehrlich SD, Sorokin A (2001) The complete genome sequence of the lactic acid bacterium Lactococcus lactis ssp. lactis IL1403. Genome Res 11:731–753
Cheirsilp B, Shimizu H, Shioya S (2001) Modelling and optimization of environmental conditions for kefiran production by Lactobacillus kefiranofaciens. Appl Microbiol Biotechnol 57:639–646
Cheirsilp B, Shimizu H, Shioya S (2003a) Enhanced kefiran production by mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. J Biotechnol 100:43–53
Cheirsilp B, Shimizu H, Shioya S (2003b) Interactions between Lactobacillus kefiranofaciens and Saccharomyces cerevisiae in mixed culture for kefiran production. J Biosci Bioeng 93:279–284
Cheirsilp B, Shimizu H, Shioya S (2007) Kinetic modeling of kefiran production in mixed culture of Lactobacillus kefiranofaciens and Saccharomyces cerevisiae. Process Biochem 42:570–579
Colonna WJ, Cano FR, Lampen JO (1975) Microheterogeneity in yeast invertase. Biochim Biophys Acta 386:293–300
Corsetti A, Rossi J, Gobbetti M (2001) Interactions between yeasts and bacteria in the smear surface-ripened cheeses. Int J Food Microbiol 69:1–10
Do TM, Plockova MI, Chumchalova JA (2001) Lactococcus lactis subsp. lactis LTM 32, a new bacteriocin-producing strain isolated from Vietnamese fermented milk. Czech J Food Sci 19:171–176
Ensgraber M, Loos M (1992) A 66-kilodalton heat shock protein of Salmonella typhimurium is responsible for binding of the bacterium to intestinal mucus. Infect Immun 60:3072–3078
Garduno RA, Garduño E, Hoffman PS (1988) Surface-associated Hsp60 chaperonin of Legionella pneumophila mediates invasion in a HeLa cell model. Infect Immun 66:4602–4610
Gill SC, von Hippel PH (1989) Calculation of protein extinction coefficients from amino acid sequence data. Anal Biochem 182:319–326
Gobbetti M (1998) The sourdough microflora: interactions of lactic acid bacteria and yeasts. Trends Food Sci Technol 9:267–274
Gomi M, Sonoyama M, Mitaku S (2004) High performance system for signal peptide prediction: SOSUIsignal. Chem-Bio Info J 4:142–147
Goulhen F, Hafezi A, Uitto VJ, Hinode D, Nakamura R, Grenier D, Mayrand D (1998) Subcellular localization and cytotoxic activity of the GroEL-like protein isolated from Actinobacillus actinomycetemcomitans. Infect Immun 66:5307–5313
Granato D, Bergonzelli GE, Pridmore RD, Marvin-Guy LF, Rouvet M, Corthesy-Theulaz IE (2004) Cell surface-associated elongation factor Tu mediates the attachment of Lactobacillus johnsonii NCC 533 (La1) to human intestinal cells and mucins. Infect Immun 72:2160–2169
Gusils C, Palacios J, Gonzalez S, Oliver G (1999) Lectin-like protein fractions in lactic acid bacteria isolated from chickens. Biol Pharm Bull 22:11–15
Hennequin C, Porcheray F, Waligora-Dupriet AJ, Collignon A, Barc MC, Bourlioux P, Karjalainen T (2001) GroEL (Hsp60) of Clostridium difficile is involved in cell adherence. Microbiology 147:87–96
Huesca MC, Borgia S, Hoffman P, Lingwood CA (1996) Acidic pH changes receptor binding specificity of Helicobacter pylori: a binary adhesion model in which surface heat shock (stress) proteins mediate sulfatide recognition in gastric colonization. Infect Immun 64:2643–2648
Huesca MC, Goodwin A, Bhagwansingh A, Hoffman P, Lingwood CA (1998) Characterization of an acidic-pH-inducible stress protein (hsp70), a putative sulfatide binding adhesin, from Helicobacter pylori. Infect Immun 66:4061–4067
Jesus MC, Urban AA, Marasigan ME, Barnett Foster DE (2005) Acid and bile-salt stress of enteropathogenic Escherichia coli enhances adhesion to epithelial cells and alters glycolipid receptor binding specificity. J Infect Dis 192:1430–1440
Kawarai T, Furukawa S, Ogihara H, Yamasaki M (2007) Mixed-species biofilm formation by lactic acid bacteria and rice wine yeasts. Appl Environ Microbiol 73:4673–4676
Kelly P, Maguire PB, Bennett M, Fitzgerald DJ, Edwards RJ, Thiede B, Treumann A, Collins JK, O’Sullivan GC, Shanahan F, Dunne C (2005) Correlation of probiotic Lactobacillus salivarius growth phase with its cell wall-associated proteome. FEMS Microbiol Lett 252:153–159
Kinoshita H, Uchida H, Kawai Y, Kawasaki T, Wakahara N, Matsuo H, Watanabe M, Kitazawa H, Ohnuma S, Miura K, Horii A, Saito T (2008) Cell surface Lactobacillus plantarum LA 318 glyceraldehyde-3-phosphate dehydrogenase (GAPDH) adheres to human colonic mucin. J Appl Microbiol 104:1667–1674
Lehle L, Cohen RE, Ballou CE (1979) Carbohydrate structure of yeast invertase. Demonstration of a form with only core oligosaccharides and a form with completed polysaccharide chains. J Biol Chem 254:12209–12218
Murooka Y, Yamashita M (2008) Traditional healthful fermented products of Japan. J Ind Microbiol Biotechnol 35:791–798
Narvhusa JA, Gadagab TH (2003) The role of interaction between yeasts and lactic acid bacteria in African fermented milk. Int J Food Microbiol 86:51–60
Peng X, Sun J, Iserentant D, Michiels C, Verachtert H (2001) Flocculation and coflocculation of bacteria by yeasts. Appl Microbiol Biotechnol 55:777–781
Shimizu H, Mizuguchi T, Tanaka E, Shioya S (1999) Nisin production by a mixed-culture system consisting of Lactococcus lactis and Kluyveromyces marxianus. Appl Environ Microbiol 65:3134–3141
Tada S, Katakura Y, Ninomiya K, Shioya S (2007) Fed-batch coculture of Lactobacillus kefiranofaciens with Saccharomyces cerevisiae for effective production of kefiran. J Biosci Bioeng 103:557–562
Tallon R, Arias S, Bressollier P, Urdaci MC (2007) Strain- and matrix-dependent adhesion of Lactobacillus plantarum is mediated by proteinaceous bacterial compounds. J Appl Microbiol 102:442–451
Williams RS, Trumbly RJ, MacColl R, Trimble RB, Maley F (1985) Comparative properties of amplified external and internal invertase from the yeast SUC2 gene. J Biol Chem 260:13334–13341
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Katakura, Y., Sano, R., Hashimoto, T. et al. Lactic acid bacteria display on the cell surface cytosolic proteins that recognize yeast mannan. Appl Microbiol Biotechnol 86, 319–326 (2010). https://doi.org/10.1007/s00253-009-2295-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-2295-y