Abstract
MAN5, the main extracellular saccharide hydrolase from Bacillus sp. MSJ-5, is an endo-β-mannanase with a demand of at least five sugar moieties for effective cleavage. It has a pH optimum of 5.5 and a temperature optimum of 50°C and is stable at pH 5–9 or below 65°C. MAN5 has a very high ability to hydrolyze konjac flour, 10 U/mg of which could completely liquefy konjac flour gum in 10 min at 50°C. HPLC analysis showed that most glucomannan in the konjac flour was hydrolyzed into a large amount of oligosaccharides with DP of 2–6 and a very small amount of monosaccharide. With the culture supernatant as enzyme source, the optimum condition to prepare oligosaccharides from konjac flour was obtained as 10 mg/ml konjac flour incubated with 10 U/mg enzyme at 50°C for 24 h. With this condition, more than 90% polysaccharides in the konjac flour solution were hydrolyzed into oligosaccharides and a little monosaccharide (2.98% of the oligosaccharides). Konjac flour is an underutilized agricultural material with low commercial value in China. With MAN5, konjac flour can be utilized to generate high value-added oligosaccharides. The high effectiveness and cheapness of this technique indicates its potential in industry.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During the past two decades, many types of oligosaccharides are actively developed for their excellent physiological properties beneficial to human health (Solange and Ismael 2007; Hirayama 2002). Several oligosaccharides such as glycosylsucrose, fructooligosaccharides, maltooligosaccharides, isomaltooligosaccharides (branched oligosaccharides), galactooligosaccharides, xylooligosaccharides, isomaltulose (palatinose), and lactosucrose are produced on an industrial scale (Sako et al. 1999; Nakakuki 2002). In contrast, mannooligosaccharides are received less attention. Mannooligosaccharides are non-digestible oligosaccharides possessing important physiological properties to behave as dietary fiber and prebiotics. They have beneficial effects on the growth of intestinal microorganisms, especially Bifidobacteria, decrease enteric pathogenic bacteria, regulate immunoreactions, and improve the integrity of intestinal mucosa, thereby increasing the human health level and the breeding animal worth (Wong and Saddler 1993; Gomes and Steiner 1998; Kobayashi et al. 1987; Dhawan and Kaur 2007; Moreira and Filho 2008). Lu et al. (2002) reported that a tetrasaccharide fraction from Amorphophallus konjac had beneficial effects on diabetes. These studies show that mannooligosaccharides are valuable oligosaccharides to be developed. Although it was reported that various mannanases from bacteria, fungi, and plants could hydrolyze mannan, glucomannan galactomannan, or galactoglucomannan to produce mannooligosaccharides (McCleary 1988; Daskiran et al. 2004; Shimahara et al. 1975; Araki 1983; Hossain et al. 1996; Reese and Shibata 1965; Yamaura et al. 1993, Dhawan and Kaur 2007; Moreira and Filho 2008), an economic and viable technique to produce mannooligosaccharides is yet rare.
A. konjac is widely planted in Asia, especially in China. Every year, about 1,300,000 tons (fresh weight) of konjac corms are cropped in China (Sheng and Teng 2008). The flour produced from konjac corms is an underutilized agricultural material with low commercial value in China, mainly used as a gelling and thickening agent and an ingredient in foods (Kurakake and Komaki 2001). Since glucomannan is the main component of konjac flour, konjac flour should be a good material to produce mannooligosaccharides.
In this paper, Bacillus sp. MSJ-5, as a mannanase-producing bacterium, was screened from the konjac field in Sichuan province of China. The β-mannanase MAN5 secreted by this strain was purified and characterized, and a cheap and effective technique to prepare oligosaccharides from konjac flour with MAN5 was proposed.
Materials and methods
Materials
Locust bean gum and xylans were purchased from Sigma. Beta-1,4-mannan and β-1,4-mannooligosaccharides (M2–M6) were purchased from Megazyme. Konjac powder was purchased from Limao Agricul Tural Products Development (Chongqing, China).
Screening and identification of strain MSJ-5 Bacillus sp.
MSJ-5 was screened from the konjac field in Sichuan province of China with the procedure described in the Electronic supplementary materials. Cellular morphology was examined using scanning electron microscopy. The 16S rRNA gene of MSJ-5 was amplified by PCR from genomic DNA and sequenced as described by Hu and Li (2007). The obtained 16S rRNA gene sequence was aligned with its closely related neighbor sequences retrieved from GenBank using CLUSTAL X (v 1.83). Neighbor-joining trees were constructed using MEGA version 3.1 by neighbor-joining method and Kimura two-parameter model. The 16S rRNA gene sequence of Bacillus sp. MSJ-5 was deposited in GenBank under the accession number FJ393326. Bacillus sp. MSJ-5 was deposited in China Center for Type Culture Collection as a patent strain under the number M208258.
Production and purification of the β-mannanase from Bacillus sp. MSJ-5
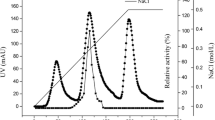
Strain MSJ-5 was inoculated in 50 ml optimized liquid medium containing (g/l) 25 konjac powder, 35 yeast extract powder, 10 soybean powder, 5 KH2PO4 (pH 7.0) in a 500-ml flask and incubated on a rotary shaker (220 rpm) at 32°C for 32 h to reach the highest β-mannanase activity in the culture. Then, the culture was centrifuged at 10,000×g, 4°C for 15 min. The supernatant was collected and used as crude mannanase enzyme. For mannanase purification, solid (NH4)2SO4 powder was added to the supernatant to 40% saturation. Precipitate was removed by centrifugation (10,000 rpm, 4°C, 10 min), and solid (NH4)2SO4 was further added to the supernatant to 60% saturation. The resulting precipitate was dissolved in 50 mM phosphate buffer (pH 6.6) and dialyzed against 20 mM phosphate buffer (pH 6.6). The enzyme solution was then purified by gel filtration on a Bio-gel P60 column, which was eluted with 20 mM phosphate buffer (pH 6.6). The fractions with β-mannanase activity were further purified by anion-exchange chromatography on a DEAE-Sepharose column, which had been pre-equilibrated with 10 mM phosphate buffer (pH 7.0). Proteins were eluted with a linear gradient of 0 to 1 M NaCl at a flow rate of 0.6 ml/min, and the fractions with β-mannanase activity were collected. Its purity and molecular mass was determined with a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) system (Bio-Rad) as described by Laemmli (1970).
Protein determination and enzyme assay
Protein content was measured by the method of Bradford (1976). Beta-mannanase activity was assayed using the method of Zhang et al. (2000). Briefly, 0.1 ml properly diluted enzyme solution was mixed with 0.9 ml locust bean gum (0.5%) in 20 mM pH 6.0 phosphate buffer, and the mixture was incubated at 50°C for 10 min. The reducing sugar released in the mixture was determined with mannose as the standard using DNS method (Miller 1959). One unit of enzyme activity was defined as the amount of enzyme used for the production of 1 µmol of reducing sugar per min.
Characterization of the β-mannanase MAN5 from Bacillus. sp. MSJ-5
To determine the optimal temperature, the activity of the purified β-mannanase toward locust bean gum was assayed at pH 5.5 in a range of 35–70°C. To assay the enzyme thermal stability, the purified β-mannanase was incubated at different temperatures (35–70°C) for 1 h, and the remaining activities were measured at pH 5.5 and 50°C. To determine the optimal pH, the β-mannanase activity of MAN5 was measured at 50°C in the range of pH 3.0–10.0 (pH 3.0–6.0, 20 mM citric acid buffer; pH 6.0–8.0, 20 mM phosphate buffer; pH 8.0–10.0, 20 mM glycine–NaOH buffer). To assay effect of pH on the enzyme stability, β-mannanase MAN5 was incubated in various buffers of different pH values (as shown above) for 1 h, and then the residual activity was measured at pH 5.5 and 50°C. The K m and V max values were determined by Lineweaver–Burk plots, which was made by linear regression with initial rates determined with 2.5–45 g/l locust bean gum or konjac flour at 50°C using an enzyme concentration ([E]) of 0.25 U/ml. k cat values were calculated with the formula \( {{k_{\text{cat}} = V_{{\max }} } \mathord{\left/ {\vphantom {{k_{\text{cat}} = V_{{\max }} } {\left[ E \right]}}} \right. } {\left[ E \right]}} \).
Hydrolysis of mannooligosaccharides, mannan, and konjac powder
Purified MAN5 was added to the sugar substrates, which were prepared in ddH2O as in the following: 0.5 U/mg β-mannanase was added to 1 mg/ml mannooligosaccharides or 5 mg/ml mannan, and 5 U/mg β-mannanase was added to 10 mg/ml konjac powder. The mixture was incubated at 50°C for 24 h for mannoseoligosaccharides and mannan and 48 h for konjac flour and then terminated by heating in boiling water for 5 min. The mixture was centrifuged at 10,000×g, 4°C for 5 min, and the supernatant was filtrated with a cellulose nitrate membrane (0.22 μm) for HPLC analysis.
HPLC analysis of sugars
The hydrolytic products were analyzed using LC-10 AD high-performance liquid chromatography (Shimadzu, Japan), equipped with an HPX-42C Aminex column (Bio-Rad) and a RID-10A refractive index detector. The column was maintained at 75°C and eluted with ddH2O at a flow rate of 0.4 ml/min.
Results
Identification of strain MSJ-5
Among the strains screened from the konjac field, strain no. 5 displayed the best mannanase production ability (Fig. S1). This strain, named MSJ-5, was selected as a good producer of mannanase. Strain MSJ-5 could form a clear hydrolytic zone on the locust bean gum-containing medium plate (Fig. S2). It is a gram-positive, rod bacterium (0.7–0.8 × 2.1–3.0 μm) without flagella (Fig. 1). It forms an oval spore in the middle of the cell after 48-h cultivation (Fig. S3). The colonies are white, circular with convex with entire margins when cultured in 36 h. After 36 h, they spread rapidly and form flowery margins because of the high movement ability of this strain (Fig. S4, S5). Blast in GenBank database showed that the 16S rRNA gene sequence of strain MSJ-5 has more than 99% identity to those of four Bacillus species (Baccillus velezensis, Bacillus polyfermenticus, Bacillus amyloliquefaciens, and Bacillus subtilis). A distance-based neighbor-joining tree was constructed with Bacillus species (Fig. 2), which also showed that strain MSJ-5 is most related to the four Bacillus species above. Based on these results, strain MSJ-5 could be determined to belong to genus Bacillus. Therefore, it was named as Bacillus sp. MSJ-5.
Neighbor-joining phylogenetic tree based on 16S rRNA gene sequences of Bacillus sp. MSJ-5 and other Bacillus species. Percent bootstrap values above 40 (1,000 replicates) are indicated at nodes. Scale bar = 0.005 substitutions per nucleotide position. Sequence alignment and comparison was performed using CLUSTAL X (v 1.83). Neighbor-joining trees were constructed using MEGA version 3.1 by neighbor-joining method and Kimura two-parameter model
Production, purification, and characterization of the mannanase from Bacillus. sp. MSJ-5
After being cultured in the optimized medium for 32 h, the activity of the β-mannanase secreted by Bacillus sp. MSJ-5 reached 651 ± 31 U/ml, which was relatively high when compared to those reported (Mao et al. 2007; Yoon and Lim 2007; Zakaria et al. 1998; Zhang et al. 2000). The crude enzyme had high activity to mannan, slight activity to soluble starch, and no activity to cellulose or xylan, suggesting that mannanase is the main saccharide hydrolase in the fermentation broth. The mannanase in the culture was purified with ammonium sulfate precipitation, gel filtration, and ion exchange chromatography as described in “Materials and methods”, which resulted in a 19-fold purification and a final yield of 18.86% (Table 1). SDS-PAGE showed the enzyme reached electrophoresis degree purity with a molecular mass of 40.5 kDa (Fig. S6). Analysis of the enzyme form by gel filtration chromatography showed that it is a momomer (Fig. S7). The purified β-mannanase from strain MSJ-5 was named MAN5.
With locust beam gum as substrate, MAN5 displayed an optimum pH of 5.5. After incubation at 50°C in pH 6–9 buffer for 1 h, the enzyme activity remained above 70%. The optimal temperature of MAN5 was 50–60°C. After incubation at 35–65°C for 1 h, the remaining activity kept above 50%, whereas the enzyme activity lost rapidly when the incubation temperature was higher than 65°C (Fig. 3) Ag+ and Fe3+ markedly inhibited the enzyme activity with the degree about 90%, while Mn2+ and Cu2+ could inhibit 35–40% the enzyme activity. Li+, Zn2+, and Fe2+ had slight inhibitory effect. Among the investigated metal ions, no metal ion had significant activating effects on the activity of MAN5. The most activating effect occurred to Co2+ with just 8.6%. One millimolar EDTA decreased the enzyme activity to 33.9% of the control (Table 2).
a, b Effects of pH (a) and temperature (b) on the activity (squares) and stability (circles) of β-mannanase MAN5. To determine the optimal temperature, the activity of the purified β-mannanase toward locust bean gum was assayed at pH 5.5 in a range of 35–70°C. To assay the enzyme thermal stability, the purified β-mannanase was incubated at different temperatures (35–70°C) for 1 h, and the remaining activities were measured at pH 5.5 and 50°C. To determine the optimal pH, the β-mannanase activity of MAN5 was measured at 50°C in the range of pH 3.0–10.0 (pH 3.0–6.0, 20 mM citric acid buffer; pH 6.0–8.0, 20 mM phosphate buffer; pH 8.0–10.0, 20 mM glycine–NaOH buffer). To assay effect of pH on the enzyme stability, β-mannanase MAN5 was incubated in various buffers of different pH values (as shown above) for 1 h, and then the residual activity was measured at pH 5.5 and 50°C
MAN5 had activity to mannan, konjac flour, and locust bean gum (β-1,4-mannan), but no activity to yeast mannan (α-1,6-mannan), showing that it is β-mannanase. MAN5 had a lower K m value to konjac powder than to locust bean gum, showing that it had higher affinity to konjac powder. However, the catalytic efficiency (k cat/K m) of MAN5 to konjac powder (glucomannan) was a little lower than to locust bean gum (galactomannan; Table 3). In addition, mannobiose (0.1–0.4 mM) had little inhibitory effect on the activity of MAN5 (Fig. S9).
Analysis of the hydrolysis of mannooligosaccharides and mannan by MAN5
One milligram per milliliter β-mannooligosaccharides with DP of 2–6 was incubated with 0.5 U/mg MAN5 at 50°C for 24 h, respectively. Then, the hydrolytic products were analyzed by HPLC. MAN5 efficiently cleaved mannooligosaccharide M5 and M6, and low activity was detected on M4. M2 and M3 were not digested at all even incubated with large amount of MAN5 for 24 h (Fig. S10). The main products released from M5 and M6 were oligosaccharides with DP of 2–3 and 2–4, respectively, with no monosaccharides. These results showed that MAN5 was a strict endo-β-mannanase exhibiting an action pattern with a demand of at least five sugar moieties for effective cleavage. During the 24 h hydrolysis of M6, the mole ratio of M2, M3, and M4 was about 1:1:1, suggesting that MAN5 seemed to preferably hydrolyze M6 as M-M↓M-M-M-M. This cleavage pattern was different from that of most of the β-mannanases reported, which preferentially hydrolyzed the central mannosidic linkage of M6 (Larsson et al. 2006; Ootsuka et al. 2006; Hrmova et al. 2006). M2 and M3 were the main hydrolysis products when M5 was hydrolyzed. M4 was detected during the hydrolysis of M5, and equal amount of M1 was not detected (Table 4), indicating that MAN5 could not cleave a monosaccharide from the end of a carbohydrate chain, and the formation of M4 during M5 hydrolysis should result from transglycosylation.
Five milligrams per milliliter β-1,4-mannan was incubated with 0.5 U/mg MAN5 at 50°C, and samples were taken at 10 min, 1 h, and 24 h, respectively, for product analysis by HPLC. MAN5 displayed the same hydrolysis pattern as hydrolyzing M6 and mainly produced M2, M3, and M4 from β-1,4-mannan (Fig. S11), which was different from most β-mannanases whose main products released from β-1,4-mannan were M2 and M3 (Larsson et al. 2006; Le Nours et al. 2006; Tamaru et al. 1995). There were very few oligosaccharides with DP value above 4 accumulated, perhaps due to MAN5's preferable hydrolysis of M5 and M6 to oligosaccharides with DP of 2, 3, and 4. The amount of M2, M3, and M4 increased rapidly from 10 min to 1 h and remained steady from 1 to 24 h. The amount of monosaccharide remained at very low level throughout the whole process, further showing that MAN5 was an endo-β-mannanase.
The amount of oligosaccharides and monosaccharide released from the hydrolysis of 10 mg/ml konjac flour by 10 U/mg β-mannanase MAN5 at 50°C for 48 h. The amount of oligosaccharides and monosaccharide was represented by the peak area in HPLC spectra, which was calculated by the software of class-vp 6.14 provided by the HPLC system (Shimadzu, Japan)
Analysis of oligosaccharides produced by the hydrolysis of konjac flour by β-mannanase MAN5
Ten milligrams per milliliter konjac flour in ddH2O was prepared for the hydrolysis of MAN5. When konjac flour was dissolved in water in a concentration of 10 mg/ml, a gum-like solution was formed. However, the gum-like solution was liquefied to water-like solution in 10 min when incubated with 10 U/mg MAN5 at 50°C, indicating that MAN5 had high ability to hydrolyze konjac flour. After 48 h hydrolysis, products were analyzed by HPLC. The main saccharide in konjac flour is glucomannan, which is composed of β-1,4-linkage mannose and glucose residues in the ratio of 1.6:1 (Cescutti et al. 2002). HPLC analysis showed that most glucomannan component in the konjac flour solution was hydrolyzed, and a large amount of oligosaccharides of DP 2–6 were produced with a very little amount of monosaccharide after 48 h hydrolysis (Fig. 4). On the HPLC spectrum, the retention time of glucose is shorter than that of mannose, suggesting that gluco-mannooligosaccharides may have shorter retention time than mannooligosaccharides. Since the retention time of the highest peak in the product was shorter than that of M3 and longer than that of M4 on the HPLC spectrum, it could be speculated that the dominant product should be gluco-mannotrisaccharides. In addition, there is a small peak at the position corresponding to M3 on the HPLC spectrum, showing that the product contained a little M3. These results suggested MAN5 was a good endo-β-mannanase, which could hydrolyze konjac flour with high effectiveness to produce oligosaccharides of DP 2–6.
Optimization of oligosaccharides preparation from konjac flour by β-mannanase MAN5
In order to develop an economic and viable technology, the conditions to prepare oligosaccharides from konjac flour with MAN5 were optimized. Since mannanase is the main saccharide hydrolase in the fermentation broth, the culture supernatant of Bacillus sp. MSJ-5 was used as enzyme source in order to reduce the cost. Firstly, an optimum of substrate concentration was studied. Various concentrations of konjac flour in the range of 1–30 mg/ml were hydrolyzed by 0.1 U/mg the enzyme for 1 h. The reduced sugar concentration in the reaction mixture was measured, and the ratio of the reduced sugar concentration to the konjac flour concentration was calculated. The result showed that the ratio no longer increased when the substrate concentration was more than 10 mg/ml (Fig. S12). The same result was obtained when the enzyme concentration increased (data not shown). Therefore, 10 mg/ml should be an optimum substrate concentration. Then, an optimum of enzyme concentration was studied with 10 mg/ml konjac flour as substrate. The products were analyzed by HPLC after the substrate was hydrolyzed by 1, 5, and 10 U/mg enzyme for 12 h, respectively. When 5 U/mg enzyme was used, the amount of oligosaccharides with DP of 2–6 accounted for 72.6% of the amount of all the saccharides in the reaction mixture according to the peak area in the HPLC spectrum, and it accounted for 77% when 10 U/mg inoculated enzyme was used (Fig. S13). Therefore, 5–10 U/mg was the optimum of enzyme amount for konjac flour hydrolysis. Finally, an optimum of hydrolytic time was studied with 10 mg/ml konjac flour and 10 U/mg enzyme. Ten milligrams per milliliter konjac flour was incubated with 10 U/mg enzyme at 50°C, and samples were taken for product analysis by HPLC in 20 min and 1, 2, 12, 24, and 48 h, respectively (Fig. 5). When konjac flour was hydrolyzed for 24 h, the amount of oligosaccharides with DP of 2–6 reached 90.9% of the amount of all the saccharides in the reaction mixture according to the peak area in the HPLC spectrum, and the amount of oligosaccharides increased only a little when the time increased to 48 h (Table 5). Therefore, 24 h was an optimum time for konjac flour hydrolysis. Taken together, the optimum condition to prepare oligosaccharides from konjac flour with the crude enzyme of MAN5 was that 10 mg/ml konjac flour was incubated with 10 U/mg enzyme at 50°C for 24 h. With this condition, more than 90% polysaccharides in the konjac flour solution were hydrolyzed into oligosaccharides with DP of 2–6, and only a very small amount of monosaccharide (2.98% of the oligosaccharides) was produced (Table 5).
Discussion
Because of their functional properties beneficial to human health, oligosaccharides are widely used in foods, beverages, and confectionary, especially in functional food. Oligosaccharides are now mainly produced with enzyme technology from saccharides such as starch, chitin, xylan, and mannan, etc. (Sako et al. 1999; Nakakuki 2002). Glucomannan is a proper material to produce oligosaccharides since it is composed of β-1,4-linkage mannose and glucose residues in the ratio of 1.6:1 (Cescutti et al. 2002; Moreira and Filho 2008). Glucomannan is the main component of konjac flour, which is produced in a large amount in China. Since the price of konjac flour is very low (30–50$/ton), konjac flour should be a cheap and good material to produce oligosaccharides. However, there have been few reports on oligosaccharide production with konjac flour.
To prepare oligosaccharides from konjac flour by enzyme technology, a β-mannanase, which can effectively hydrolyze konjac flour to produce oligosaccharides, is essential. In this article, an endo-β-mannanase MAN5 with high konjac flour-hydrolyzing ability was purified and characterized. MAN5 is secreted by Bacillus sp. MSJ-5 screened from konjac field. Mannanase seems to be the main extracellular saccharide hydrolase secreted by this strain because no other cellulase or hemicellulase activity was detected in the strain culture. Therefore, the culture supernanant can be used as mannanase without purification. Similar to a lot of reported β-mannanases (Mendoza et al. 1994; Zakaria et al. 1998; Zhang et al. 2000; Hägglund 2002), the purified MAN5 has a pH optimum of 5.5 and a temperature optimum of 50°C and is stable at pH 5–9 or below 65°C. End product such as mannobiose has little inhibition to MAN5 activity. All these characters make it suitable to be applied as an enzyme in a technical process. MAN5 is a strict endo-β-mannanase with a demand of at least five sugar moieties for effective cleavage. It preferably hydrolyzes M6 as M-M↓M-M-M-M, which is uncommon in the cleavage patterns of the β-mannanases reported. Most β-mannanases preferentially hydrolyze the central mannosidic linkage of M6 (Larsson et al. 2006; Ootsuka et al. 2006; Hrmova et al. 2006). Transglycosylation, a common action of many β-mannanases (Schröder et al. 2006; Puchart et al. 2004; Le Nours et al. 2006), also occurred in the hodrolysis of mannoseoligosaccharides by MAN5. The most remarkable characteristic of MAN5 is its high ability to hydrolyze konjac flour. An enzyme amount of 10 U/mg could completely liquefy konjac flour gum in 10 min at 50°C. Moreover, HPLC analysis showed that most glucomannan in the konjac flour was hydrolyzed into oligosaccharides with DP of 2–6 with a very small amount of monosaccharide, which may result from transglycosylation.
Since β-mannanase MAN5 has high ability to hydrolyze konjac flour to produce oligosaccharides, and mannanase is the main saccharide hydrolase in strain MSJ-5 culture, an economic and viable technology to prepare oligosaccharides from konjac flour with Bacillus sp. MSJ-5 culture as enzyme source was explored. By optimizing the concentration of konjac flour and enzyme and hydrolytic time, the optimum condition to prepare oligosaccharides from konjac flour with the crude enzyme of MAN5 was that 10 mg/ml konjac flour was incubated with 10 U/mg enzyme at 50°C for 24 h. With this condition, more than 90% saccharides in the konjac flour solution were hydrolyzed into oligosaccharides with DP of 2–6, and only a very small amount of monosaccharide (2.98% of the oligosaccharides) was produced. To our knowledge, this is the first report about a viable technique to prepare oligosaccharides from konjac flour. Although it is a laboratory-scale result, the high effectiveness and cheapness of this technique indicates its potential in industry.
References
Araki T (1983) Purification and characterization of an endo-β-mannanase from Aeromonas sp F-25. J Fac Agric Kyushu Univ 27:89–98
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Chem 72:248–254
Cescutti P, Campa C, Delben F, Rizzo R (2002) Structure of the oligomers obtained by enzymatic hydrolysis of the glucomannan produced by the plant Amorphophallus konjac. Carbo Res 337:2505–2511
Daskiran M, Teeter RG, Fodge D, Hsiao HY (2004) An evaluation of endo-β-D-mannanase (Hemicell) effects on broiler performance and energy use in diets varying in β-mannan content. Poult Sci 83:662–668
Dhawan S, Kaur J (2007) Microbial mannanases: an overview of production and applications. Crit Rev Biotechnol 27(4):197–216
Gomes J, Steiner W (1998) Production of a high activity of an extremely thermostable β-mannanase by the thermophilic eubacterium Rhodothermus marinus, grown on locust bean gum. Biotechnol Lett 20:729–733
Hägglund P (2002) Mannan-hydrolysis by hemicellulases. Lund University, Lund, pp 24–25
Hirayama M (2002) Novel physiological functions of oligosaccharides. Pure Appl Chem 74:1271–1279
Hossain MZ, Abe J, Hizukuri S (1996) Multiple forms of β-mannanase from Bacillus sp. KK01. Enz Microb Tech 18:95–98
Hrmova M, Burton RA, Lahnstein J, Fincher GB (2006) Hydrolysis of (1, 4)-β-D-mannans in barley (Hordeum vulgare L.) is mediated by the concerted action of (1, 4)-β-D-mannan endohydrolase and β-D-mannosidase. Biochem J 399:77–90
Hu ZY, Li Y (2007) Pseudidiomarina sediminum sp. nov., a marine bacterium isolated from coastal sediments of Luoyuan Bay in China. Int J Syst Evol Microbiol 57:2572–2577
Kobayashi Y, Echigen R, Mada M, Mutai M (1987) Effects of hydrolyzates of konjac mannan and soybean oligosaccharides on intestinal flora in man and rats. In: Mitsuoka T (ed) Intestinal flora and food factors. Gakkai Shuppan Centre, Tokyo, pp 79–97
Kurakake M, Komaki T (2001) Production of β-mannanase and β-mannosidase from Aspergillus awamori K4 and their properties. Curr Microbiol 42:377–380
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nat 227:680–685
Larsson AM, Anderson L, Xu B, Muñoz IG, Usón I, Janson JC, Stålbrand H, Ståhlberg J (2006) Three-dimensional crystal structure and enzymic characterization of β-mannanase Man5A from blue mussel Mytilus edulis. J Mol Biol 357:1500–1510
Le Nours J, Anderson L, Stoll D, Stålbrand H, Lo Leggio L (2006) The structure and characterization of a modular endo-beta-1, 4-mannanase from Cellulomonas fimi. Biochem 44:12700–12708
Lu XJ, Chen XM, Fu DX, Cong W, Ouyang F (2002) Effect of Amorphophallus konjac oligosaccharides on STZ-induced diabetes model of isolated islets. Life Sci 72:711–719
Mao SM, Zhang HY, Zhang XW (2007) Clone and expression of β-mannanase gene from Bacillus subtilis. J Agric Biotechnol 15:360–361
McCleary BV (1988) β-D-Mannanase. Methods Enzymol 160:596–610
Mendoza NS, Arai M, Kawaguchi T, Yoshida T, Joson LM (1994) Purification and properties of mannanase from Bacillus subtilis. World J Microbial Biotechnol 10:551–555
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428
Moreira LR, Filho EX (2008) An overview of mannan structure and mannan-degrading enzyme systems. Appl Microbiol Biotechnol 79:165–178
Nakakuki T (2002) Present status and future of functional oligosaccharide development in Japan. Pure Appl Chem 74:1245–1251
Ootsuka S, Saga N, Suzuki K, Inoue A, Ojima T (2006) Isolation and cloning of an endo-β-1, 4-mannanase from Pacific abalone Haliotis discus hannai. J Biotechnol 125:269–280
Puchart V, Vršanská M, Svoboda P, Pohl J, Ögel ZB, Biely P (2004) Purification and characterization of two forms of endo-β-1, 4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749). Biochim Biophy Acta 1674:239–250
Reese ET, Shibata Y (1965) β-Mannanases of fungi. Can J Microbiol 11:167–183
Sako T, Matsumoto K, Tanaka R (1999) Recent progress on research and applications of non-digestible galacto-oligosaccharides. Int Dai J 9:69–80
Schröder R, Wegrzyn TF, Sharma NN, Atkinson RG (2006) LeMAN4 endo-β-mannanase from ripe tomato fruit can act as a mannan transglycosylase or hydrolase. Planta 224:1091–1102
Sheng DX, Teng JX (2008) Present status and future of konjac industry. China Agric Infor 7:39–40
Shimahara H, Suzuki H, Sugiyama N, Nisizawa K (1975) Partial purification of β-mannanase from the konjac tubers and their substrate specificity in relation to the structure of konjac glucomannan. Agric Biol Chem 39:301–312
Solange IM, Ismael MM (2007) Non-digestible oligosaccharides: a review. Carbohy Polymers 68:587–597
Tamaru Y, Araki T, Amagoi H, Mori H, Morishita T (1995) Purification and characterization of an extracellular β-1, 4-mannanase from a marine bacterium, Vibrio sp. strain MA-138. Appl Environ Microbiol 61:4454–4458
Wong KKY, Saddler JN (1993) Applications of hemicellulases in the food, feed, and pulp and paper industries. In: Coughlan MP, Hazlewood GP (eds) Hemicellulose and hemicellulases. Portland, London, pp 127–143
Yamaura I, Nozaki Y, Matsumoto T, Kato T (1993) Purification and some properties of endo-1, 4-β-D-mannanase from a mud snail, Pomacea insularus (de Ordigny). Biosci Biotech Biochem 57:1316–1319
Yoon KH, Lim BL (2007) Cloning and strong expression of a Bacillus subtilis WL-3 mannanase gene in B. subtilis. J Microbiol Biotechnol 17:1688–1694
Zakaria MM, Yamamoto S, Yagi T (1998) Purification and characterization of an endo-1, 4-β-mannanase from Bacillus subtilis KU-1. FEMS Microbiol Lett 158:25–31
Zhang J, He ZM, Hu K (2000) Purification and characterization of β-mannanase from Bacillus licheniformis for industrial use. Biotechnol Lett 22:1375–1378
Acknowledgments
The work was supported by the Program for New Century Excellent Talents in University (NCET-04-0637), Foundation for Young Excellent Scientists in Shandong Province (2004BS06001), and COMRA Program (DYXM-115-02-2-6).
Author information
Authors and Affiliations
Corresponding author
Additional information
Min Zhang and Xiu-Lan Chen contributed equally to this work.
Electronic supplementary materials
Below is the link to the electronic supplementary material.
ESM 1
(DOC 5718 kb)
Rights and permissions
About this article
Cite this article
Zhang, M., Chen, XL., Zhang, ZH. et al. Purification and functional characterization of endo-β-mannanase MAN5 and its application in oligosaccharide production from konjac flour. Appl Microbiol Biotechnol 83, 865–873 (2009). https://doi.org/10.1007/s00253-009-1920-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1920-0