Abstract
The co-culture of the suspension cells of Taxus chinensis var. mairei and its endophytic fungi, Fusarium mairei, in a 20-L co-bioreactor was successfully established for paclitaxel production. The co-bioreactor consists of two-unit tanks (10 L each) with a repairable separate membrane in the center, culturing Taxus suspension cells in one tank and growing fungi in another. By optimizing the co-culture conditions, there was a desirable yield of paclitaxel in Taxus cell cultures. The Taxus cell cultures by co-culture produced 25.63 mg/L of paclitaxel within 15 days; it was equivalent to a productivity of 1.71 mg/L per day and 38-fold higher than that by uncoupled culture (0.68 mg/L within 15 days). The optimum conditions for co-culture in the co-bioreactor were: B5 medium, inoculating fungi when Taxus cells had grown for 5 days in the co-bioreactor, hydrophilic separate membrane in the center of the co-bioreactor, and air flow rate of 1:0.85 v/v/m in fungus cultures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paclitaxel is an excellent anticancer drug. A major obstacle to the clinical use of this drug is its limited supply. Scientists have been seeking new ways to improve the production of this drug. Several methods have been developed for paclitaxel production, e.g., total chemical synthesis (Nicolaou et al. 1994; Holton et al. 1994), semisynthesis from its precursor (Commercn et al. 1995; Patel 1998), plant cell culture (Goleniowski 2000; Zhong 2002), etc. Additionally, certain endophytic fungi associated with Taxus produce paclitaxel, although the amounts are very small compared to those produced by the various Taxus species (Strobel et al. 1996; Li et al. 1998; Xu et al. 2006). Among these systems, plant cell suspension culture is considered as the most promising alternative approach to mass production of paclitaxel (Gibson et al. 1993) and paclitaxel production by plant cell suspension culture has been commercialized. The synthesis of second metabolite (such as paclitaxel) in plants is part of the plant defense responses to environmental stresses and pathogen attacks. The strategies of improving the yield of paclitaxel in plant cell suspension culture should be based on this mechanism (Zhao et al. 2005). Many elicitors and signal molecules, e.g., methyl jasmonate (MJ), arachidonic acid, silver ion, chitosan, La3+ ion, fungal mycelia extracts, etc., have been used to improve taxoid production by plant cell suspension culture based on this principle (Yukimune et al. 1996; Zhang and Wu 2003).
We derived a paclitaxel-producing endophytic fungus from the inner bark of China maire yew and identified it as Fusarium mairei (Xu et al. 2006). Symbiosis of plant–endophyte is a common phenomenon in their natural ecological environment. Some observations have reported that endophytic fungi induced the secondary metabolite accumulation in plant. When plant was inoculated by endophytic fungi, some secondary metabolites related to plant defenses were observed to accumulate in plant cells, such as terpenoid (Akiyama and Hayashi 2002), alkaloid (Rojas-Andrade et al. 2003), etc. Additionally, endophytic fungi also regulated plant growth via mediating the plant hormones in plant cells (Barker and Tagu 2000). In fact, endophytic fungi and their hosts exhibit a reciprocal relationship in a natural ecological system. So, it is believed that endophytic fungi may play a role in paclitaxel biosynthesis in the yew tree. Although the previous works have reported many strategies of improving the yield of paclitaxel in plant cell suspension cultures, e.g., using elicitors (Wu and Lin 2003; Furmanowa et al. 2000; Khosroushahi et al. 2006), novel bioreactor (Son et al. 2000), immobilized yew cells (Bentebibel et al. 2005), perfusion and continuous cultivation (Seki et al. 1997), etc., they all ignored the natural interactions of endophytic fungi and Taxus cells. Little information are available regarding utilization of plant–fungus interactions in improving paclitaxel production in Taxus cell suspension cultures. Based on these regards and the current investigations, we established a co-culture system of plant–fungus to produce paclitaxel via coupled culturing of Taxus suspension cells and endophytic fungi in a co-bioreactor. Practically, the co-culture was applied widely in many biotechnological processes, especially in those processes involving more than two kinds of microbes, e.g., the co-cultures of bacteria and fungi (Chávez-Gómez et al. 2003) and bacterial co-culture (Vatsala et al. 2008), etc. In plant secondary metabolite area, the previous works have reported that the co-culture of plant–plant enhanced the secondary metabolite synthesis (Łuczkiewicz and Kokotkiewicz 2005). To the best of our knowledge, those co-culture systems were binary processes in different devices. There are no commercial devices available which could be used for co-culture performed in one bioreactor for large-scale experiments and no reported works on the use of live microbial cells to stimulate the secondary metabolite production by co-culture of plant suspension cells and fungi.
In this paper, the conditions were developed for co-culture of Taxus cells and paclitaxel-producing endophytes. Also, the stimulation of paclitaxel production by live fungi and the counteracting effect of plant cell and fungus growth were analyzed.
Materials and methods
Seed cultures of plant cell and endophytic fungus
Taxus chinensis var. mairei (line C206) cells were grown under darkness on a gyratory shaker at 100 rpm and 25 ± 1°C, in liquid Gamborg’s B5 medium (Gamborg et al. 1968) supplemented with sucrose 25 g/L, naphthalene acetic acid 2 mg/L, 6-benzylamino purine 0.15 mg/L, and casein acid hydrolysate 1 g/L. An aliquot (20 mL) of a 12-day-old subcultured suspension cultures (approximately 6 g of fresh cells weight) was inoculated into 80 mL of B5 medium in a 500-mL Erlenmeyer flask. Further subculturing was done once at 12-day intervals. The seed cultures were subcultured for four generations in flasks prior to being inoculated into a co-bioreactor.
Fusarium mairei (strain Y178; China patent no. ZL200510038424.8) was screened from China maire yew and maintained in our laboratory. The fungi can produce ∼200 μg/L of paclitaxel in its paclitaxel-producing medium (Xu et al. 2006). The seed cultures of the fungi were performed by transferring the tubes of solid cultures to a 500-mL Erlenmeyer flask containing 100 mL B5 medium, growing on a gyratory shaker at 170 rpm and 25 ± 1°C for 3 days before being inoculated into a co-bioreactor.
Cultures in a co-bioreactor and conditions for co-culture
When enough cellular biomass in a linear growth phase was available, Taxus cell seed cultures were transferred to the co-bioreactor under sterile condition. Fungus seed cultures were inoculated to the co-bioreactor according to designated experiment timings. The co-culture conditions established are summarized in Table 1.
Configuration of the co-bioreactor
As shown in Fig. 1, the co-bioreactor (China patent no. ZL200410014227.8) consists of two-unit turbine-stirred tanks with a repairable separate membrane in the center of the tanks. The chemicals in the tanks can exchange through the separate membrane, but the cells cannot pass through. The volume of each tank is 10 L. Sterilizing medium was carried out by steam at 121°C for 30 min. The temperature of the cultures in the co-bioreactor was controlled by circulating water through the outside jacket. Sparging air volume was adjusted using an air flow meter to give a constant flow rate. The ferment conditions are shown in Table 1.
Paclitaxel analysis
In this set of experiments, paclitaxel only in plant cell cultures was considered. The paclitaxel in the fungus cultures of the co-bioreactor was not calculated since it is not confirmed whether the paclitaxel was produced by the fungi or derived from Taxus cell cultures through the center membrane.
For the extraction of cell-associated paclitaxel from Taxus cells, 100 mg of dried cells were powdered in a glass mortar with a pestle, soaked with 20 mL methanol for 12 h, and mixed with 20 mL distilled water. The mixture was extracted three times with 20 mL of trichloromethane. The trichloromethane phase was separated from the aqueous phase and evaporated at 45°C under vacuum. The remaining paclitaxel were re-dissolved in 1 mL of methanol and filtered through a 0.2-μm filter prior to high-performance liquid chromatography (HPLC).
Extracellular paclitaxel was extracted three times from the culture medium using the equivolume of trichloromethane.
Quantification of the paclitaxel was performed via a reverse-phase HPLC system (Agilent) with a XDB-C18 column (4.6 × 250 mm, 5 μm) and a mobile phase consisting of methanol–water at 65:35 (v/v) with a flow rate of 1 mL/min and detected by a UV detector at 227 nm (Yuan et al. 2002; Wang et al. 2001). Identification of the paclitaxel was accomplished by comparison of retention times with authentic standards (Sigma).
Statistics
The experimental data are reported as the mean of the three independent experiments. The significance of differences among experimental points was determined by two-sample paired t tests. P < 0.05 was considered statistically significant. Results are expressed as mean ± SD.
Results
Cell growth and paclitaxel production of Taxus cultures by co-culture system and uncoupled culture system in a co-bioreactor
In a co-culture system, F. mairei was inoculated into the co-bioreactor when Taxus cells had grown for 5 days. Uncoupled culture was performed also in a co-bioreactor but with no fungal inoculums. The complete identical conditions for coupled and uncoupled culture were established as in Table 1. The growth of Taxus cell is indicated by growth index, which was defined as the ratio of the final wet cell weight to the inoculum wet weight. The results are shown in Fig. 2. Compared to shake-flask culture (growth index 3.42 at day 15), the maximum growth of Taxus cell using uncoupled culture at day 15 (growth index, 2.64) was 23% lower (Fig. 2a). But no significant difference in cell growth was observed between shake-flask culture and co-culture (growth index 3.18 at day 15, P > 0.05). The scale-up from shake flask to bioreactor usually results in reduced productivity (Alvarez et al. 2005); but, compared to shake-flask culture, the co-culture was more efficient in paclitaxel production (Fig. 2b), giving a yield of paclitaxel at 25.63 mg/L in Taxus cultures at 15 days, which was equivalent to a rate of 1.71 mg/L per day, 27-fold higher than shake-flask culture (0.96 mg/L at 15 days) and 38-fold higher than uncoupled culture (0.68 mg/L at 15 days). The paclitaxel yield by co-culture began to increase sharply from day 7 (the second day after inoculating fungi), but the Taxus cultures exhibited a small decline in paclitaxel content from 18 mg/L at day 8 to 15.62 mg/L at day 10. This decline was likely a result of paclitaxel passing the middle separate membrane from tank A (Taxus cultures) to tank B (fungal cultures). Furthermore, paclitaxel increases only slightly from day 12 (24.02 mg/mL) to day 15 (25.63 mg/L). So, it may be presumed that the optimal harvest time of paclitaxel was during days 7–10 after inoculating fungi in the co-culture system.
Medium studies in co-culture
In this set of experiments, co-culture was performed with MS or B5 mediums containing identical hormones under the above-indicated co-culture conditions (Table 1), and F. mairei was inoculated into the co-bioreactor when Taxus cells had grown for 5 days. Figure 3 shows that B5 medium was more suitable for Taxus cell growth in co-culture. The growth ratio of Taxus cell using B5 medium was 3.12 at 15 days, while only 2.35 by using MS medium. The previous reports suggested the existence of an inverse relationship between cell growth and paclitaxel accumulation (Seki et al. 1997; Ketchum et al. 1999), but in our works, B5 medium led to a desirable paclitaxel yield (25.72 mg/L) and also a higher Taxus cell growth compared to MS medium (paclitaxel, 12.8 mg/L).
Effects of separate membrane in the center of the co-bioreactor on the cell growth and paclitaxel production of Taxus cultures
The separate membrane in the center of the co-bioreactor may control the substance exchange between tanks A and B, which substances likely work on the growth of Taxus cells, the fungi growth, and the paclitaxel accumulation. Two kinds of separate membrane, one 0.25-μm hydrophilic pyroxylin filter and another 0.25-μm lipophilic filter, were tested in this experiment with the MS medium. Except the separate membrane, the other co-culture conditions were the same as shown in Table 1. Figure 4 shows that hydrophilic pyroxylin filter considerably favored cell growth and paclitaxel production in Taxus cell cultures. The Taxus cell cultures using hydrophilic filter presented a cell growth index of 2.47 and a paclitaxel content of 12.8 mg/L at day 15, while those were 2.15 and 7.11 mg/L, respectively, in Taxus cell cultures using lipophilic filter. It can be deduced that a characteristic of effective chemicals produced by endophytic fungi was being hydrophilic; they regulated the cell growth of Taxus cultures and elicited paclitaxel formation. Lipophilic filter hindered the exchange of the effective chemicals between tanks and resulted in low paclitaxel yield.
Cell growth and paclitaxel accumulation of Taxus cell cultures in a co-bioreactor with different separate membranes. Experimental medium was MS, and F. mairei was inoculated into the co-bioreactor when Taxus cells had grown for 5 days. Samples were taken at day 15. All data represent mean ± SD (n = 3)
Air flow rate of fungus cultures in a co-culture
Endophytic fungi in a co-culture would produce a series of complex substances that induce the paclitaxel production and regulate the cell growth of Taxus. The formation of these substances was closely related to the culture conditions of fungi, especially aeration parameters. Figure 5 shows that lower air flux of fungus cultures was conducive to fungus growth but inhibited plant cell growth and paclitaxel formation. The paclitaxel yield of Taxus cell cultures increased synchronically with the air flux when the air flux of fungus cultures was <6 L/min, but the cultures would produce a lot of foam when the air flux of fungus cultures was >6 L/min. Thus, the optimal air flux of fungus cultures was 6 L/min, equivalent to the air flow rate of 1:0.85 v/v/m (based on 7 L of culture volume).
Effects of the aeration in fungal cultures on cultures. Experimental medium was B5 medium. F. meivai was inoculated into the co-bioreactor when Taxus cells had grown for 5 days. Paclitaxel labeled in graph indicates the paclitaxel yield in Taxus cell cultures. Samples were taken at day 15. All data represent mean ± SD (n = 3). DW=dry weight
Inoculation timing of endophytic fungi
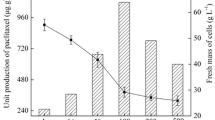
According to the previous reports, the optimal inducing time by elicitors in Taxus cell suspension cultures was at late exponential growth phase of Taxus cells (Wang et al. 2001). As a similar reason, the timing of fungus inoculation is an important factor for co-culture and only an optimal timing of fungus inoculation can result in a desirable paclitaxel yield. In our work, three timings, 5, 10, and 15 days after inoculating Taxus cells, were tested under the aforementioned co-culture conditions (Table 1). Graphs labeled accordingly in Fig. 6 show that Taxus cell cultures presented a slightly higher volume yield of paclitaxel when fungi were inoculated at day 10 (26.58 mg/L) than that at day 5 (25.63 mg/L). Since the total culture time when fungi were inoculated at day 10 is 20 days and that at day 5 is 15 days (total co-culture time are all 10 days), Taxus cell cultures demonstrated a higher paclitaxel productivity of 1.71 mg/L per day when fungi were inoculated at day 5 and that was 1.33 mg/L per day at day 10. From this result, it seems evident that the fungus inoculation at day 5 not only enhanced paclitaxel production but also shortened the culture period, thus resulting in higher paclitaxel productivity. Additionally, the growth of fungi decreased with its inoculation time. The biomass of fungi at day 15 (15.26 g/L) decreased by 50% compared with day 5 (32.75 g/L). The over-decrease in fungi biomass would affect its active chemicals production and resulted in low paclitaxel yield.
Effects of fungus inoculation timing on cultures. Experimental medium was B5 medium. The co-culture times after inoculating fungi are all 10 days. Samples were taken at the finished day of the co-culture. Paclitaxel labeled in graph indicates the paclitaxel yield in Taxus cell cultures. All data represent mean ± SD (n = 3). DW=dry weight
Discussion
The present works show that the growth of Taxus cells by co-culture was higher than that by uncoupled culture (Fig. 2a); it is suggested that endophytic fungi stimulated the growth of Taxus cell. We found that the efficient chemical stimulating Taxus cell growth was gibberellic acid as F. mairei produce ∼2.7 mg/L of gibberellic acid in B5 or MS medium (data not shown). On the other hand, the plant cell cultures also effected the growth of fungi obviously. Figure 6 indicates short-time cultures of Taxus cell resulting in higher fungi biomass, likely due to the reason that substances from Taxus cell cultures inhibited the growth of fungi, and these substances increased with the culture time of the plant cells.
Oligosaccharide is a well-known elicitor in potentiating paclitaxel production (Linden and Phisalaphong 2000). According to the results in Fig. 2b, endophytic fungi strongly stimulated the paclitaxel formation of Taxus cell cultures in the co-bioreactor, which was possibly attributed to the induction of the oligosaccharide since F. mairei may produce an oligosaccharide with molecular masses ∼2 kDa in B5 or MS medium (data not shown).
From the present results, the co-culture of plant cells and fungi proved to be an efficient method of promoting paclitaxel yield, but the control of the growth of fungi is the key of this method. The over-growth of fungi would cause an inhibition or death of plant cells as well as exhaustion of nutrients from the medium. We controlled the growth of fungi via the relatively small inoculum size of fungus (1.5%, v/v), a higher air flux of fungus cultures (Fig. 5), and the appropriate timing of fungus inoculation (Fig. 6). In this way, the metabolites produced by live fungi switched on the secondary metabolism of Taxus cells from primary metabolism, and the secondary metabolites of Taxus cells depressed the over-growth of fungi. Although we must hinder the over-growth of fungi in co-culture, the over-low biomass of fungi did not favor the growth of Taxus cell and paclitaxel production (Fig. 6). The fungi biomass should be controlled to an optimal level.
The co-culture system supplied a similar symbiosis circumstance for plant cell and its endophytic fungus. The exchange of available substances (e.g., elicitors produced by fungi to plant, inhibitors produced by plant cells to fungi, etc.) via the separate membrane in the middle of the system regulated their growths and enhanced the paclitaxel accumulation. In batch elicitation culture, stimulating effects of elicitors to plant cells would be short-lived and very limited (Wang et al. 2001; Wang and Zhong 2002), but those in a co-culture system was uninterrupted and long-lived. Thus, compared to batch elicitation culture, the co-culture system showed higher paclitaxel yield. Bentebibel et al. (2005) reported a paclitaxel productivity of 2.71 mg/L per day using immobilized cells elicited with MJ in a 5-L turbine-stirred bioreactor; it was fivefold higher than that of the control cells. The paclitaxel productivity of 1.71 mg/L per day obtained in our work was lower than that reported by Bentebibel, but its increased extent (27-fold more than that using shake-flask culture) was much higher.
In conclusion, the novelty of this work was to establish a co-culture system of Taxus cells and endophytic fungi for paclitaxel production. Under the optimum culture conditions, the co-culture system not only strongly improved the production of paclitaxel but also shortened the culture time. The optimal co-culture conditions are: B5 medium, hydrophilic separate membrane, fungus inoculation at day 5, and air flow rate of 1:0.85 v/v/m in fungus cultures.
References
Akiyama K, Hayashi H (2002) Arbuscular mycorrhizal fungus-promoted accumulation of two new triterpenoids in cucumber roots. Biosci Biotechnol Biochem 66:762–769
Alvarez MM, Guzmán A, Elías M (2005) Experimental visualization of mixing pathologies in laminar stirred tank bioreactors. Chem Eng Sci 60:2449–2457
Barker SJ, Tagu D (2000) The roles of auxins and cytokinins in mycorrhizal symbioses. J Plant Growth Reg 19:144–154
Bentebibel S, Moyano T, Palazon J, Cusidó RM, Bonfill M, Eibl R, Piñol T (2005) Effects of immobilization by entrapment in alginate and scale-up on paclitaxel and baccatin III production in cell suspension cultures of Taxus baccata. Biotechnol Bioeng 89:647–655
Chávez-Gómez B, Quintero R, Esparza-García F, Mesta-Howard AM, Zavala Díaz de la Serna FJ, Hernández-Rodríguez CH, Gillén T, Poggi-Varaldo HM, Barrera-Cortés J, Rodríguez-Vázquez R (2003) Removal of phenanthrene from soil by co-cultures of bacteria and fungi pregrown on sugarcane bagasse pith. Bioresour Technol 89:177–183
Commercn A, Bourzat JD, Didier E, Lavelle F (1995) Practical semisynthesis and antimitotic activity of docetaxel and side-chain analogs. ACS Symp Ser 583:233–246
Furmanowa M, Olędzka H, Sykłowska-Baranek K, Józefowicz J, Gieracka S (2000) Increased taxane accumulation in callus cultures of Taxus cuspidata and Taxus x media by some elicitors and precursors. Biotechnol Lett 22:1449–1452
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gibson DM, Ketchum REB, Vance NC, Christen AA (1993) Initiation and growth of cell lines of Taxus brevifolia (Pacific yew). Plant Cell Rep 12:479–482
Goleniowski ME (2000) Cell lines of Taxus species as source of the anticancer drug taxol. Biocell 24:139–144
Holton RA, Somoza C, Kim HB, Liang F, Biediger RJ, Boatman PD, Shindo M, Smith CC, Kim S, Nadizadeh H, Suzuki Y, Tao C, Vu P, Tang S, Zhang P, Murthi KK, Gentile LN, Liu JH (1994) First total synthesis of taxol. I. Functionalization of the B ring. J Am Chem Soc 116:1597–1598
Ketchum REB, Gibson DM, Croteau RB, Shuler ML (1999) The kinetics of taxoid accumulation in cell suspension cultures of Taxus following elicitation with methyl jasmonate. Biotechnol Bioeng 62:97–105
Khosroushahi AY, Valizadeh M, Ghasempour A, Khosrowshahli M, Naghdibadi H, Dadpour MR, Omidi Y (2006) Improved taxol production by combination of inducing factors in suspension cell culture of Taxus baccata. Cell Biology Int 30:262–269
Li JY, Sidhu RS, Ford EJ, Long DM, Hess WM, Strobel GA (1998) The induction of taxol production in the endophytic fungus—Periconia sp from Torreya grandifolia. J Indust Microbiol Biotechnol 20:259–264
Linden JC, Phisalaphong M (2000) Oligosaccharides potentiate methyl jasmonate-induced production of paclitaxel in Taxus canadensis. Plant Sci 158:41–51
Łuczkiewicz M, Kokotkiewicz A (2005) Co-cultures of shoots and hairy roots of Genista tinctoria L. for synthesis and biotransformation of large amounts of phytoestrogens. Plant Sci 169:862–871
Nicolaou KC, Yang Z, Liu JJ, Ueno H, Nantermet PG, Guy RK, Claiborne CF, Renaud J, Couladouros EA, Paulvannan K, Sorensen EJ (1994) Total synthesis of taxol. Nature 367:630–634
Patel RN (1998) Tour de paclitaxel: biocatalysis for semisynthesis. Annu Rev Microbiol 98:361–395
Rojas-Andrade R, Cerda-Garcia-Rojas CM, Frias-Hernandez JT, Dendooven L, Olalde-Portugal V, Ramos-Valdivia AC (2003) Changes in the concentration of trigonelline in a semi-arid leguminous plant (Prosopis laevigata) induced by an arbuscular mycorrhizal fungus during the presymbiotic phase. Mycorrhiza 13:49–52
Seki M, Ohzora C, Takeda M, Furusaki S (1997) Taxol (paclitaxel) production using free and immobilized cells of Taxus cuspidata. Biotechnol Bioeng 53:214–219
Son SH, Choi SM, Lee YH, Choi KB, Yun SR, Kim JK, Park HJ, Kwon OW, Noh EW, Seon JH, Park YG (2000) Large-scale growth and taxane production in cell cultures of Taxus cuspidata (Japanese yew) using a novel bioreactor. Plant Cell Rep 19:628–633
Strobel G, Yang X, Sears J, Kramer R, Sidhu RS, Hess WM (1996) Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallichiana. Microbiol 142:435–440
Vatsala TM, Mohan Raj S, Manimaran A (2008) A pilot-scale study of biohydrogen production from distillery effluent using defined bacterial co-culture. Int J Hydrogen Energy 33:5404–5415
Wang ZY, Zhong JJ (2002) Repeated elicitation enhances taxane production in suspension cultures of Taxus chinensis in bioreactors. Biotechnol Lett 24:445–448
Wang CG, Wu JY, Mei XG (2001) Enhancement of taxol production and excretion in Taxus chinensis cell culture by fungal elicitation and medium renewal. Appl Microbiol Biotechnol 55:404–410
Wu J, Lin L (2003) Enhancement of taxol production and release in Taxus chinensis cell cultures by ultrasound, methyl jasmonate and in situ solvent extraction. Appl Microbiol Biotechnol 62:151–155
Xu F, Tao WY, Cheng L, Guo LJ (2006) Strain improvement and optimization of the media of taxol-producing fungus Fusarium mairei. Biochem Eng J 31:67–73
Yuan YJ, Li C, Hu ZD, Wu JC (2002) A double oxidative burst for taxol production in suspension cultures of Taxus chinensis var. mairei induced by oligosaccharide from Fusarium oxysporum. Enzyme Microb Technol 30:774–778
Yukimune Y, Tabata H, Higashi Y, Hara Y (1996) Methyl jasmonate-induced over-production of paclitaxel and baccatin III in Taxus cell suspension cultures. Nat Biotechnol 14:1129–1132
Zhang CH, Wu JY (2003) Ethylene inhibitors enhance elicitor-induced paclitaxel production in suspension cultures of Taxus spp. cells. Enzyme Microb Technol 32:71–77
Zhao J, Davis LC, Verpoorte C (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Zhong JJ (2002) Plant cell culture for production of paclitaxel and other taxanes. J Biosci Bioeng 94:591–599
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (grant nos. 30370044 and 30470016) and National “863” High-Tech Project of China (grant no. 2007AA021501).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, YC., Tao, WY. & Cheng, L. Paclitaxel production using co-culture of Taxus suspension cells and paclitaxel-producing endophytic fungi in a co-bioreactor. Appl Microbiol Biotechnol 83, 233–239 (2009). https://doi.org/10.1007/s00253-009-1856-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-009-1856-4